 by Zoe Diana Draelos, MD; Steven Hall, PharmD; and Carey Munsick, MS
by Zoe Diana Draelos, MD; Steven Hall, PharmD; and Carey Munsick, MS
Dr. Draelos is with Dermatology Consulting Services, PLLC, in High Point, North Carolina. Dr. Hall and Ms. Munsick are with Sandoz Inc., in Princeton, New Jersey.
J Clin Aesthet Dermatol. 2020;13(8):E54–E58
FUNDING: This study was funded by PharmaDerm, a division of Fougera Pharmaceuticals.
DISCLOSURES: Dr. Draelos received funding from Fougera Pharmaceuticals to conduct the research detailed in this manuscript. Dr. Hall and Ms. Munsick are employees of Sandoz Inc.
ABSTRACT: Background.Dry, flaky skin remains one of the most common and vexing of human disorders. The anterior shin is a challenging area to treat in women with scaly skin.
Objective.We sought to investigate whether a lactic acid/ceramide lotion improves the texture and appearance of dry skin through moisturization and desquamation.
Methods.This was a randomized, single-center, controlled, evaluator-blinded, within-subject comparison of a lactic acid/ceramide lotion versus no treatment. The lotion was applied twice daily for 14 days, with evaluations performed on Days 1, 2, and 14. A total of 56 healthy female subjects, older than 50 years of age, with Fitzpatrick Skin Types I through VI and dry, rough skin on the anterior shins (defined as Grades 3–4 by investigator evaluation) were included in the study. Assessments of change in skin appearance following desquamation and moisturization by D-SQUAME® analysis and an investigator assessment on the Dry Skin Scale were performed on Days 1, 2, and 14. Additionally, subjects completed a self-assessment questionnaire on Day 1 and Day 14. Safety was assessed via spontaneous reporting and review of subject diaries.
Results.Clinical and statistically significant superiority to no treatment in terms of enhancing moisturization and desquamation per D-SQUAME® analysis at Day 2 and Day 14 (-2.51 and -3.07 from baseline, respectively; p<0.0001) was achieved. Treatment success as assessed by the investigator (via the Dry Skin Scale) and subject’s self-assessment also indicated statistically significant improvements were achieved with the lactic acid/ceramide lotion (p<0.0001). No adverse events were reported during the study.
Conclusion.The lactic acid/ceramide lotion provided a statistically significant and clinically meaningful improvement in moisturization and desquamation.
ClinicalTrials.gov Identifier: NCT04085809
Key words: Desquamation, moisturization, D-SQUAME, lactic acid, ceramides
Dry, flaky skin remains one of the most common and vexing of human disorders. Although there is no unambiguous definition of dermatosis, it is characterized by a rough, scaly, and flaky skin surface that often becomes fissured, particularly during the winter months.1 The anterior shin is a challenging area to treat in women with scaly skin. The application of traditional lotion formulations results in improvement through emolliency, where oily ingredients, such as mineral oil or dimethicone, smooth down the desquamating skin scale and make it more even to the eye. However, improvement is temporary; once the emollient is removed from the skin surface by friction or clothing, the scaliness becomes visible again.1 Longer-lasting improvement requires removal of the skin scale by correcting the desquamatory failure that resulted in the anterior shin skin scale retention.2 The enzymes that induce skin exfoliation require hydration, which can be difficult to achieve in dehydrated corneocytes.1 One approach is to enhance hydration by digesting the skin scale keratin protein and increasing the water-binding capacity of the keratin, which can be achieved with the active ingredient lactic acid.1 The enhanced skin scale hydration will increase desquamation, improving skin smoothness by removing the skin scale. However, the application of lactic acid alone fails to ameliorate the symptoms of dry skin, and coformulation with an occlusive agent is required to help retain the humectant-bound water within the surface layers of the stratum corneum. Lotions that contain barrier lipids (i.e., ceramides) and lactic acid provide synergistic relief from dry skin.1
Further, lactic acid can be supplemented with other ingredients that increase the water-holding capacity of the skin, such as humectants.3 Glycerin is one of the most effective and widely used humectants, not only capable of holding water, but also modulating aquaporins.4 Moisturization can also be enhanced by using ceramides, an important component of the intercellular lipids and the first substance produced by the skin after barrier damage has occurred.5
We examined the ability of a lactic acid, glycerin, and tri-ceramide (ceramide NP, ceramide AP, and ceramide EOP) formulation to improve the skin texture and quality through desquamation and moisturization. The improvement in the skin scale was documented with adhesive tape discs that collected loose skin scales over time to demonstrate the initial and continuing skin scale reduction with a novel topical formulation.6
The research also evaluated the overall skin appearance on Days 1, 2 and 14, using the Dry Skin Scale (DSS) created for this study by the investigator based on the European Group on Efficacy Measurement of Cosmetic and Other Topical Products assessment, the Overall Dry Skin Score, and the Specified Symptoms Sum Score.7 In addition, the subjects reported perceptions of their own skin via subject self-assessments performed before and after 14 days of treatment. Safety was assessed via the evaluation of any adverse events (AEs) reported during the study.
Methods
This study was conducted at Dermatology Consulting Services, PLLC in High Point, North Carolina. Institutional Review Board approval (Allendale Institutional Review Board, Old Lyme, Connecticut) was obtained prior to commencement of the study (NCT04085809).
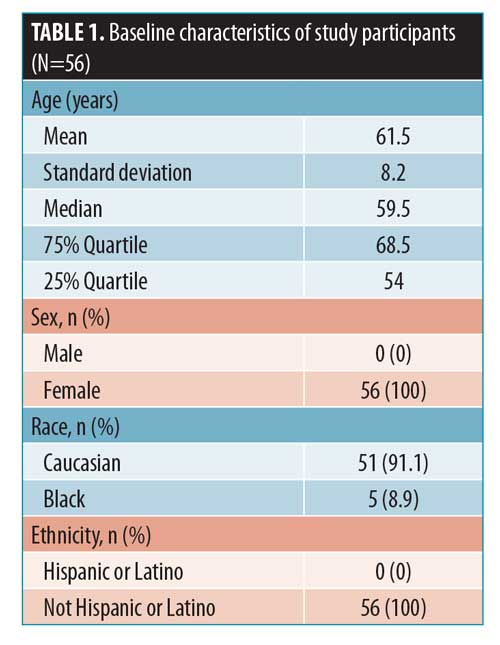
This was a randomized, single-center, controlled, evaluator-blinded, within-subject comparison study of a lactic acid/ceramide lotion (AmLactin® Rapid Relief; Fougera, Princeton, New Jersey) versus no treatment in healthy female subjects. Fifty-six healthy female subjects, aged 50 years or older, with Fitzpatrick Skin Types I through VI and dry rough, skin on the anterior shins (defined as Grades 3 and 4 on a five-point ordinal scale) were enrolled. Written informed consent was obtained from all subjects prior to inclusion in the study. Baseline subject demographic and characteristics are provided in Table 1.
Eligible subjects used a bland bar cleanser (Dove Sensitive Skin Beauty Bar, Unilever, United Kingdom), had to be willing to shower and not bathe for duration of the study, and agreed to refrain from shaving their legs within 72 hours of Day 1 and throughout the remainder of the study. Meanwhile, key exclusion criteria for the study were as follows:
- Visible skin disease at the assessment site
- Unwillingness or inability to refrain from using sunscreens, cosmetics, creams, ointments, lotions, or any products on the shins during the study
- Was a woman who had given birth within three months prior to the study
- Was a woman who was pregnant, planning to become pregnant during the study, or breast-feeding
- Had damaged skin in or around the test sites, including from sunburn, excessively deep tans, scars, excessive hair, psoriasis and/or active atopic dermatitis, or other disfiguration
- Had received treatment for any type of internal cancer within five years prior to study entry
- Was being treated for skin cancer or hepatitis at the time of the study
- Had any condition that might have compromised the study results and/or place the subject at risk, including but not limited to diabetes, human immunodeficiency virus, or lupus
- Was currently or planned to sunbathe or use tanning salons during the study period
- Was participating or had participated in an investigational drug study within 28 days prior to the study
- Had any known sensitivity to adhesives
All subjects had a template-located target site identified on both anterior shins to ensure the assessments were consistently evaluated on the same area for the moisturization and desquamation potential of the treatments. One shin was treated with the lactic acid/ceramide lotion and the opposite shin was used as an untreated control. The investigator was blinded to the study treatment.
The study product was applied to the randomized leg twice-daily for 14 days. Evaluations of moisturization and desquamation were performed clinically using tape-stripping (D-SQUAME®; CuDerm, Dallas, Texas) as well as dermatological evaluation of the skin using the DSS. Subjects rated their skin attributes of the lower randomized (treated) leg on Day 1 (pretreatment) and on Day 14. The site personnel provided the questionnaire to each participant prior to randomization and again on Day 14, prior to any clinical assessments being completed.
Statistical analysis. Subject demographics, D-SQUAME®, DSS, and subject self-assessment questionnaire data were summarized. A sample size of 56 subjects was derived based on the effect size to detect a change from baseline that was at least one unit greater for the D-SQUAME® assessment in the lactic acid/ceramide lotion treatment arm relative to in the control (no treatment) arm. The desired ?-value was 0.05 and the power was 90 percent. The primary statistical analysis assessed the desquamation potential of AmLactin® Rapid Relief by comparing the mean changes from baseline of the anterior shins of the subjects of this treatment versus no treatment using the D-SQUAME® assessment (evaluation performed based on the scaliness level). The analysis was based on the standard t-test and statistical significance was concluded if the resulting p-value was less than or equal to 0.05 (i.e., ? was 0.05).
Efficacy, safety, and additional assessments. Assessments of the change in skin appearance through desquamation and moisturization were made via D-SQUAME® analysis and an investigator assessment of the DSS. The assessments were conducted by a trained, blinded evaluator once at baseline (Day 1), Day 2, and Day 14.
D-SQUAME® adhesive discs were used to assess the desquamation of the skin at the investigator-targeted site on both the right and left anterior shins. The D-SQUAME® tapes were placed individually on both shins. A constant-pressure plunger was placed over the tape for 10 seconds. The D-SQUAME® tape was then removed by placing forceps on the tab with gentle pulling. The removed tapes were placed on a black background for evaluation and archiving. Scaliness was assessed on a scale of 1 to 5 points, with increasing scaliness at higher scores as shown in the product information.8
The blinded investigator used the DSS evaluation of the skin to assess the overall appearance of the skin. The scale assessed parameters on an ordinal scale, with 0=none, 1=minimal, 2=mild, 3=moderate and 4=severe at screening/Day 1 (baseline), Day 2 (following the morning application), and Day 14. The parameters assessed included skin dryness, tactile skin texture/roughness, visual skin texture/roughness, desquamation/flakiness, and lack of luminosity/radiance. These parameters were assessed on both legs and an overall evaluation number was calculated by averaging the five parameters for each leg together.
Safety. Safety was assessed by the review of any AEs reported during the study and subject diaries.
Additional assessments. Photographs of the D-SQUAME® discs and shins of five randomized subjects were collected for all study visits and selected by the investigator. Photos were captured on a D90 SLUE camera (Nikon, Tokyo, Japan). Subjects rated their skin attributes on Day 1 (pretreatment) and Day 14 of the lower randomized (treated) leg (Table 2). The site personnel provided the questionnaire prior to randomization and again on Day 14, prior to any clinical assessments.

Results
The primary efficacy analysis assessed the desquamation potential of the lactic acid/ceramide lotion by comparing the mean changes from baseline in the tape-retrieved skin scale of the treated versus untreated anterior shin after 14 days of treatment. The D-SQUAME® scale was evaluated on a five-point ordinal scale (1–5 points), where lower values indicated enhanced desquamation. For the lactic acid/ceramide lotion arm, there was a mean reduction of 3.07 points from baseline to Day 14 compared to a reduction of 0.45 points for the untreated control arm (Table 3).
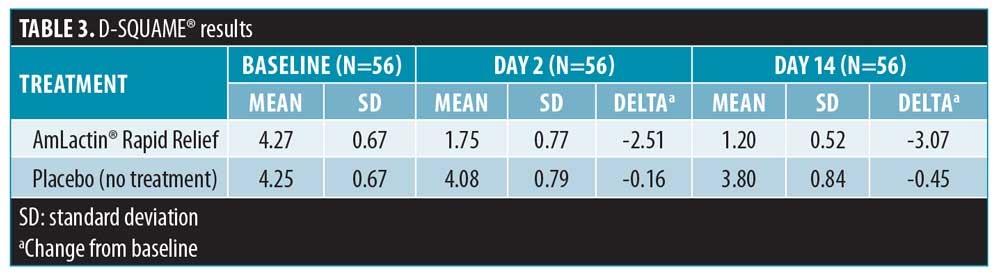
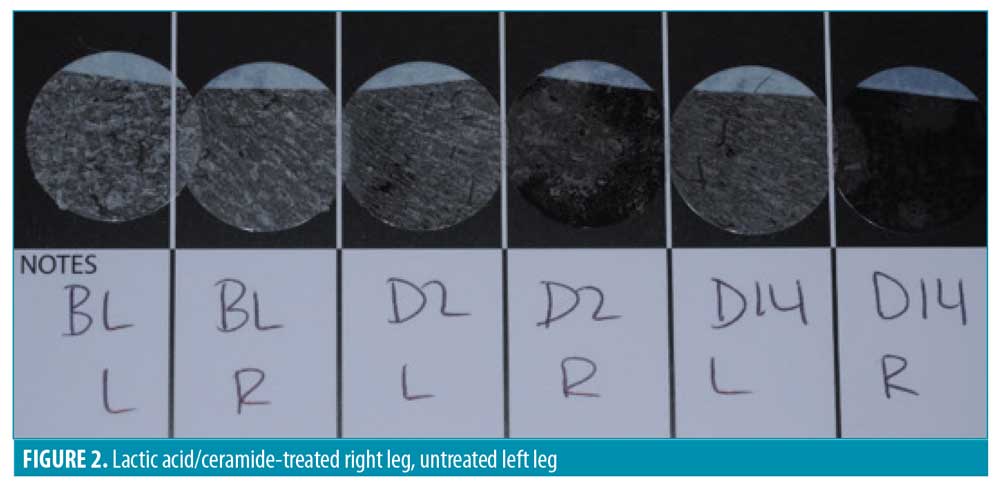
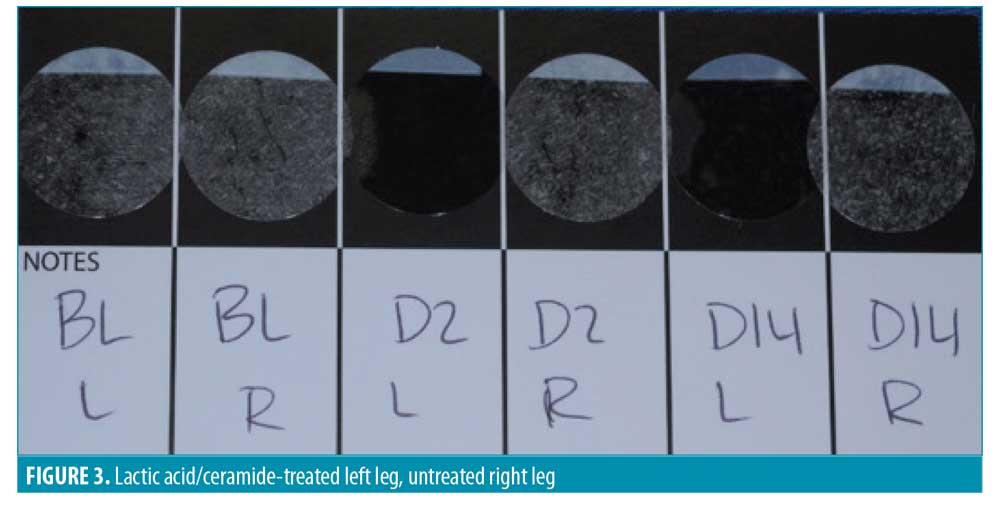
D-SQUAME® photographs. Figures 1 through 3 show sequential tape discs for three subjects. The photographs visually illustrate the progressive desquamation induced by the lactic acid/ceramide formulation. Full results for a representative subject, including sequential photographs, are shown in Figure 4.

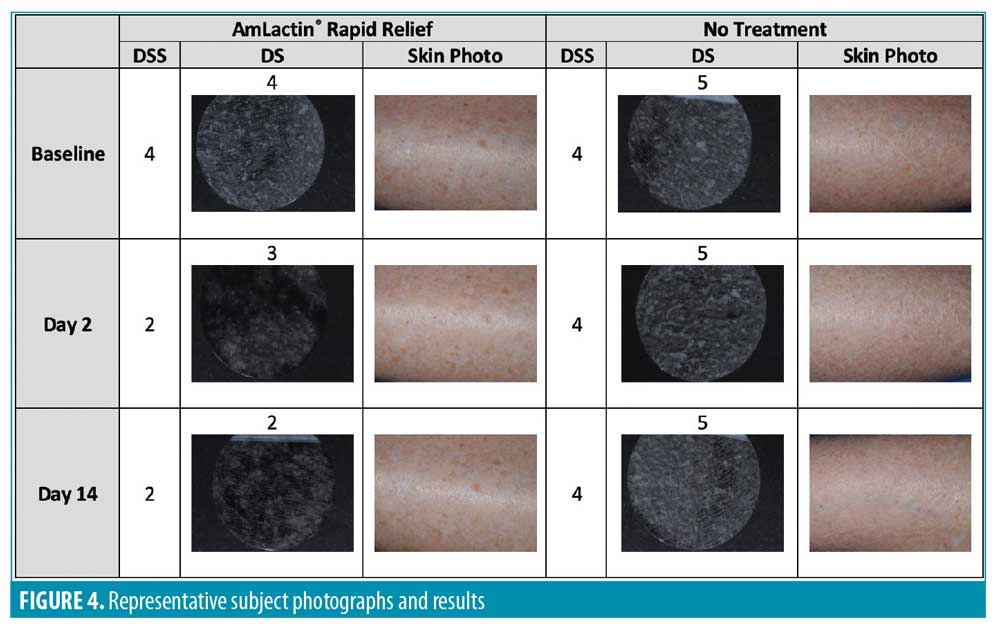
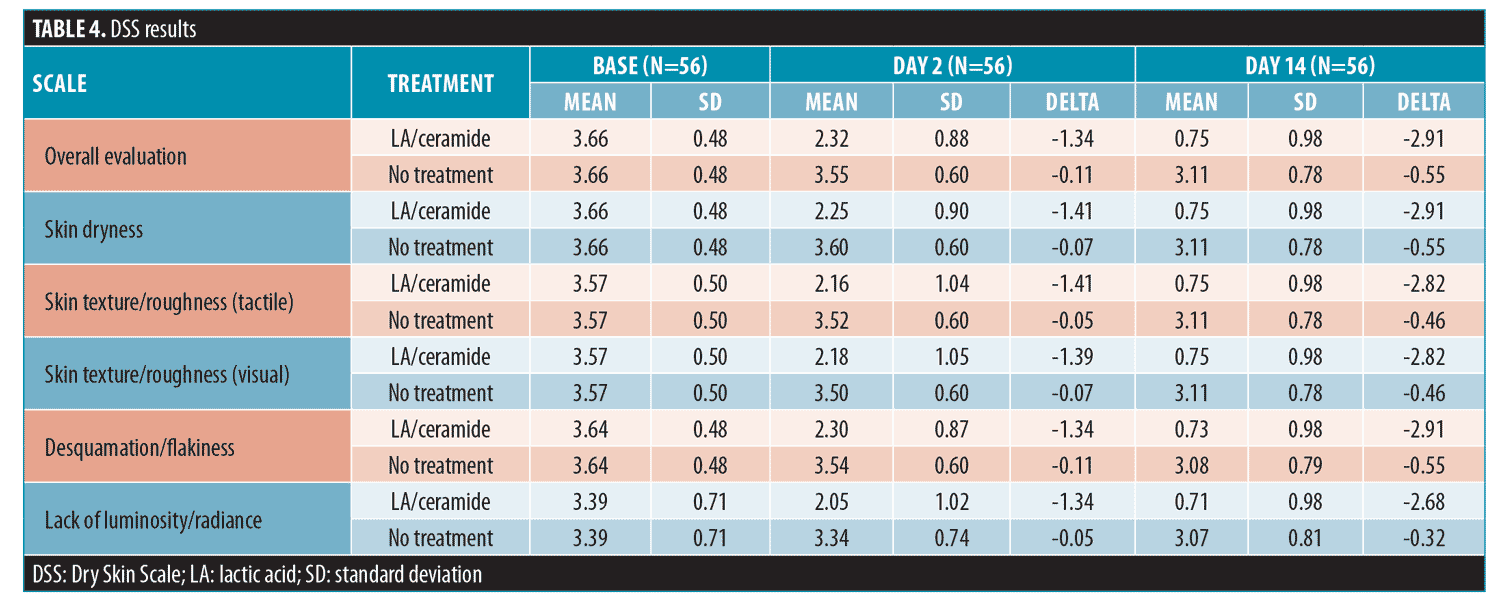
The DSS was assessed at baseline, Day 2, and Day 14 for both legs by the blinded investigator. The scale included the following measures: skin dryness, tactile skin texture/roughness, visual skin texture/roughness, desquamation/flakiness, and lack of luminosity/radiance. The measures were assessed separately and an overall evaluation of the DSS that included an average of the five assessments for each leg was performed (Table 4). Every subscale and the overall evaluation of the DSS revealed a significant improvement for the lactic acid/ceramide-treated leg versus no-treatment leg at Days 2 and 14 (all p<0.0001).
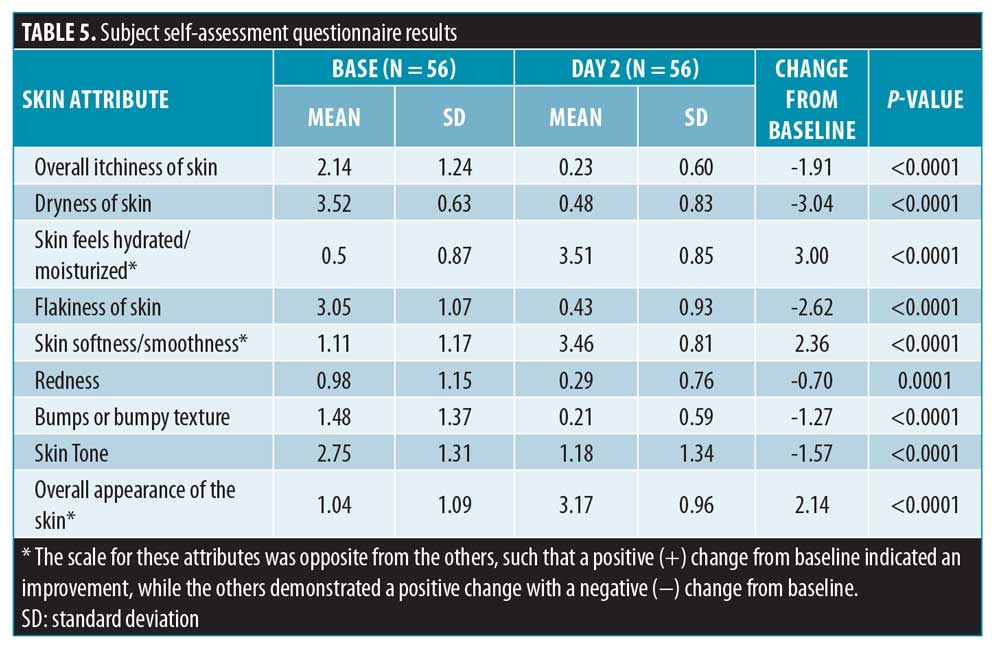
A self-assessment questionnaire was completed by each subject on Days 1 (pre-treatment) and 14 based on the randomized (treated) leg. Each of the nine questions was scored on a scale from 1 to 10 (Table 5). Subject-reported ratings improved for all attributes from 0.7 to 3 and all changes were statistically significant.
Safety. There were no AEs, serious AEs, or deaths during the study.
Discussion
This was a randomized, single-center, controlled, evaluator-blinded, within-subject comparison study of a lactic acid/ceramide lotion versus no treatment in healthy female subjects. The study product was applied to the randomized leg twice-daily for 14 days and the moisturization and desquamation were assessed clinically using D-SQUAME® and the dermatologist evaluation of the skin. Fifty-six subjects were screened and randomized, with all 56 (100%) successfully completing the study with no adherence issues and no adverse events. All collected data was used in the final analysis.
The lactic acid/ceramide lotion yielded a clinically and statistically significant difference in all assessments measured in this study. For the D-SQUAME® analysis (primary efficacy analysis), the treated leg showed -2.51 and -3.07 changes from baseline to Day 2 and Day 14, respectively, compared to the changes for no treatment of -0.16 and -0.45—the larger decrease indicates enhanced moisturization and desquamation. The t-tests for both measures were significant, with p<0.0001. The DSS was applied at baseline and Days 2 and 14 for the treated and untreated legs. When all the assessment values were averaged for a cumulative DSS assessment score, the lactic acid/ceramide lotion-treated leg had improvements of 1.34 and 2.91 from baseline to Days 2 and 14, respectively, compared to those scores for no treatment of 0.11 and 0.55. For each measured attribute, there was a 1.34- to 1.41-point and 2.68- to 2.91-point improvement from baseline on Days 2 and 14, respectively, with the lactic acid/ceramide lotion treatment and a 0.05- to 0.11-point and 0.32- to 0.55-point improvement from baseline when using no treatment on Days 2 and 14, respectively. The differences observed with the lactic acid/ceramide lotion treatment were all clinically meaningful and statistically significant.
Additionally, a subject self-assessment questionnaire was developed and used to gain insight into how the subjects felt regarding certain characteristics of their skin posttreatment (Day 14) with the lactic acid/ceramide lotion. All attributes were rated on a scale of 0 to 4 by the subject at baseline and Day 14. Overall, all of the attributes improved in a statistically significant manner, with p<0.0001. The overall itchiness of the skin improved by 1.91 points, the dryness of skin improved by 3.04 points, the feeling of skin hydration/moisturization improved by 3.00 points, the flakiness of the skin improved by 2.62 points, the skin softness/smoothness improved by 2.36 points, bumps or bumpy texture improved by 1.27 points, skin tone improved by 1.57 points, redness improved by 0.70 points, and the overall appearance of the skin improved by 2.14 points.
Limitations. This study was limited by the lack of comparisons to other currently marketed moisturizing products, the limited duration of treatment, single-center design, use of modified validated scales, and limited diversity in the study population race.
Conclusion
The tested lactic acid/ceramide-containing lotion provided a statistically significant and clinically meaningful improvement in desquamation, resulting in enhanced moisturization.
References
1. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders, In Loden M, Mailbach H, eds. Dry skin and moisturizers: chemistry and function. 2nd ed. Boca Raton, FL: CRC Press; 2006: 771–778.
2. Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003; 4(11): 771–788.
3. Black D, Boyer J, Lagarde JM. Image analysis of skin scaling using D-SQUAME samplers: comparison with clinical scoring and use for assessing moisturizer efficacy. Int J Cosmet Sci. 2006;28(1):35–44.
4. Murata K, Mitsuoka K, Hirai T, et al. Structural determinants of water permeation through aquaporin. Nature. 2000;407(6804):599–605.
5. Huang HC, Chang TM. Ceramide 1 and ceramide 3 act synergistically on skin hydration and the transepidermal water loss of sodium sulfate-irritated skin. Int J Dermatol. 2008;47(8):812–819.
6. Serup J, Winther A, Blichmann C. A simple method for the study of scale pattern and effects of a moisturizer—qualitative and quantitative evaluation of D-SQUAME® tape compared with parameters of epidermal hydration. Clin Exp Dermatol. 1989;14(4):277–282.
7. Serup J. EEMCO guidance for the assessment of dry skin (xerosis) and ichthyosis: clinical scoring systems. Skin Res Technol. 1995;1(3):109–114.
8. Cosderma. D-SQUAME® instructions. Available at: https://cosderma.com/lequipement-de-cosderma/dsquame.html. Accessed on August 11, 2020.

