 J Clin Aesthet Dermatol. 2018;11(11):25–35
J Clin Aesthet Dermatol. 2018;11(11):25–35
by Andrew Alexis, MD, MPH; James Q. Del Rosso, DO; Seemal R. Desai, MD, FAAD; Jeanine B. Downie, MD, FAAD; Zoe Diana Draelos, MD; Christina Feser, DO; Rion Forconi, MD; Joseph F. Fowler Jr., MD; Michael Gold, MD; Joely Kaufman-Janette, MD; Edward Lain, MD; Mark Lee, MD; Mark Ling, MD; Ava T. Shamban, MD; WM. Philip Werschler, MD FAAD, FAACS; and AnnaMarie Daniels, BS
Dr. Alexis is with Mount Sinai St. Luke’s in New York, New York. Dr. Del Rosso is with JDR Dermatology Research in Las Vegas, Nevada. Dr. Desai is with ACRC Trials/Innovative Dermatology in Plano, Texas. Dr. Downie is with Image Dermatology in Montclair, New Jersey. Dr. Draelos is with Dermatology Consulting Services in High Point, North Carolina. Drs. Feser and Forconi are with International Clinical Research in Sanford, Florida. Dr. Fowler is with DS Research in Louisville, Kentucky. Dr. Gold is with the Tennessee Clinical Research Center in Nashville, Tennessee. Dr. Kaufman-Janette is with the Skin Research Institute in Coral Gables, Florida. Dr. Lain is with the Austin Institute for Clinical Research in Heatherwilde, Texas. Dr. Lee is with Progressive Clinical Research in San Antonio, Texas. Dr. Ling is with MedaPhase in Newnan, Georgia. Dr. Shamban is with Medical and Cosmetic Dermatology in Santa Monica, California. Dr. Werschler is with Premier Clinical Research in Spokane, Washington. Ms. Daniels is with BioPharmX in Menlo Park, California.
Abstract: Background and objectives. Acne vulgaris is a highly prevalent and multifactorial skin disorder that can adversely impact health-related quality of life. Factors that contribute to the pathogenesis of acne include pilosebaceous proliferation of proinflammatory Propionibacterium acnes (P. acnes) bacteria, presence of circulating androgens, excess sebum production, abnormal follicular keratinization, and multiple inflammatory cascades. Oral tetracyclines—especially doxycycline and minocycline—are frequently prescribed for the treatment of moderate-to-severe acne, given their anti-inflammatory properties and their effect on P. acnes reduction. Notwithstanding their established efficacy in the management of acne vulgaris, there is a desire to limit systemic exposure to antibiotics given growing concerns regarding bacterial resistance as well as the potential for serious side effects. This report describes outcomes of two randomized, vehicle-controlled trials (Phases IIa and IIb) of BPX-01, a topical minocycline gel, in the treatment of moderate-to-severe acne.
Methods. In Study 1 (NCT02709096), at a single center, 33 subjects with highly fluorescing facial skin were randomized 2:1 to BPX-01 1% or vehicle control once-daily treatment for four weeks. Changes in P. acnes quantitative bacteriological cultures were assessed, as well as cutaneous tolerance to the study drug by both subjects and the investigator. In Study 2 (NCT02815332), subjects with moderate-to-severe inflammatory nonnodular acne (n=226) at 15 centers were randomized 1:1:1 to treatment with BPX-01 1%, BPX-01 2%, or vehicle control once-daily for 12 weeks. The primary endpoint was reduction in the number of inflammatory lesions; other endpoints included the number of noninflammatory lesions, Investigator’s Global Assessment (IGA) of severity, and subjective ratings (investigator and subject) of acne. In both studies, cutaneous tolerability and safety were assessed, and plasma minocycline levels were tracked with a highly sensitive assay.
Results. In Study 1, BPX-01 treatment reduced P. acnes colonization by 90.9 percent, which exceeded the reduction in the vehicle control group (65.53%; p=0.020). In Study 2, treatment with BPX-01 2% reduced the number of inflammatory lesions by 58.5 percent, exceeding the reduction in the vehicle control group (43.8%; p=0.0256). Trends toward an improvement preferential to BPX-01 2% were observed in the other endpoints. Across both studies, BPX-01 treatment was well-tolerated, with no photosensitivity, postinflammatory hyperpigmentation, or skin discoloration reported. A single subject (out of 259 study participants ) was identified to have detectable levels of plasma minocycline at low levels (42ng/mL) after 12 weeks of treatment but had no signs or symptoms associated with systemic administration of minocycline.
Conclusion. BPX-01 appears to exhibit an effectiveness profile for reduction of inflammatory (nonnodular) acne lesions similar to that of oral minocycline formulations. However, because BPX-01 is topical and exhibits negligible systemic exposure, the likelihood of adverse events associated with oral minocycline use is much lower. These results demonstrate effectiveness of BPX-01 topical minocycline gel in reducing P. acnes colonization, suggesting that the BPX-01 2% formulation is a promising treatment for moderate-to-severe nonnodular, inflammatory acne vulgaris in both reduction of inflammatory lesions and also overall improvement in facial acne according to IGA.
KEYWORDS: Acne vulgaris, Propionibacterium acnes, P. acnes, minocycline, topical antibiotic, plasma concentration levels
Introduction
Acne vulgaris is a highly prevalent skin disorder, affecting approximately 85 percent of the population at some time; it most often appears between the ages of 12 years and 24 years, but can develop as early as the age of nine years and can persist well past the age of 25 years. It is chronic and multifactorial, best described as an inflammatory dermatosis.1–3 The clinical spectrum of acne includes the presence of inflammatory papules, pustules, or nodules and comedones (open and closed; noninflammatory) involving the face, chest, and back. Acne scarring and postinflammatory hyperpigmentation are potential long-term sequelae of the disease. Its deleterious effect on health-related quality of life is considerable.4 During puberty, surges in androgens cause an overproduction of sebum along with abnormal keratinocyte desquamation of the epidermal lining of the pilosebaceous follicle. Blocked by excess sebum and skin debris, the obstructed acne-affected follicle is an ideal environment for the proliferation of Propionibacterium acnes (P. acnes) bacteria, which in turn induce local inflammatory reactions via innate immune responses.5,6
The management of acne involves the use of topical and/or oral therapies that target one or more of the aforementioned pathogenic factors. The most effective acne therapies work by targeting the sebaceous gland and decreasing sebum production. Clinicians often use a combination therapy approach to acne management, employing topical treatments such as antibacterials, antibiotics, and retinoids prior to prescribing oral antibiotics, hormone therapy, or oral isotretinoin. Light and laser devices have also shown utility.3 Designing rational therapeutic regimens based on the predominant types of acne lesions and overall clinical severity is the recommended approach.7
Recent work with topical agents has demonstrated a significant reduction in P. acnes levels as soon as 24 hours after application of a 5% benzoyl peroxide + 1% clindamycin phosphate gel combination treatment, with 99.9-percent inhibition occurring after two weeks of treatment. In the same study, two weeks of treatment with clindamycin phosphate 1% solution alone resulted in 77-percent inhibition of P. acnes levels.8 Separately, a 2.5% benzoyl peroxide + 0.1% adapalene combination gel inhibited P. acnes populations after two week and four weeks of treatment (by 1.1 and 1.6 log10 reductions in colony-forming units per square centimeter of skin [cfu/cm2], respectively). The treatment was effective in reducing both antibiotic-sensitive and antibiotic-resistant P. acnes subpopulations.9
The application of oral antibiotics, although commonplace, can be problematic amid concerns for limiting antibiotic exposure in light of the development of widespread antibiotic resistance patterns.10 The skin naturally harbors a diverse microbiota that can be important for natural cutaneous immune functions and can also interact with the microbial flora elsewhere in the body. The use of oral antibiotics for acne treatment can alter systemic levels of host-protective bacteria, for example through the increase in the emergence of antibiotic-resistant P. acnes and other cutaneous bacterial strains (e.g., Staphylococcus epidermidis) as well as changes in the gastrointestinal tract microbiome.11 This is especially important given the evolution of methicillin-resistant Staphylococcus aureus (MRSA) strains.12 Recent data suggest that there might be far-reaching complications of long-term antibiotic therapy. Statistical analyses have shown worrisome associations of oral antibiotic therapy for acne with, among others, the development of inflammatory bowel disease13 and lupus erythematosus.14 Thus, judicious antibiotic use is increasingly important in the treatment of dermatologic conditions that might require long-term therapy.
Minocycline is an antibiotic currently available in multiple oral dosage forms for treating various bacterial infections. Minocycline reduces the growth of bacteria through the inhibition of protein synthesis by blocking aminoacyl-transfer-ribonucleic acid (RNA) binding to the messenger RNA–ribosome complex. Minocycline is active against a number of gram-positive and gram-negative organisms, including P. acnes. The mechanism through which minocycline ameliorates acne is not fully elucidated, although inflammation might be decreased along with bacterial counts to reduce the size and quantity of lesions.15,16 Minocycline given systemically has been particularly successful in the treatment of moderate-to-severe inflammatory acne vulgaris. However, potentially serious side effects associated with oral minocycline include (but are not limited to) hepatotoxicity; metabolic effects; photosensitivity; discoloration of the teeth, skin, and mucous membranes; lupus-like syndrome; drug hypersensitivity syndrome; central nervous system (CNS) effects such as vertigo and dizziness; and pseudotumor cerebri.17–20 There is currently no commercially available topical dosage form of minocycline.
BPX-01 is a newly developed topical minocycline hydrochloride (HCl) gel. In preclinical studies, uptake of BPX-01 has been confirmed to penetrate the epidermis to gain access to pilosebaceous units, with local minocycline levels above the minimum inhibitory concentration (MIC) value for P. acnes.20–22 The current report describes studies for the reduction in baseline P. acnes populations with BPX-01 1% minocycline topical gel and for the safety and efficacy of treatment with BPX-01 1% and 2%. Portions of this report have previously been presented as posters at scientific meetings.23–25
Methods
Study 1: Phase IIa safety and effectiveness. A Phase IIa randomized, controlled trial was conducted at a single center in 2016. The study was publicly registered on ClinicalTrials.gov (NCT02709096), was institutional review board (IRB)-approved, and all subjects gave informed consent. The objectives of the study were to evaluate the safety (adverse events [AEs], skin reactions) of treatment with BPX-01 and its ability to reduce P. acnes. Subjects were assessed at baseline, after four weeks of treatment, and after another two weeks without treatment (i.e., at 6 weeks after baseline).
Eligible subjects were adults aged between 18 and 40 years who showed a high degree of fluorescence of the face under a Wood’s lamp at the screening visit. Subjects were excluded on the basis of a medical condition or skin disease that would interfere with study conduct or interpretation, current or planned pregnancy during the study, recent use of topical or systemic antibiotics, or concurrent participation in another clinical trial. Subjects agreed to refrain from using antimicrobial topical products during the study.
The study medication was BPX-01 (BioPharmX Inc., Menlo Park, California), a hydrophilic preparation of minocycline HCl in a topical gel. Subjects were assigned to use a 1% formulation of BPX-01, provided in single-dose aluminum tubes calculated to deliver approximately 1g of the drug product, or the matching vehicle control, at each daily application. Subjects were instructed to apply the assigned gel to the entire clean face once per day for four weeks.
Subjects were randomized 2:1 to receive BPX-01 1% or the vehicle control. Double-blinding was employed via a randomization scheme provided by the study sponsor.
Quantitative bacteriologic cultures were obtained according to a modification of the standard technique.26 The cheeks of subjects were cleaned, and a 4cm2 area was vigorously swabbed with a wash solution. The sample was diluted and cultured anaerobically in agar for seven days. Total densities of P. acnes were reported as log10 cfu/cm2.
Local application site reaction assessments of erythema, edema, scaling/peeling, and hyperpigmentation were made by the investigator on a four-point scale (0=none to 3=severe). Cutaneous tolerability ratings of burning/stinging, tightness, and itching were completed by the subjects on a four-point scale (0=none to 3=severe). Serum chemistry and hematology panels were completed at baseline and throughout the study at the designated time points. AEs, including headaches and changes in vision, which are indicative of AEs associated with minocycline use, were tracked at every study visit.27–29 Minocycline plasma levels were obtained using a highly sensitive assay with a limit of quantification of 10ng/mL.
Analysis populations included the safety group (all randomized subjects), modified intent-to-treat (MITT) group (completing all visits), and per-protocol (PP) group (no missing visits or evaluations and no major protocol deviations). Standard data management procedures were employed. Analysis included descriptive statistics (means, standard deviations (SDs), and proportions) and hypothesis testing with two-sided paired t-tests with equal variance and significance defined as p<0.05.
Study 2: Phase IIb effectiveness and dosage. This study was a multicenter, Phase IIb randomized, controlled trial. The study was registered publicly (ClinicalTrials.gov: NCT02815332) and enrolled subjects across 15 study centers in the United States between August 2016 and January 2016. The study had the oversight of IRBs, and all subjects gave written informed consent prior to any study procedures. The primary objective of the study was to evaluate the efficacy in terms of quantitative reduction in inflammatory lesions of two dosages of the topical study medication versus the efficacy of vehicle gel in a double-blinded fashion. Secondary objectives were to evaluate changes in investigator’s rating of acne severity, circulating plasma levels of the study medication, and safety. After completing screening assessments, subjects were evaluated at a baseline pretreatment visit and after two, four, eight, and 12 weeks of treatment.
Potential participants were recruited from the clinical populations of the investigators, using the local media and via targeted online advertising. Subjects of both sexes were between the ages of 9 and 40 years, inclusive, and had moderate-to-severe inflammatory, non-nodular acne vulgaris. This was defined as 20 to 60 inflammatory lesions on the face, 0 to 100 noninflammatory lesions on the face, and an Investigator’s Global Assessment (IGA, a five-point scale from 0=clear skin to 4=severe acne30) rating of 3 or 4 at baseline. Exclusions for participation included current or planned pregnancy or medical conditions and/or medication use (including acne treatments) that could compromise safety or study data interpretation. Subjects agreed to maintain their dosages of any allowed medications as well as to continue their customary use of make-up, facial cleansers, facial creams, and other facial care during the study period. All concomitant medications were tracked throughout the study.
As in the Phase IIa study, BPX-01 topical gel was provided in 1-g aluminum tubes. Subjects were assigned to use 1% or 2% formulations of BPX-01 or the matching vehicle control at each daily application. Subjects were instructed to apply the assigned gel to the entire clean face at least 30 minutes before bedtime every day for 12 weeks.
Subjects were randomized 1:1:1 to receive BPX-01 1%, BPX-01 2%, or the vehicle control. The randomization scheme was generated a priori by the study statistician and administered by an online interactive web response system. Randomization was stratified by study site to ensure an approximately equal distribution. The study was double-blinded. Because the three topical preparations were in identical tubes, subjects were unaware of their assignment. Furthermore, because the randomization list was locked and medication dispensing was based only on randomization codes, investigators and study staff were also blinded to subject assignments.
The primary endpoint was the absolute mean change in the number of inflammatory lesions on the face after 12 weeks of treatment, relative to baseline. The secondary endpoint was the proportion of subjects with at least a two-grade reduction in their baseline IGA rating, to 0 (“clear”) or 1 (“almost clear”), after 12 weeks of treatment. Exploratory endpoints included changes in the number of inflammatory and noninflammatory lesions and IGA ratings at other time points, changes in the Patient Global Impression of Severity (PGI-S; a 7-point scale31) ratings, and PGI-Improvement (PGI-I; a 7-point scale31) ratings.
Safety endpoints were the incidence of treatment-emergent AEs during the study; shifts from baseline in hematology and laboratory tests; changes from baseline in local tolerability (e.g., erythema, scaling, edema, tightness, discomfort); incidence of minocycline-induced skin hyperpigmentation; and the incidence of visual disturbances or headaches suggestive of pseudotumor cerebri, which can indicate systemic minocycline exposure.27–29
Adherence by participants with the daily dosing schedule was confirmed by review of their diaries and the utilization of test products. The pharmacokinetic endpoint was plasma level of minocycline at baseline and after four and 12 weeks of treatment, using a highly sensitive assay with a limit of quantification of 10ng/mL. Subjects completed a satisfaction questionnaire at the 12-week exit visit.
The sample size for the primary efficacy endpoint was calculated to detect differences using one-way analysis of variance with two contrasts (BPX-01 1% vs. vehicle and BPX-01 2% vs. vehicle) at 90-percent statistical power. With effect sizes and subject withdrawal rates based on previous work, this resulted in an estimated sample size of 225 subjects (75 per group). The study was not powered to detect a difference in other measures.
Efficacy analyses used the ITT population, which included all randomized subjects who received at least one dose and who provided evaluable data for at least one postbaseline visit for the primary endpoint. The primary and secondary endpoints used an analysis of covariance (ANCOVA) with the absolute change from baseline as dependent variable; study site, treatment group, and site-by-treatment interaction as fixed effects; and baseline value as the covariate. Imputation was applied as the last observation carried forward for any missing value. Pairwise comparisons of treatment groups used the Dunnett’s procedure of adjustment for multiple comparisons. Statistical significance was assessed at the two-sided five-percent level. Exploratory endpoints also used ANCOVA at p<0.05, but without imputation. The efficacy endpoints involving proportions of IGA were analyzed using a Cochran-Mantel-Haenszel test stratified by site. All safety data were tabulated according to MedDRA classification and by severity, seriousness, and relationship to study medication. All analyses used the Statistical Analysis System (SAS Institute Inc., Cary, North Carolina) software. Unless otherwise noted, descriptive statistics are presented as means, SDs, and proportions.
Results
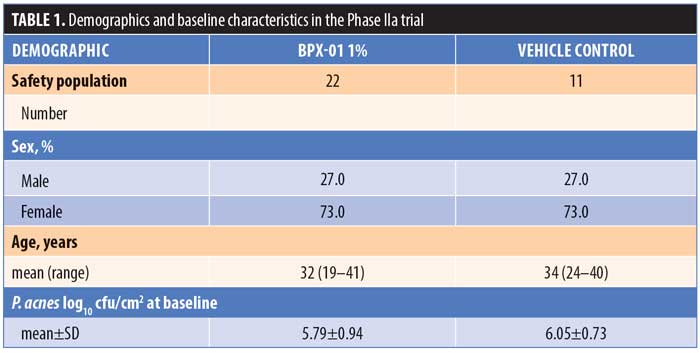
Study 1. A total of 45 people were screened and 33 subjects were enrolled. Twenty-two subjects were randomized to BPX-01 1% and 11 subjects were randomized to the vehicle control. Of these, 31 subjects (93.9%) finished the study; one subject (vehicle group) developed kidney stones and was unable to complete the study visits, while another subject (BPX-01 group) was lost to follow-up (Figure 1). There were 33 subjects included in the safety population (100%), 30 subjects in the MITT population (90.9%), and 25 subjects (75.7%) in the PP population. Subjects were an average age of 32 years in the BPX-01 group and 34 years in the vehicle group, and 73 percent of the subjects were women. At baseline, P. acnes density was 5.79 log10 cfu/cm2 in the active group and 6.05 log10 cfu/cm2 in the vehicle group (Table 1).
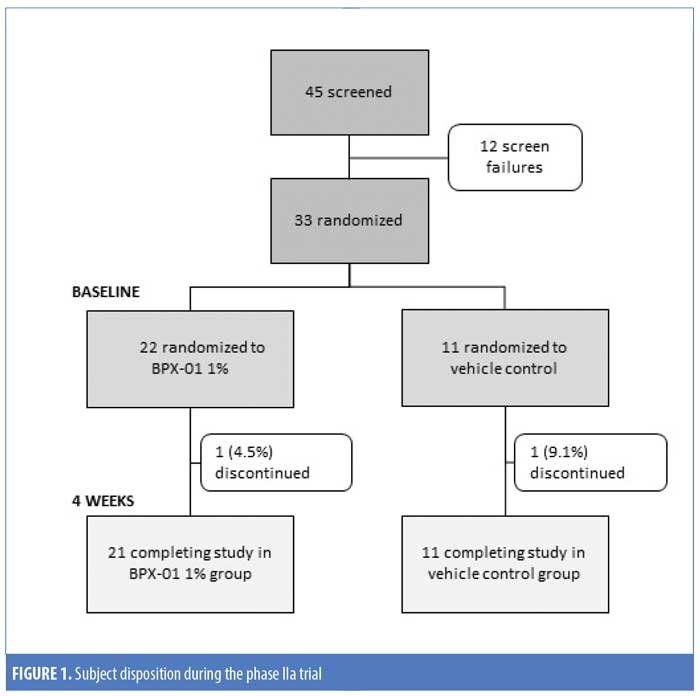
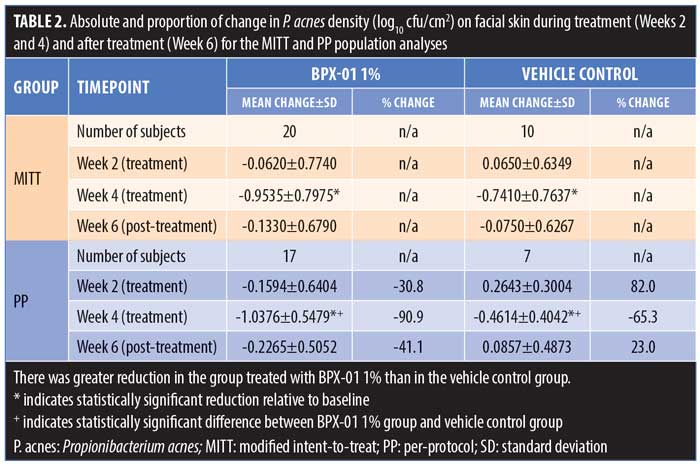
There was a statistically significant decrease in P. acnes during the four weeks of BPX-01 1% treatment in both the MITT and PP populations, with a mean decrease of 0.9535 log10 cfu/cm2 in the MITT group (p<0.0001) and a mean decrease of 1.0376 log10 cfu/cm2 in the PP group (p<0.0001). This was correlated with a 90.9-percent reduction in colonization. Although there were also statistically significant decreases after four weeks in the vehicle arm, P. acnes density was reduced statistically significantly more in the BPX-01 1% treatment group (p=0.020; PP population) (Table 2).
No drug-related AEs occurred. One serious AE, kidney stones with severe abdominal pain, occurred. Although this was not considered related to the study, the subject was withdrawn from the study due to an inability to continue with the study schedule. Additionally, there were five minor AEs that were deemed not related to the study: upper respiratory infection (two subjects), ear pain, pharyngitis, and sinus infection. There were no occurrences of headache or changes in vision. Hematology and blood chemistry assessments were made at baseline and after four weeks of treatment. No clinically significant hematologic or blood chemistry abnormalities were noted or attributed to the drug product or vehicle at any time point.
No adverse cutaneous effects (e.g., erythema, edema, scaling-peeling, hyperpigmentation) were reported by the investigators during the treatment or posttreatment phases, although one incident each of hyperpigmentation and edema were observed at baseline with subsequent resolution within two weeks. Subject-reported cutaneous effects (burning/stinging, tightness, itching) were also tracked; a single subject in the BPX-01 group reported tightness after one week of treatment but not at any of the later time points. Minocycline was not detected in the plasma of any subject at any time point during the study.
At the end of the study, subjects were asked which group (BPX-01 1% or vehicle control) they believed themselves to be assigned. Of 31 subjects, 18 (58.1%) responded correctly and 13 (41.9%) responded incorrectly. Thus, it was concluded that blinding was adequately maintained during the study.
Study 2. A total of 286 subjects were screened and 226 subjects were enrolled. Of these, 219 (96.9%) were randomized, received at least one dose of their assigned treatment, and completed at least one postbaseline follow-up visit, stratified as follows: 73 subjects in the BPX-01 1% group, 72 subjects in the BPX-01 2% group, and 74 subjects in the vehicle control group. A total of 171 subjects (75.7%) completed the 12-week study. Rates of early discontinuation were approximately equal across the three groups (22.7–27.6%) and were most commonly due to study nonadherence and loss to follow-up (Figure 2).
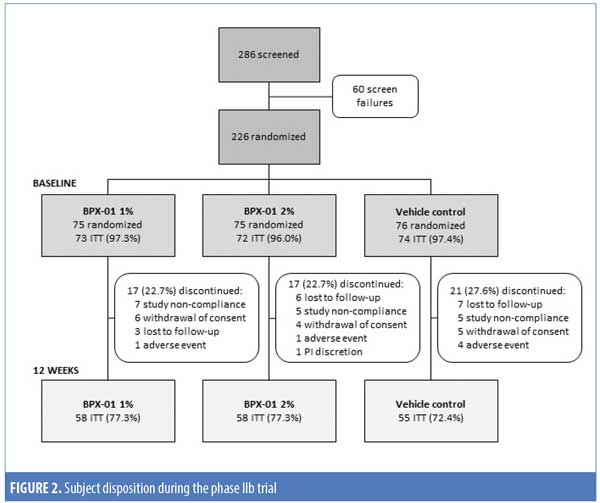
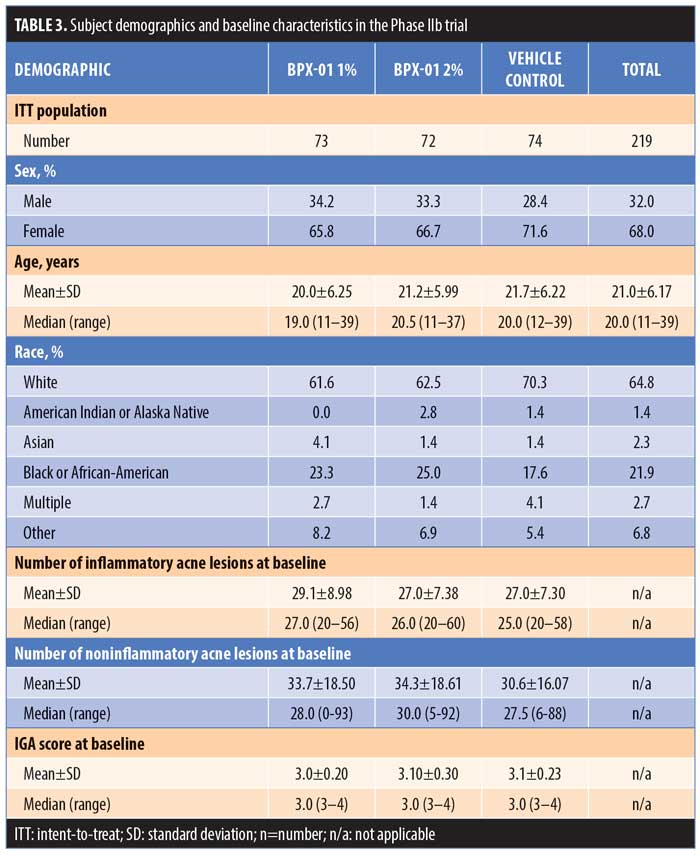
Subjects were an average age of 21.0±6.17 years and 68.0 percent were women, with White (64.8%) and African-American (21.9%) reported as the most common races. At baseline, subjects had 20 to 60 inflammatory lesions, 0 to 93 noninflammatory lesions, and were rated as either “moderate” or “severe” (3 or 4) on the IGA. Subject characteristics were comparable across the three treatment groups (Table 3).
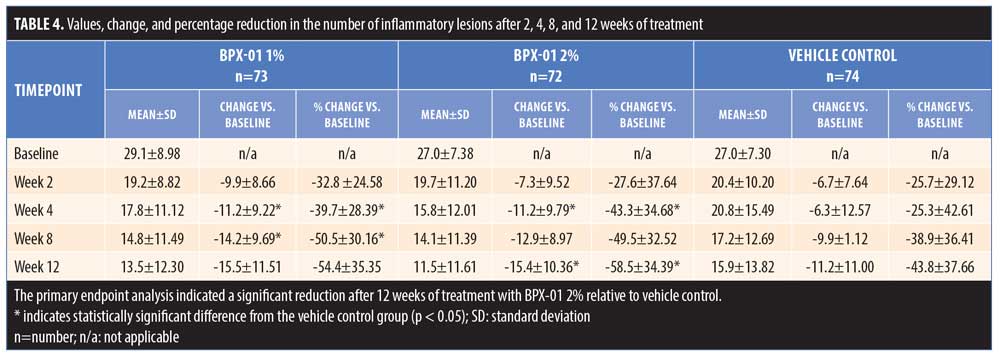
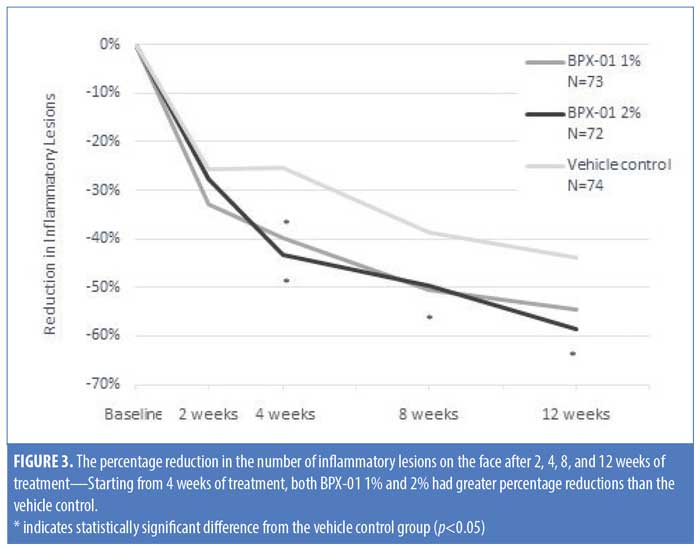
The primary endpoint analysis indicated that the absolute mean change in the number of inflammatory lesions on the face after 12 weeks of treatment, relative to baseline, was greater in the BPX-01 2% group than in the vehicle control group (-15.4 lesions vs. -11.2 lesions; p=0.0352). Improvements in lesion number occurred rapidly in the BPX-01-treated groups; for both the 1% and 2% preparations, more than 25-percent reduction was achieved prior to the two-week assessment. Treatment for four weeks resulted in a 43.3-percent reduction of lesions in the BPX-01 2% group. After 12 weeks of treatment, the percent reduction in number of inflammatory lesions was significantly higher in the BPX-01 2% group than in the vehicle control group (i.e., nearly 15% greater at 58.5% reduction vs. 43.8% reduction; p=0.0256). Differences in efficacy with BPX-01 1% compared with vehicle control, although trending toward a beneficial treatment effect (-15.5 lesions vs. -11.2 lesions, 54.4-percent reduction; p=0.054), did not reach statistical significance (Table 4 and Figure 3).
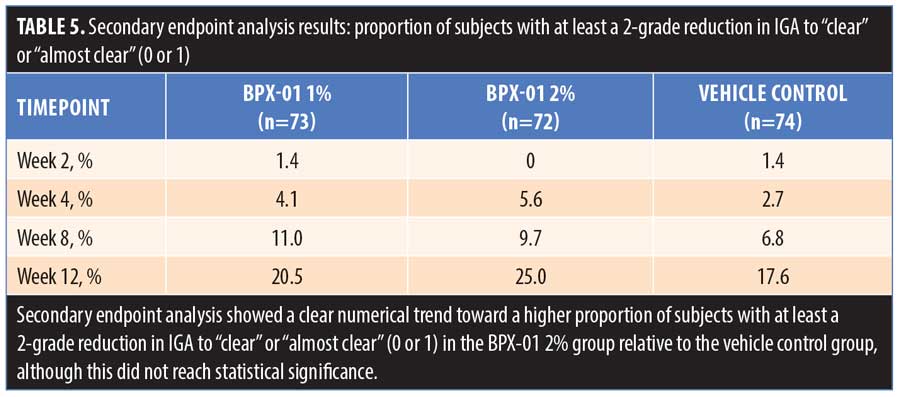
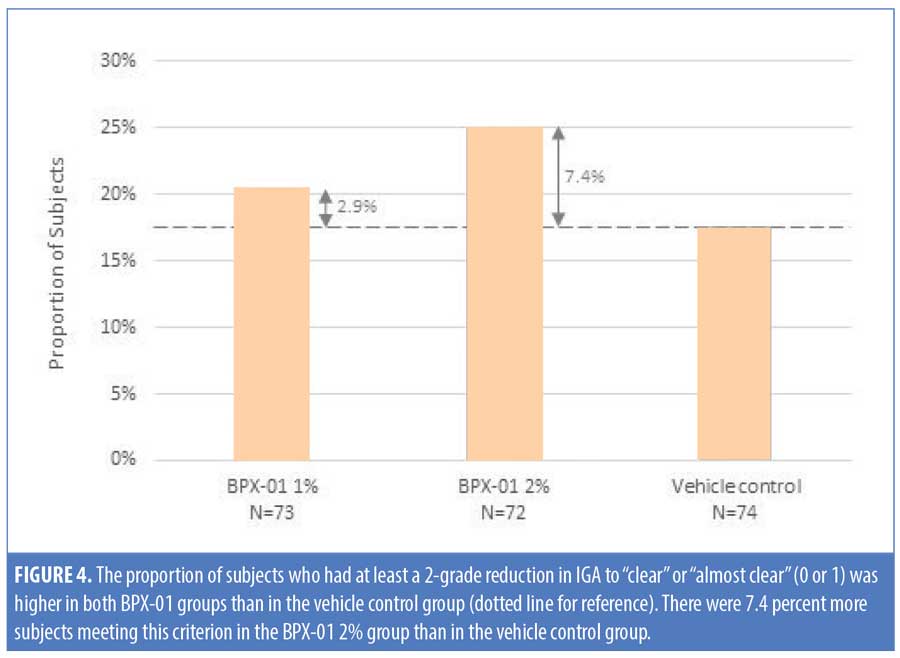
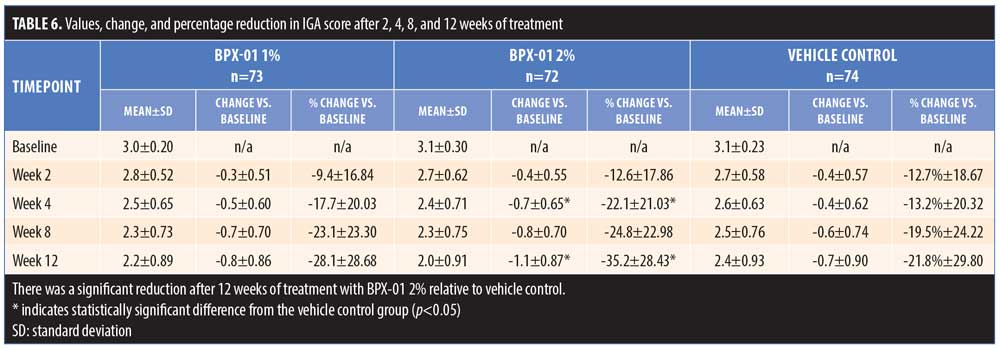
The secondary endpoint analysis indicated that the proportion of subjects with at least a two-grade reduction in IGA to “clear” or “almost clear” (0 or 1) trended larger for the BPX-01 2% group than the vehicle control group (7.4% larger: 25.0% vs. 17.6%; p=0.5445) (Table 5 and Figure 4). Mean IGA ratings in each group over time were then compared. From baseline to 12 weeks, IGA scores declined by 1.1 points±0.87 points in the 2% group, representing a 35.2-percent improvement from baseline. In contrast, IGA scores in the vehicle control group declined by 0.7±0.90 points, representing a 21.8-percent improvement; the difference between the two was statistically significant (p=0.0077). There appeared to be a dose-dependent effect on IGA scores, as outcomes for the 1% group were approximately midway between those of the BPX-01 2% and vehicle control groups, while the IGA of the 1% group relative to baseline did not reach statistical significance (Table 6).
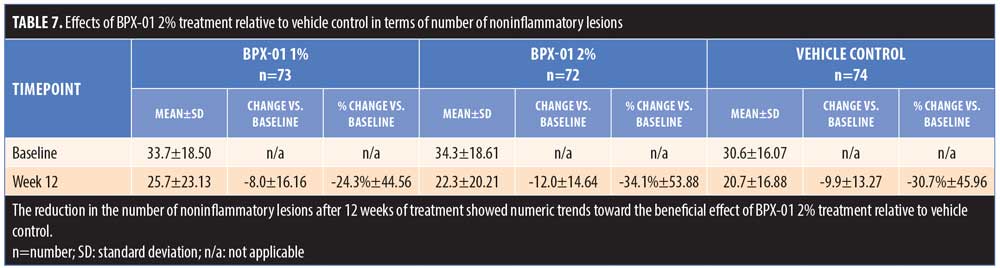
The mean change in the number of noninflammatory lesions on the face after 12 weeks of treatment, relative to baseline, was greater in the BPX-01 2% group than in the vehicle control group (-12.0 lesions vs. -9.9 lesions; p=0.7882). Similarly, the percentage reduction in the number of noninflammatory lesions was higher in the BPX-01 2% group than in the vehicle control group (34.1% reduction vs. 30.7% reduction). The group differences, however, were not statistically significant (p=0.8946) (Table 7).
The mean change in PGI-S ratings after 12 weeks of treatment, relative to baseline, was greater in the BPX-01 2% group than in the vehicle control group (-0.9 severity rating vs. -0.6 severity rating; p=0.6184) (Table 8). After 12 weeks of treatment, the proportion of subjects who selected “very much improved” (the most favorable rating) on the PGI-I was 12.1% for the BPX-01 1% group, 19.0 percent for the BPX-01 2% group, and 10.9 percent for the vehicle control group.
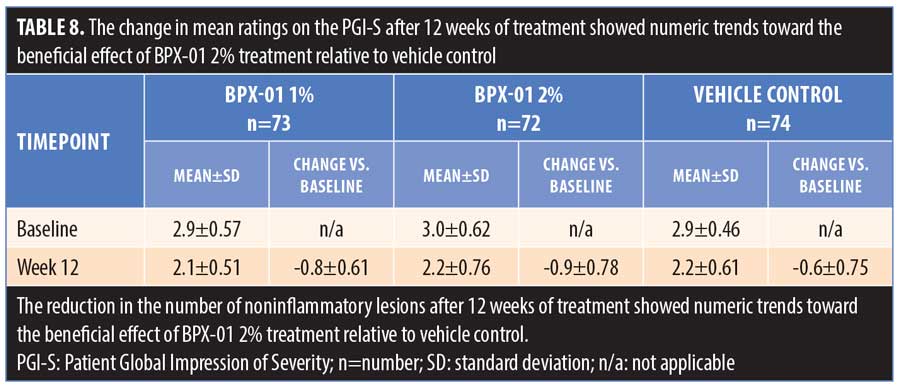
The study treatment was generally safe and well tolerated. In total, 57 subjects (25.2%) reported at least one AE, of which eight subjects (3.5%) experienced a study-drug-related AE. One subject (1.3%) in the BPX-01 2% group experienced a severe migraine, which was recorded as a related AE given that its presentation was consistent with what could occur in association with pseudotumor cerebri. However, this relationship was never established as the subject had a history of migraines as well as head trauma, no detectable plasma minocycline level, and refused further neurologic workup that might have enabled a more definitive diagnosis or identification of a causal relationship with treatment. This subject was withdrawn from the study. Another subject (1.3%) in the BPX-01 2% group experienced mild dizziness. In the vehicle control group, one subject (1.3%) reported a moderate headache. The other related AEs were mild or moderate perturbations of laboratory values or application site reactions, the majority of which occurred in the vehicle control group. Among unrelated AEs, the most common (occurring at a >2% frequency in any group) across all three study arms included nasopharyngitis, upper respiratory tract infection, urinary tract infection, headache, and influenza. Few changes in blood chemistry or hematology were noted. Two serious AEs, neither considered study-related, were reported: a major depressive episode (one subject [1.3%] in the BPX-01 1% group) and suicidal thoughts (one subject [1.3%] in the vehicle control group) (Table 9).
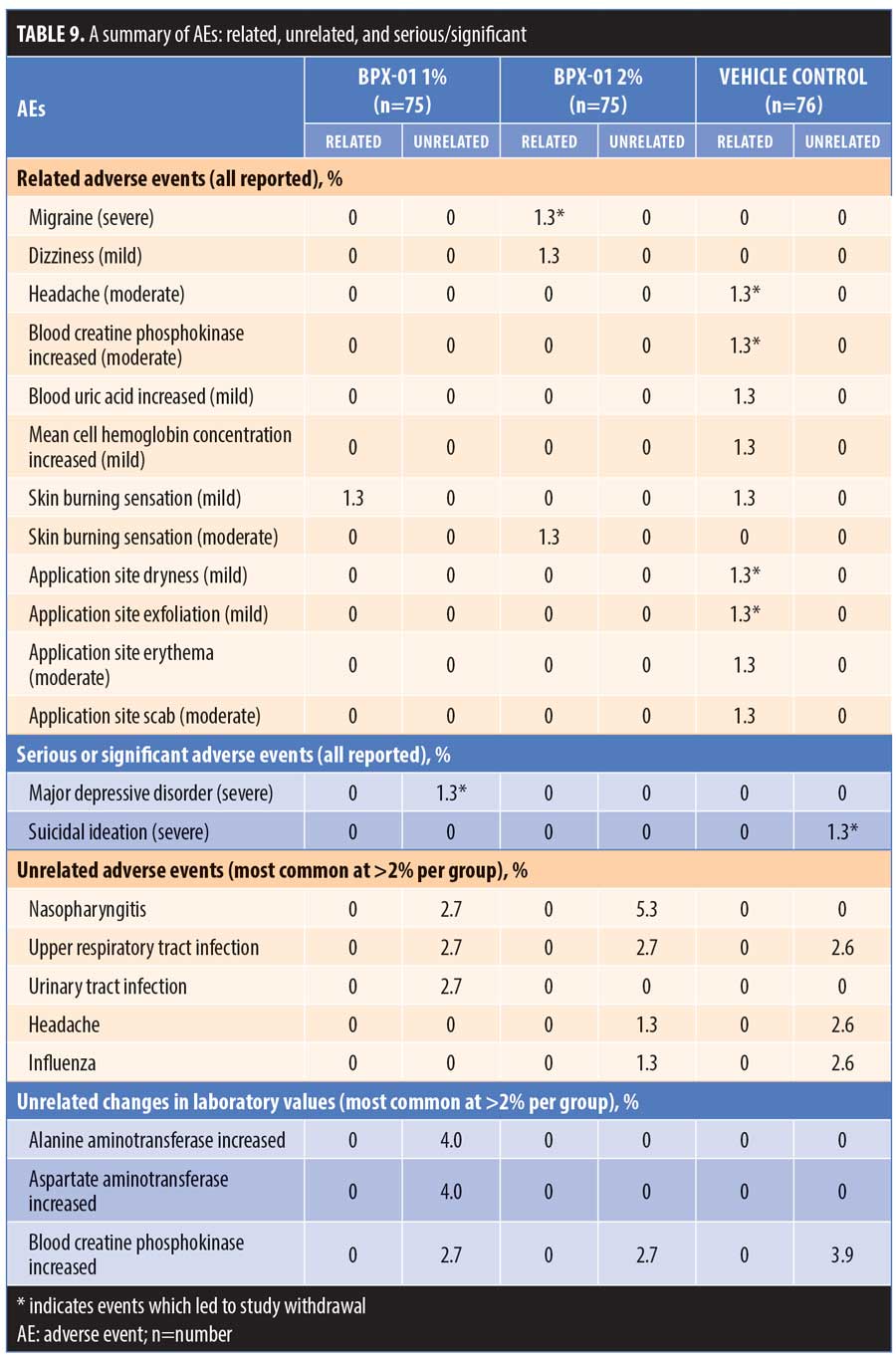
There were no reports of photosensitivity or postinflammatory hyperpigmentation, and no staining and/or skin discoloration was observed. Investigator and subject assessments of local tolerability showed no increases relative to baseline in erythema, scaling/peeling, edema, burning, stinging, tightness, or itching in any of the groups during the 12-week treatment period (Table 10).
Plasma concentrations of minocycline were assessed at baseline and after four and 12 weeks of treatment. The assays of all subjects at all time points were below the limit of quantification of 10ng/mL, with one time point exception. This single exception occurred with one subject in the BPX-01 2% group who was measured at 42.40ng/mL at the 12-week assessment. This subject had no AEs. All groups had similar levels of adherence with dosing schedules and exposure to the assigned treatment; the assigned medication was applied on at least 97.4 percent of the study days across all groups.
When asked, “Would you consider using the study medication again?,” 86.2 percent of subjects in the BPX-01 1% group and 81.0 percent in the 2% group answered affirmatively versus 76.4 percent in the vehicle group. When asked to respond on a scale from 1 (least favorable) to 5 (most favorable) regarding the question, “How easy is it to apply the study medication in the evening?,” 79.3 percent of subjects in both the BPX-01 1% and 2% groups selected 4 or 5 (the two most favorable responses) versus 92.7 percent in the vehicle group. Using the same scale, subjects were asked, “Do you like the study medication?” The top two favorable responses were selected by 60.3 percent of subjects in the BPX-01 1% group and 62.1 percent of the 2% group as compared with by 49.1 percent of the vehicle group.
Subjects were asked whether they believed they were assigned to receive BPX-01 medication (independent of dosage) or the vehicle. Subjects correctly selected the active treatment 75.4 percent of the time in the BPX-01 1% group and 74.1 percent of the time in the BPX-01 2% group. In the vehicle group, 43.6 percent correctly identified their treatment as vehicle.
Discussion
In the Phase IIa study, treatment with BPX-01 reduced P. acnes colonization of facial skin by 90.9 percent, which was significantly more than the reduction achieved with the vehicle alone (p<0.0001). In the Phase IIb study, treatment with BPX-01 2% reduced the number of inflammatory lesions by 58.5 percent, which was significantly more than the percentage reduction seen with the vehicle alone (p<0.0256). Other improvements specific to BPX-01 treatment included a reduction in the number of noninflammatory lesions, a higher proportion of subjects with two-point reductions in IGA scores to “clear” or “almost clear’,” favorable ratings on the PGI-S and PGI-I, and high subject satisfaction ratings. Across both studies, BPX-01 was well-tolerated with few AEs. There were no reports of photosensitivity, postinflammatory hyperpigmentation, or skin discoloration. A single subject with a history of migraine cephalgia developed a migraine headache episode during treatment with the 2% BPX-01 formulation.
A branded, extended-release minocycline (ERM; Solodyn®; Valeant Pharmaceuticals, Laval, Canada) is an oral minocycline formulation approved specifically for the treatment of moderate-to-severe acne vulgaris in patients 12 years of age and older.17 At a dosage of 1mg/kg/day, this formation has been shown to reduce the potential for acute vestibular AEs.32 In pivotal trials, ERM was found to reduce the number of inflammatory acne lesions by 40 to 50 percent, in contrast with reductions of approximately 30 percent acheived with placebo.28,29,33 Twelve weeks of treatment with ERM were required to reach maximum effectiveness.33 In this report, topically applied BPX-01 2% reached an inflammatory lesion reduction level of 58.5 percent after 12 weeks of treatment, with a 43.3-percent reduction achieved after four weeks of treatment, in contrast with a 43.8-percent improvement after 12 weeks in the vehicle-only group. It should be noted that the current study cannot be directly compared with the studies performed involving ERM due to differences in study design, populations, and study conduct. However, it is reasonable to note that BPX-01 treatment resulted in rapid improvement in both the 1% and 2% treatment arms, resulting in better than 25-percent improvement within two weeks. This is likely to be interpreted by subjects as an early, clinically important improvement and might augment adherence to clinical treatment regimens.34
The Phase IIb study showed trends toward improvements in a variety of quantitative, clinical, and patient-reported outcomes, including reductions in the number of noninflammatory lesions, IGA ratings, and PGI of severity and improvement. Because the study was not powered for these endpoints, the differences between groups could not be assessed adequately for statistical significance. The converging trends across multiple measures, however, provide strong support for the effectiveness of BPX-01 over the vehicle control. Subjects also reported a high degree of satisfaction with using the product and its treatment results.
ERM results in steady-state minocycline plasma concentrations of 2,630ng/mL as compared to 2,920ng/mL for nonmodified, immediate-release formulations of oral minocycline.35 In contrast, minocycline could not be detected in the plasma of 99.6 percent of the subjects in the BPX-01 studies despite a highly sensitive assay that could detect concentrations of minocycline at an order of magnitude lower than that observed with ERM. The single subject with detectable levels had a plasma minocycline concentration of 42ng/mL; in contrast, a single oral dose results in an average plasma concentration of 758ng/mL.36 It is well established that oral administration of minocycline is associated with a broad array of potential AEs, including some rare yet severe reactions such as drug hypersensitivity syndrome, lupus-like reactions, hepatotoxicity, and pseudotumor cerebri.18–20,27–29 As there is virtually no detectable plasma minocycline level with BPX-01 treatment, it is not anticipated that systemic side effects related to minocycline would occur.
Limitations. The studies in this report had a number of potential limitations. The small sample size of the Phase IIa study could mean that the results are not generalizable to the larger population. However, because subjects were selected according to commonly utilized criteria and because differences in effectiveness between the treatment groups were based on quantitative assessment of P. acnes colonization, the study’s proof-of-concept was confirmed. The Phase IIb study was limited by having not been statistically powered enough to demonstrate a difference between groups for the secondary endpoint, the proportion of subjects with IGA reduction of at least two points to “clear” or “almost clear,” which is admittedly a stringent threshold and more commonly used in larger Phase III studies than in Phase II studies. When the change in IGA was analyzed as a continuous variable, statistically significant differences were observed between the BPX-01 and vehicle groups. Supporting endpoints also did not reach statistical significance when compared with outcomes in the vehicle control group due to the sample size. However, the consistent trends across multiple assessments provide converging evidence that BPX-01 is an effective treatment. Lastly, there were large effect sizes for the vehicle control group; placebo effects are common in acne studies and can be attributed to, for example, careful study-mandated skin care and regular physician visits that occur for all subjects.37 Thus, it is the incremental clinical effects of treatment that are of relevance. It should be noted, however, that the amelioration of acne in the vehicle-only group exceeds that generally seen in placebo groups of oral treatments, suggesting some benefit inherent to the vehicle.
Effectiveness outcomes in this report appear to be dose-dependent, since a reduction in the number of inflammatory lesions was observed in both the BPX-01 1% and 2% groups, but reached statistical significance relative to the vehicle control group only for the higher 2% concentration. This is in contrast with outcomes seen with ERM, in which higher rates of acute vestibular AEs, but not better clinical outcomes, were observed with higher dosages.29 Because the topical application of BPX-01 does not result in systemic exposure or accumulation of minocycline, and thus avoids minocycline-related AEs associated with oral administration, it might be that BPX-01 can achieve higher pilosebaceous concentrations than would be possible with oral minocycline formulations; however, this is not quantifiable. The topical gel delivers solubilized minocycline directly to the pilosebaceous unit, allowing for rapid absorption at the targeted site.21,22 This, along with the trends toward improvements in noninflammatory lesions and investigator and subject ratings in the 2% group, supports continued investigation in the form of a Phase III trial of a similar design employing the BPX-01 2% formulation.
Conclusion
BPX-01 topical minocycline gel reduced cutaneous P. acnes colonization. Clinically, BPX-01 2% resulted in better outcomes than the vehicle control in the treatment of moderate-to-severe, non-nodular inflammatory acne vulgaris. The treatment was well-tolerated and there was a high rate of subject satisfaction. This treatment might provide an effective new option with a favorable safety profile, a potential for high patient adherence, and avoidance of AEs associated with oral minocycline use.
Acknowledgments
The authors thank Allison Foster, PhD, an independent medical writer, for her intellectual contribution to the drafting of the manuscript.
References
- Leyden JJ. A review of the use of combination therapies for the treatment of acne vulgaris. J Am Acad Dermatol. 2003;49(Suppl 3):S200–S210.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485.
- Das S, Reynolds RV. Recent advances in acne pathogenesis: implications for therapy. Am J Clin Dermatol. 2014;15(6):479–488.
- Martin AR, Lookingbill DP, Botek A, Light J, Thiboutot D, Girman CJ. Health-related quality of life among patients with facial acne—assessment of a new acne-specific questionnaire. Clin Exp Derm. 2001;26(5):380–385.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(Suppl 1):S1–S37.
- Weiss JS. Acne: evolving concepts of pathogenesis need to guide therapeutic developments. J Drugs Dermatol. 2013;12:S66.
- Thiboutot DM, Dreno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2S1):S1–S23.
- Leyden JJ. Effect of topical benzoyl peroxide/clindamycin versus topic clindamycin and vehicle in the reduction of Propionibacterium acnes. Cutis. 2002;69(6):475–480.
- Leyden JJ, Preston N, Osborn C, Gottschalk RW. In-vivo effectiveness of adapalene 0.1% / benzoyl peroxide 2.5% gel on antibiotic-sensitive and resistant Propionibacterium acnes. J Clin Aesthet Dermatol. 2011;4(5):22–26.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report. Part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9(4):18–24.
- Del Rosso J, Gallo RL, Thiboutot D, et al. Status report. Part 2: Perspectives on antibiotic use and the microbiome and review of microbiologic effects of selected specific therapeutic agents commonly used by dermatologists. J Clin Aesthet Dermatol. 2016;9(5):11–17.
- Del Rosso JQ, Rosen T, Thiboutot D, et al. Status report. Part 3: Current perspectives on skin and soft tissue infections with emphasis on methicillin-resistant staphylococcus aureus, commonly encountered scenarios when antibiotic use may not be needed, and concluding remarks on rational use of antibiotics in dermatology. J Clin Aesthet Dermatol. 2016;9(6):17–24.
- Margolis DJ, Fanelli M, Hoffstad MA, Lewis JD. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2610–2616.
- Margolis DJ, Hoffstad O, Bilker W. Association or lack of association between tetracycline class antibiotics used for acne vulgaris and lupus erythematosus. Br J Dermatol. 2007;157(3): 540–546.
- Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260.
- Jonas M, Cunha B. Minocycline. Ther Drug Monit. 1982;4(2):137–145.
- Valeant Pharmaceuticals North America LLC. Solodyn prescribing information 2016. Available at: http://www.valeant.com/Portals/25/Pdf/PI/Solodyn-PI.Pdf.
- Brown RJ, Rother KI, Artman H, et al. Minocycline-induced drug hypersensitivity syndrome followed by multiple autoimmune sequelae. Arch Dermatol. 2009;145(1):63–66.
- Gough A, Chapman S, Wagstaff K, et al. Minocycline induced autoimmune hepatitis and systemic lupus erythmatosus-like syndrome. BMJ. 1996;312(7024):169–172.
- Ochsendorf F. Minocycline in acne vulgaris: benefits and risks. Am J Clin Dermatol. 2010;11(5):327–341.
- Sawant T, Hermsmeier M, Lac D, et al. Effects of minocycline against Propionibacterium acnes-induced inflammation in human keratinocytes. Paper presented at: American Society of Cell Biology (ASCB) annual meeting; 2016; San Francisco, California.
- Nagavarapu U, Hermsmeier M, Lac D, et al. Development and assessment of BPX-01, a novel topical minocycline gel for treatment of acne vulgaris. Paper presented at: American Academy of Dermatology (AAD) annual meeting; 2017; Orlando, Florida.
- Daniels AM. BPX-01 minocycline 1% topical gel for the treatment of acne vulgaris. Paper presented at: Skin Disease Education Foundation (SDEF) 17th Annual Las Vegas Dermatology Seminar; 2016; Las Vegas, Nevada.
- Daniels AM. BPX-01 minocycline 1% topical gel for reduction of Propionibacterium acnes. Paper presented at: 12th Annual Coastal Dermatology Symposium; 2016; Monterey, California.
- BioPharmX BPX-01 OPAL Study Group, Daniels AM. Results from a randomized, double-blind, vehicle-control study to assess the safety and efficacy of BPX-01 minocycline topical gel in the treatment of moderate-to-severe inflammatory acne vulgaris. Paper presented at: Alabama Dermatology Society Summer Symposium; 2017; Sandestin, Florida.
- Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Investigative Dermatol. 1965;45(6):498–503.
- Chiu AM, Chuenkongkaew WL, Cornblath WT, et al. Minocycline treatment and pseudotumor cerebri syndrome. Am J Ophthalmol. 1998;126(1): 116–121.
- Fleischer ABJ, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(4 Suppl):21–31.
- Stewart DM, Torok HM, Weiss JS, et al. Dose-ranging efficacy of new once-daily extended-release minocycline for acne vulgaris. Cutis. 2006;78(4 Suppl):11–20.
- Stein Gold L, Weiss J, Rueda MJ, et al. Moderate and severe inflammatory acne vulgaris effectively treated with single-agent therapy by a new fixed-dose combination adapalene 0.3% / benzoyl peroxide 2.5% gel: a randomized, double-blind, parallel-group, controlled study. Am J Clin Dermatol. 2016;17:293–303.
- Guy W. Early Clinical Drug Evaluation Unit (ECDEU) assessment manual for psychopharmacology. Revised. NIMH publication DHEW publNO (Adm) 76-338. Bethesda, MD: National Institute of Mental Health; 1976.
- Bowe WP, Glick JB, Shalita AR. Solodyn and updates on topical and oral therapies for acne. Curr Derm Rep. 2012;1(3):97–107.
- Torok HM. Extended-release formulation of minocycline in the treatment of moderate-to-severe acne vulgaris in patients over the age of 12 years. J Clin Aesthet Dermatol. 2013;6(7):19–22.
- Del Rosso JQ. When do efficacy outcomes in clinical trials correlate with clinical relevance? Analysis of clindamycin phosphate 1.2%-benzoyl peroxide 3.75% in moderate to severe acne vulgaris. Cutis. 2016;98(1):21–25.
- Plott RT, Wortzman MS. Key bioavailability features of a new extended-release formulation of minocycline hydrochloride tablets. Cutis. 2006;78(4 Suppl):6–10.
- Minocycline hydrochloride tablets package insert. Available at: https://www.drugs.com/pro/minocycline-tablets.html. Accessed March 22, 2018.
- Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl estradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89(4):615–622.

