 J Clin Aesthet Dermatol. 2018;11(11):15–19
J Clin Aesthet Dermatol. 2018;11(11):15–19
by Neal D. Bhatia, MD; David M. Pariser, MD; Leon Kircik, MD; Tina Lin, PharmD; Susan Harris, MS; Lindsey Mathew; and Radhakrishnan Pillai, PhD
Dr. Bhatia is with Therapeutics Clinical Research in San Diego, California. Dr. Pariser is with Virginia Clinical Research, Inc. in Norfolk, Vriginia. Dr. Kircik is with the Indiana University School of Medicine in Indianapolis, Indiana; Physicians Skin Care, PLLC in Louisville, Kentucky, and the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Lin is with Ortho Dermatologics in Bridgewater, New Jersey. Mses. Harris and Mathew are with Bausch Health in Bridgewater, New Jersey. Dr. Pillai is with Dow Pharmaceutical Sciences Inc. in Petaluma, California.
FUNDING: Ortho Dermatologics funded the activities of Konic Ltd. (West Sussex, England, United Kingdom) pertaining to this manuscript. Konic Lts. provided editorial services.
DISCLOSURES: Dr. Bhatia is an advisor, consultant, investigator, and speaker with Ortho Dermatologics. Dr. Pariser is an adviser and investigator with Ortho Dermatologics. Dr. Kircik is an adviser, consultant and investigator with Ortho Dermatologics. Drs. Lin and Pillai and Mses. Harris and Mathew are employees of Bausch Health.
Abstract: Background. Topical corticosteroids (TCS) represent a mainstay of psoriasis treatment, though more potent formulations are not recommended for use for more than 2 to 4 weeks.
Objective.We sought to investigate the safety and efficacy of once-daily halobetasol propionate 0.01%/tazarotene 0.045% (HP/TAZ) lotion and halobetasol propionate 0.05% cream in patients with moderate-to-severe plaque psoriasis.
Methods.This was a multicenter, randomized, double-blind, parallel-group, vehicle-controlled Phase II study (n=154). Patients were randomized (2:2:1) to HP 0.01%/TAZ 0.045% lotion, halobetasol propionate 0.05% cream, or vehicle, applied topically once daily for two weeks. Efficacy assessments included treatment success, impact on individual psoriasis signs (i.e., erythema, plaque elevation, and scaling) at target lesion, and body surface area (BSA). Safety and adverse events (AEs) were evaluated throughout.
Results. HP/TAZ lotion was significantly more effective than vehicle and was comparable to halobetasol propionate 0.05% cream in reducing erythema, plaque elevation, and scaling. Of the HP/TAZ patients, 32.8 percent achieved treatment success versus 34.0 and 3.3 percent in the halobetasol and vehicle groups. At Week 2, treatment success was seen in 34.4 percent (erythema), 54.1 percent (plaque elevation), and 60.7 percent (scaling) of patients versus in 43.5, 50.8, and 50.8 percent receiving halobetasol. A 25.0- and 24.8-percent reduction in BSA occurred, respectively. The most frequently reported, treatment-related AE was application site pain.
Conclusions.The efficacy of HP/TAZ lotion was comparable to a higher concentration of 0.05% halobetasol cream, showing rapid treatment success after two weeks with good safety.
Keywords: Psoriasis, topical, halobetasol, tazarotene, clinical trial
Introduction
Psoriasis is a chronic, immune-mediated disease that varies widely in its clinical expression and affects approximately two percent of the population.1–3 Many sufferers have localized disease, where topical therapy can provide the cornerstone of treatment.4 In addition, the majority of patients have mild or moderate disease severity (75–90%).5,6 In cases of mild or moderate disease severity, the use of topical corticosteroids (TCS) is commonplace.7 However, long-term safety remains a concern, particularly when using more potent formulations, with an associated increased risk of local cutaneous adverse events (AEs). These AEs include skin atrophy, telangiectasia, and, infrequently, hypothalamic-pituitary-adrenal (HPA) axis suppression.7–9 The use of halobetasol propionate cream (Ultravate® 0.05%; Ranbaxy Laboratories, Inc., Jacksonville, Florida) is not recommended for more than two consecutive weeks due to potential HPA axis suppression.
Tazarotene has also been shown to be an effective treatment for psoriasis.10,11 Use of topical tazarotene is limited by its potential irritancy. However, combining it with a TCS is one option that might prevent these irritant effects while simultaneously providing a synergistic therapeutic benefit and a potential decrease in steroid-induced atrophy.12,13
Recently, data on halobetasol propionate 0.01%/tazarotene 0.045% (HP/TAZ) lotion were published.14 HP/TAZ lotion was more effective than individual active ingredients or the vehicle and appeared to be well-tolerated following eight weeks of daily treatment. Here, we further investigate the safety and tolerability of HP/TAZ lotion in comparison with a commercially available formulation of halobetasol propionate.
Methods
This was a multicenter, double-blind, randomized, parallel-group Phase II study conducted to assess the safety, tolerability, and efficacy of HP/TAZ lotion and halobetasol propionate 0.05% (Ultravate®) cream in patients with a clinical diagnosis of moderate-to-severe psoriasis (with an Investigator Global Assessment [IGA] score of 3 or 4 and an affected Body Surface Area [BSA] of 3–12%). Both cream and lotion vehicle-matched formulations were used to ensure blinding and equal randomization. Areas of the face, scalp, and axillae or other intertriginous areas were not included or treated.
The present study is registered on ClinicalTrials.gov (NCT02785172) and was conducted from April 2016 to December 2016.
Patients were randomized (2:2:1) to receive HP/TAZ lotion, halobetasol cream, or vehicle applied topically to the affected area once daily for two weeks. Assessments were carried out at baseline and Week 2.
Patients. Patients were of either sex and 18 years of age or older. A target lesion was defined primarily to assess signs of psoriasis and was required to measure 16cm2 to 100cm2 with a score of 3 or more for two or more signs of psoriasis (e.g., erythema, plaque elevation, and scaling), a summed score of 8 or more, with no sign scoring less than 2.
Patients were excluded from the study if they had pustular psoriasis or had used phototherapy, photochemotherapy, or systemic psoriasis therapy within the last four weeks (or biologics within the last three months). Patients who had received topical treatment within 14 days prior to the baseline visit or who were diagnosed with skin conditions that would interfere with the interpretation of results were also excluded. Tazarotene is not recommended for use by pregnant women, women attempting to become pregnant, or women who are breast-feeding. These patients were also excluded from the study.
Patients were considered adherent with the dosing regimen if they applied 80 to 120 percent of the total number of expected doses during the treatment period.
Study oversight. Patients provided written informed consent before study-related procedures were performed, and protocols and consent were approved by the relevant institutional review boards or ethics committees at all investigational sites. The study was conducted in accordance with the principles of Good Clinical Practice (GCP) and the Declaration of Helsinki.
Statistical and analytical plan. The primary goal was to assess treatment effect differences between the combination and halobetasol treatments with respect to IGA score. Data for the two vehicle arms (cream and lotion) were pooled. All patients randomized and given the study drug were included in the intent-to-treat (ITT) analysis set. The ITT analysis set was considered primary for the evaluation of efficacy. Data were analyzed with a Cochran–Mantel–Haenszel (CMH) test, with three pairwise tests comparing the HP/TAZ lotion and halobetasol cream to the vehicle and the HP/TAZ lotion to the halobetasol cream. Multiple imputation (MCMC) was used to represent missing values. Summary statistics for Week 2 represented average values obtained from averaging the summary statistics generated from each imputed dataset. P values for the BSA data were calculated using a Wilcoxon rank-sum test, with no imputation of missing data.
All AEs were recorded and classified using the Medical Dictionary for Regulatory Activities (MedDRA version 18.0). A Fisher’s exact test was used to compare the incidences of treatment-emergent AEs (TEAEs) and treatment-related AEs occurring with frequencies of one percent or higher. All patients who were randomized, receiving at least one confirmed dose of study drug and undergoing at least one postbaseline safety assessment were included in the safety analysis set.
Efficacy assessment. IGA score was based on a five-point scale ranging from 0 (clear) to 4 (severe) and was assessed at each visit to describe the severity of overall psoriasis of the treatable areas. The primary efficacy endpoint was improvement in IGA score at Week 2, including the percent of patients judged as being “treatment successes” with at least a two-grade improvement from baseline in IGA score and an IGA score equating to “clear” or “almost clear.”
Signs of psoriasis (i.e., erythema, plaque elevation, and scaling) were assessed using individual five-point scales ranging from 0 to 4. Target lesion improvement was assessed based on the percent of patients with at least a two-grade improvement from baseline in the score for each of the key signs of psoriasis. BSA was assessed at each study visit.
Safety and tolerability assessment. Safety evaluations, including AEs, vital signs, laboratory evaluations, and physical examinations, were performed. Information on reported and observed AEs was obtained at each visit. Tolerability was evaluated through assessments of selected local signs and symptoms (e.g., itching, dryness, and burning/stinging) using individual four-point scales. Treatment areas were examined by the investigator at each visit for the presence/absence of significant known drug-related AEs, skin atrophy, striae, telangiectasia, and folliculitis. Any local skin reaction requiring the use of a concomitant therapy or any instance of study drug interruption or discontinuation was to be reported as an AE.
Results
Patient disposition. In all, 154 patients from 15 study centers in the United States were randomized to HP/TAZ lotion (n=61), halobetasol propionate 0.05% cream (n=63), or vehicle (n=30) and included in the ITT population.
Overall, 100.0 percent (n=61), 98.4 percent (n=62), and 96.7 percent (n=29) of patients treated with HP/TAZ lotion, halobetasol propionate cream, or vehicle, respectively, completed the study.
Patient demographics and baseline characteristics. Demographic data were comparable across the treatment arms. Mean age ranged from 49.5 years to 51.3 years. Most patients were male (59.7%, n=92) and Caucasian (77.9%, n=120) (Table 1).
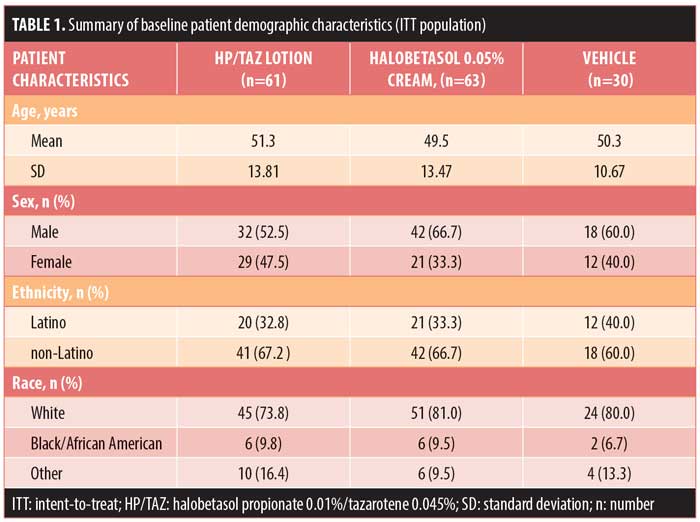
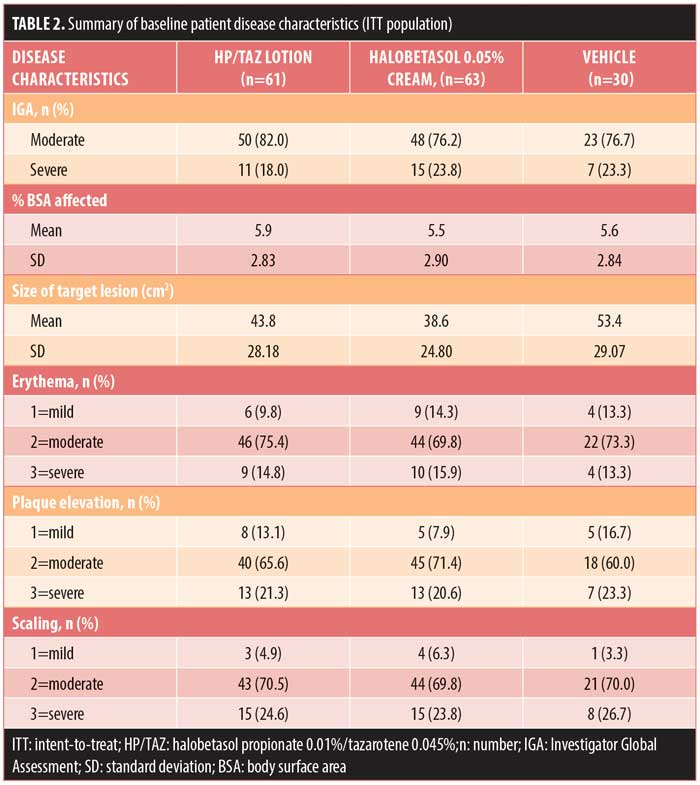
Baseline disease characteristics were also comparable across the treatment groups. Patients had a baseline IGA score of moderate (78.6%, n=121) or severe (21.4%, n=33) and a mean BSA of 5.5 to 5.9 percent (range: 3–12%). The mean target lesion size was 38.6cm2 to 53.4cm2 (range: 16–100cm2). The majority of patients had moderate erythema (72.7%, n=112), plaque elevation (66.9%, n=103), and scaling (70.1%, n=108) at the target lesion site at baseline (Table 2).
Efficacy results: IGA of disease severity. The HP/TAZ lotion was significantly more effective than the vehicle and comparable to halobetasol propionate cream in achieving at least a two-grade improvement from baseline in the IGA score and a score of “clear” or “almost clear” at Week 2. Overall, 32.79 percent of patients achieved this primary efficacy outcome of treatment success versus 33.97 percent and 3.33 percent, respectively, in the halobetasol propionate and vehicle groups (P=0.002 versus vehicle) (Figure 1).
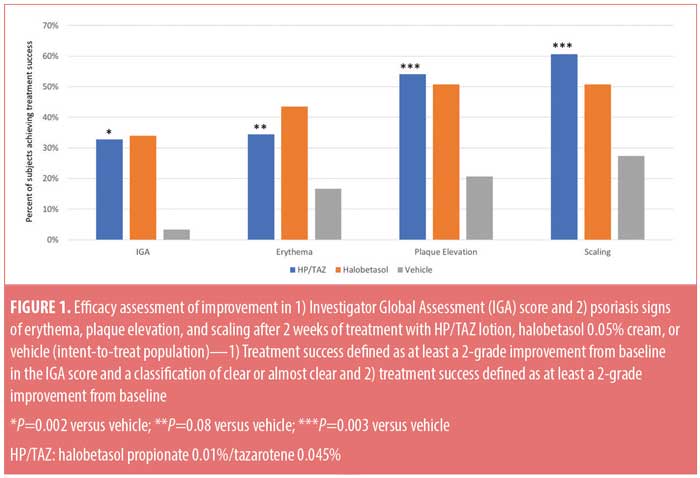
Efficacy results: severity of psoriasis signs at target lesion site. The HP/TAZ lotion was statistically superior to the vehicle in achieving treatment success and numerically more effective than halobetasol propionate in improving plaque elevation and scaling. At Week 2, at least a two-grade improvement from baseline was achieved by 34.43 percent (erythema, P=0.08 versus vehicle), 54.10 percent (plaque elevation, P=0.003 versus vehicle), and 60.66 percent (scaling, P=0.003 versus vehicle) of patients in comparison with 43.49, 50.79, and 50.79 percent treated with halobetasol propionate (all nonsignificant versus HP/TAZ) and 16.67, 20.67, and 27.33 percent treated with vehicle, respectively (Figure 1).
Efficacy results: body surface area. There was a 25.0-percent reduction in mean baseline-affected BSA following HP/TAZ lotion treatment versus 24.8 percent (halobetasol propionate), and 5.0 percent (vehicle, P<0.001) at Week 2.
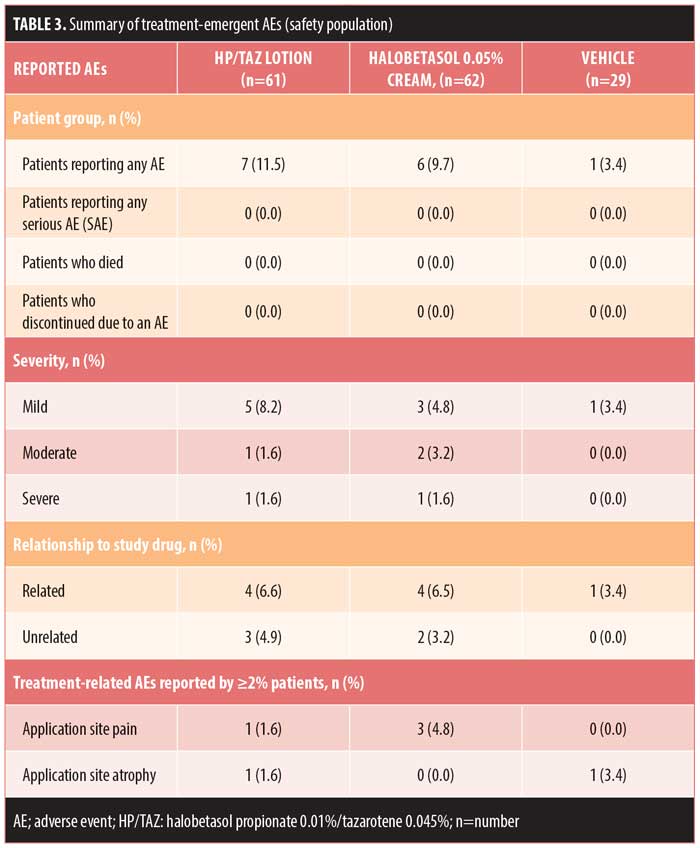
Safety evaluation. Overall, 14 (9.2%) patients reported AEs, with the majority (n=9) reporting AEs that were mild or treatment-related. No patient discontinued treatment due to an AE, and no serious AEs were reported. The most common treatment-related AEs (>2% incidence) were application site pain (halobetasol propionate, 4.8%, n=3) and application site atrophy (vehicle, 3.4%, n=1) (Table 3). Mild skin atrophy occurred in two patients (one treated with vehicle [right foot instep, 3.4%] and one with HP/TAZ [right patella, 1.6%]). In both cases, the AE was reported at the end of treatment (Days 14 and 15, respectively), and the issue was resolved at study’s end.
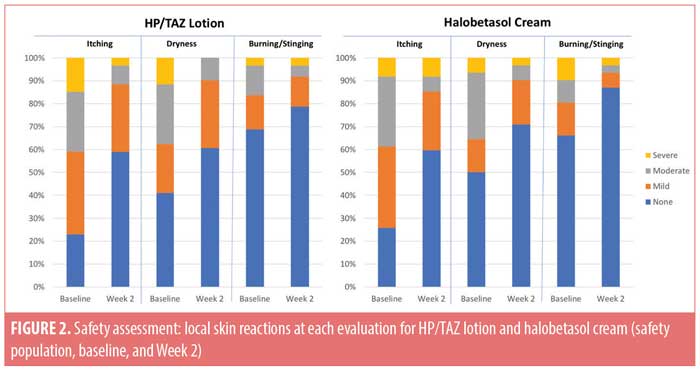
Local skin reactions. Local skin reactions are not uncommon in psoriasis patients. At baseline, 40.1 percent of patients reported moderate-to-severe itching, 36.8 percent reported moderate-to-severe dryness, and (less commonly) 18.4 percent reported moderate-to-severe burning/stinging. Following study treatment, improvement was observed in both active treatment groups. Only 11.5 percent and 14.5 percent of patients treated with HP/TAZ lotion or halobetasol propionate cream still reported moderate-to-severe itching, while 9.8 percent and 9.7 percent reported moderate-to-severe dryness and 8.2 percent and 6.5 percent reported moderate-to-severe burning/stinging, respectively. The majority of patients reported no itching, dryness, or burning/stinging (59.0% and 59.7%, 60.7% and 71.0%, and 78.7% and 87.1%, respectively) at Week 2 (Figure 2).
Discussion
Halobetasol propionate 0.01%/tazarotene 0.045% (HP/TAZ) lotion has been shown to be consistently more effective than its individual active ingredients or vehicle in achieving treatment success and reducing psoriatic signs of erythema, plaque elevation, and scaling at the target lesion.14 Over the first two weeks, HP/TAZ lotion proved significantly more effective than tazarotene 0.045% lotion (all efficacy assessments) and significantly more effective than halobetasol 0.01% lotion in improving plaque elevation (P=0.002) and scaling (P=0.028).
Commercially, halobetasol propionate 0.05% cream is indicated for the topical treatment of plaque psoriasis. Halobetasol propionate 0.05% cream is applied once or twice daily, and its use beyond two consecutive weeks is not recommended due to the potential for HPA axis suppression. With a fixed combination containing only 0.01% halobetasol, we were able to demonstrate both comparable and rapid treatment success in comparison with 0.05% halobetasol monotherapy, when both were applied once daily for two weeks. Improvements in plaque elevation and scaling were greater with HP/TAZ lotion, most likely due to the keratolytic action of tazarotene, despite a five-fold lower halobetasol concentration than the monotherapy, emphasizing the importance of tazarotene in the HP/TAZ combination. The numerically greater effect on erythema seen with halobetasol cream, as compared with HP/TAZ, is consistent with the results of other studies showing the efficacy of TCS on erythema and would be expected given the higher monotherapy concentration (0.05% vs. 0.01%). Erythema has been shown to be less responsive to tazarotene, perhaps due to the retinoid-induced erythema typically seen and the lack of vasoconstrictive effects.
Not only did the initial Phase II study with HP/TAZ lotion show a synergistic benefit in combining the two active ingredients, but the safety data were also consistent with the known safety profile of halobetasol propionate and tazarotene and did not reveal any new safety concerns with the combination product following daily use for eight weeks.14 In this shorter two-week study, both treatments were well tolerated, although more patients reported application site pain with halobetasol. Labeling restrictions for halobetasol did not allow for a longer-term comparative study to be performed. The efficacy seen with HP/TAZ lotion in our study suggests a broader utility of this combination topical treatment in plaque psoriasis, and data from a long-term safety study over 12 months are currently being evaluated.
Formulation development is key to attaining balance between efficacy and safety. Vehicles can significantly impact efficacy and adherence and increase cosmetic elegance.15 Formulation can also impact other aspects of treatment. A risk of rebound (a worsening of disease following treatment discontinuation) is not uncommon with TCS and might be formulation-dependent16; 77 percent of patients treated with clobetasol lotion maintained success at the four-week follow-up visit as compared with 49 percent of those treated with cream.17 Phase II clinical data on HP/TAZ lotion have shown better sustained efficacy at four weeks after treatment when compared with halobetasol 0.01% lotion formulated in the same vehicle. Unfortunately, our study did not include a post-treatment evaluation to assess differences between lotion and cream formulations. However, it is likely that the presence of tazarotene would complicate the evaluation, as tazarotene is known to provide a sustained efficacy post-treatment. Lotions have also been shown to be more acceptable to patients. For example, as compared with clobetasol cream, clobetasol lotion demonstrated significantly better ratings on six measures of cosmetic acceptability (all, P<0.05).17 Patient preference studies regarding HP/TAZ lotion are currently underway.
Conclusion
HP/TAZ lotion affords rapid treatment success comparable to a higher concentration of halobetasol propionate 0.05% cream when applied once daily for two weeks, with a numerically greater effect on plaque elevation and scaling. HP/TAZ lotion was well tolerated with minimal safety concerns.
Acknowledgments
We thank Brian Bulley, MSc (Konic Ltd., United Kingdom) for assistance with the preparation of this manuscript.
References
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007;25(6):535–546.
- Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007;8(1):1–12.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- Samarasekera EJ, Sawyer L, Wonderling D, et al. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br J Dermatol. 2013;168(5):954–967.
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity:
a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. - Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659.
- Levin E, Gupta R, Butler D, et al. Topical steroid risk analysis: differentiating between physiologic and pathologic adrenal suppression. J Dermatolog Treat. 2014;25(6):501–506.
- Lam LH, Sugarman JL. Adrenal suppression with chronic topical corticosteroid use in psoriasis patients. J Drugs Dermatol. 2016;15(8):945–948.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40(1):64–66.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: Two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48(5):760–767.
- Kaidbey K, Kopper SC, Sefton J, et al. A pilot study to determine the effect of tazarotene gel 0.1% on steroid-induced epidermal atrophy. Int J Dermatol. 2001;40(7):468–471.
- Lebwohl MG, Breneman DL, Goffe BS, et al. Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39(4 Pt 1):590–596.
- Sugarman JL, Stein Gold L, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16(3):194–201.
- Ceilley R. Advances in topical delivery systems in acne: new solutions to address concentration dependent irritation and dryness. Skinmed. 2011;9(1):15–21.
- Koo J, Lebwohl M. Duration of remission of psoriasis therapies. J Am Acad Dermatol. 1999;41(1):51–59.
- Decroix J, Pres H, Tsankov N, et al. Clobetasol propionate lotion in the treatment of moderate to severe plaque-type psoriasis. Cutis. 2004;74(3):201–206.

