 J Clin Aesthet Dermatol. 2020;13(9): E53–E58
J Clin Aesthet Dermatol. 2020;13(9): E53–E58
by Thilo Gambichler, MD; Konstantina Mamali, MD; and Christina Scheel, MD
Dr. Gambichler and Dr. Scheel are with the Department of Dermatology, Venereology and Allergology at Ruhr-University Bochum in Bochum, Germany. Dr, Mamali is with Cosmetic Derma Medicine in Athens, Greece.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: We provide a brief review on mid-dermal elastolysis (MDE) and summarize clinical data of 105 patients with MDE who were reported in the literature since the disease was first described in 1977. In doing so, emphasis is placed on pathomechanisms and therapeutic aspects. MDE is a rare, acquired skin disease histopathologically characterized by selective loss of elastic fibers in the mid-dermis. Lesions are commonly observed on the trunk and proximal extremities. These include well-circumscribed patches of fine wrinkles, perifollicular papular protrusions, and persistent reticular erythema. With respect to pathomechanisms, current data suggest that different cell types (e.g., macrophages, fibroblasts) might chronically overexpress matrix metalloproteinases resulting in an enhanced elastolytic activity. Together with decreased expression of the tissue inhibitors of metalloproteinases, this is thought to result in zonal degradation and loss of elastic tissue in the mid-dermis. However, the exact mechanisms leading to the enhanced elastolytic activity in MDE remain elusive. A multifactorial pathogenesis is likely, including genetic predisposition, chronic inflammation, and (auto)immune processes. Moreover, the capacity for elastic fiber renewal appears to be diminished in patients with MDE, limiting regenerative potential and informing possible treatment strategies, for example, by stimulating elastic fiber synthesis. Although the course of MDE is usually benign and asymptomatic, it can cause severe cosmetic problems. Hence, new therapeutic approaches that block increased elastolytic activity and enhance regeneration of elastic tissue observed in MDE patients are required.
Keywords: Mid-dermal elastolysis, middermal elastolysis, elastin; elastic tissue, wrinkles
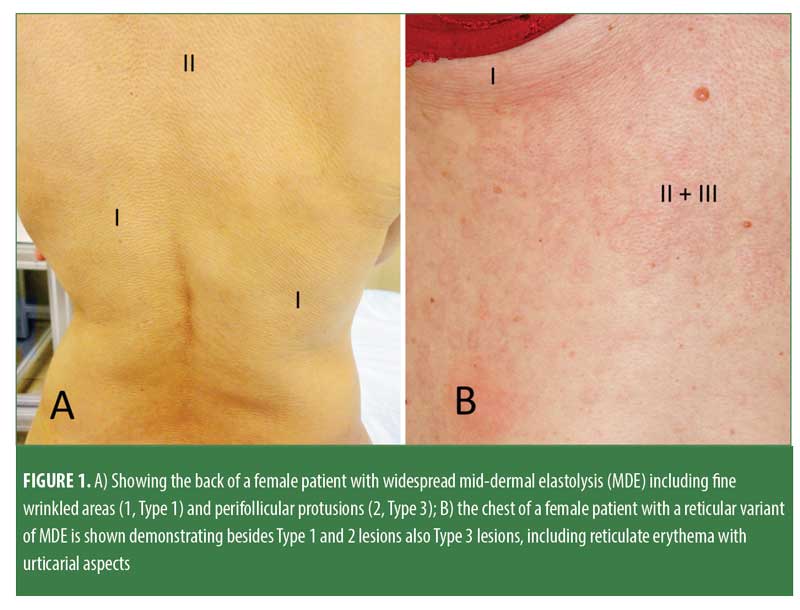
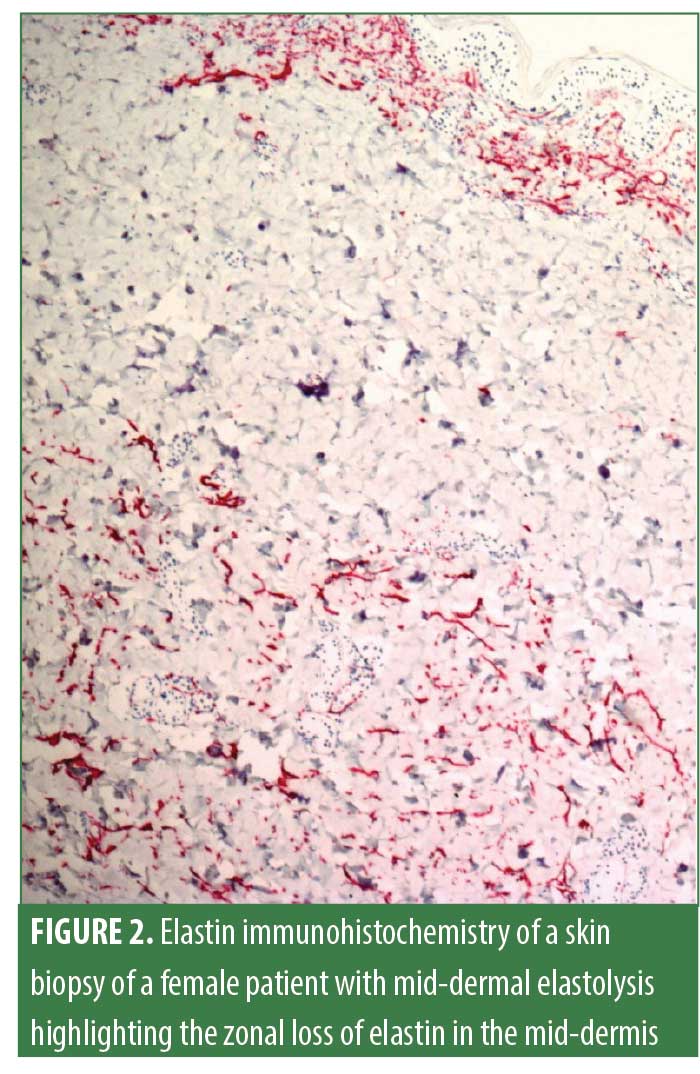
Mid-dermal elastolysis (MDE) is a rare acquired skin disease characterized by selective loss of elastic fibers in the mid-dermis. MDE usually affects younger Caucasian women. Most commonly found skin lesions in individuals with MDE include well-circumscribed patches of fine wrinkles (Type 1), perifollicular papular protrusions (Type 2), and persistent reticular erythema and wrinkles (Type 3; Figure 1). Skin lesions are predominantly observed on the trunk and proximal extremities. In most cases, hematoxylin-eosin staining does not reveal significant changes that could aid in diagnosing MDE, except for slightly thickened collagen fiber bundles and a barely visible inflammatory infiltrate consisting of few perivascular lymphocytes.1 Disease-defining histopathological findings identified by elastica stains (e.g., Orcein stain, Verhoeff-van-Gieson stain; Figure 2) include a band-like or focal loss of elastic fibers in the mid-dermis. In contrast, no histological alterations are found in the papillary and deeper reticular dermis.1 MDE is confined to the skin and is usually not associated with systemic involvement. The pathogenesis of MDE remains unclear.1 However, some insights into MDE pathomechanisms have been gained during the last 10 years.
Our latest review on MDE, which was published in the March issue 2010 of the Archives of Dermatological Research,1 was updated in 2015 by Hardin and colleagues.2 However, this update did not include several additional cases published between 2010 and 2015.3–8 Our aim for the present review was to identify and review new clinical case reports and research papers on MDE in the light of previously published cases.1,2 For this purpose, a PubMed search was performed for articles published from 2009 to January 2020 using the following keywords: mid-dermal elastolysis, mid-dermal elastolysis, and elastophagocytosis. Moreover, references from these publications were searched for additional relevant data, and cases previously reviewed by Hardin et al2 were excluded.9–12 Furthermore, eight patients were excluded from a large case report,13 whose clinical data were already reported elsewhere.14–17 Taken together, we could include 20 publications2–8,13,15,16,18–27 with clinical data of 26 new patients with MDE and present their findings in the light of previously reported cases.1
Basics on Elastic Tissue Formation
Fibrillar collagen and elastin are two of the basic extracellular matrix (ECM) components significantly contributing to the maintenance of skin structure, elasticity, and, hence, resilience. They each form an architecturally distinct meshwork that confers elastic recoil properties to the skin. The formation of elastic fibers is complex and not yet fully understood. Tropoelastin, the crucial building component of elastin, is expressed by the ELN gene and the mature form of the protein is secreted into the ECM.28,29 Then tropoelastin accumulates on the cell surface, first as small particles, then as larger spherules, which are associated coacervates of tropoelastin. Thereafter, tropoelastin is subjected to oxidation by lysyl oxidase (LOX) and LOX-like enzymes at a subset of lysines which subsequently participate in aldol condensation and Schiff base reactions to form cross-links.28,29 The forming elastin is introduced to microfibrils (fibrillin-1) in the ECM by members of the fibulin protein family where the elastin fibers are assembled. The resulting protein elastin is a highly stable and robust structure with an impressive ability to confer recoil to human tissues, such as the skin.28,29 A dense mass of elastic fibers characterizes the reticular dermis and is crucial for the overall elasticity of the skin. In general, the most mature, thicker elastin fibers are found deep in the dermis, where they function as an interpenetrating elastin network.28–31
As mentioned above, the fundamental building component of elastin is tropoelastin encoded by the ELN gene, whose expression is highest before birth and during the first years of life. Thereafter, tropoelastin gene expression and thus, elastin formation, decreases significantly. In middle-aged subjects, only a small fraction of elastin is still produced.28–31 Hence, adults have to rely mostly on the elastin that was deposited in the first few years of life. Consequently, damage to the elastin in the skin of adults following injuries (e.g., burns), loss during the aging process, or through elastolytic disorders (e.g., MDE), the lack of robust endogenous elastin synthesis will not be repaired efficiently leading to lost skin elasticity and wrinkle formation.28–31
Clinicopathological Features of 105 Patients with Mid-dermal Elastolysis
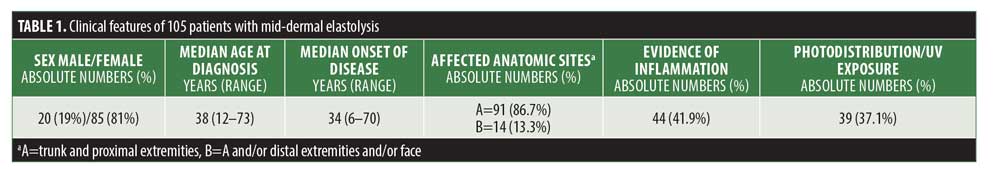
As shown in Table 1, the sex distribution remains similar compared with previous reports. More than 80 percent of reported patients with MDE were female and only 20 percent male, indicating that MDE is a female-centric skin disease. MDE is usually observed in middle-aged women, with a median onset of disease at 34 years (6–73 years). The most affected anatomic sites include the trunk and proximal extremities (86.7%). Clinical and/or histological evidence for inflammation was observed in 41.9 percent of patients. Significant ultraviolet (UV) exposure prior to the onset of MDE and/or photodistribution of MDE lesions was described in 37.1 percent of patients. In this regard, UV exposure has not yet been shown to represent a definitive pathogenetic driver in MDE.1,2
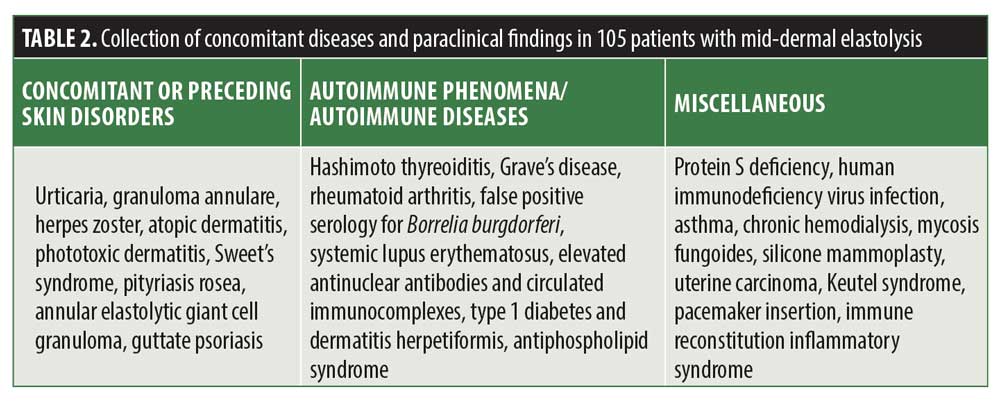
In Table 2, we demonstrated the wide range of concomitant or preceding disorders and paraclinical findings observed in patients with MDE. Importantly, many of these findings were reported only once and do not necessarily represent a true association with MDE. However, the association between MDE and preceding granuloma annulare deserves particular attention, since it has already been observed in four independent cases over the decades.6,21,32,33 These cases support the existing but scant evidence in the literature that MDE results from destruction of dermal elastic fibers through inflammatory processes.1,2,25,27 Importantly, granuloma annulare is one form of dermatitis capable of culminating in this entity, but others have also been implicated, such as sarcoidosis and Sweet’s syndrome.
In up to 30 percent of MDE cases, macrophages and/or elastophagocytosis and in up to 17 percent of cases giant cells have been described.1,2 Microscopically, elastophagocytosis can be observed in the cytoplasm of histiocytes and multinucleated giant cells. Generally believed to be a characteristic feature of certain granulomatous disorders, such as annular elastolytic giant cell granuloma, or elastolytic disorders, such as MDE, this feature has also been described in other cutaneous inflammatory conditions, cutaneous malignancies, infectious entities, and secondary to certain medications.11
Another interesting point is that Subtype 3 of MDE, which is clinically characterized by frank inflammation as indicated by reticulate erythema, is more common in men at older age (>50 years). Thirteen patients previously reported in the literature may be included into MDE Subtype 3. Of these 13 patients, five (38.5%) were female27,34–36 and eight (61.5%) male.37–42 Elastophagocytosis has been observed more frequently in the reticular variant of MDE27,40-42 when compared with Subtypes 1 and 2.1,2
As shown in Table 2, MDE might be associated with different immune processes, such as rheumatoid arthritis, Hashimoto thyroiditis, Grave’s disease, systemic lupus erythematosus, Type 1 diabetes, dermatitis herpetiformis, and the presence of antiphospholipid antibodies. Moreover, HIV-infection and immune reconstitution inflammatory syndrome was reported in patients with MDE.1,2,20,25 Another interesting finding is the occurrence of MDE following pacemaker insertion, which was observed in two independent cases. Whether the surgery or the pacemaker itself was the trigger for a consecutive inflammatory response directed specifically towards the elastic tissue remains unclear. Given the great number and variety of conditions and interventions reported to be associated with MDE, one might speculate that a common immunological pathomechanism leads to an activation of elastin-degrading processes with consecutive loss of elastic tissue.
Pathogenetic Aspects in MDE
Different hypotheses exist regarding the pathogenesis of MDE, including a correlation with smoking, sexual hormones, copper deficiency, decreases in serum elastase inhibitor (alpha1-antitrypsin), and the occurrence of anti-elastin antibodies. However, these hypotheses are predominantly based on anecdotal evidcence.1,2 In order to better understand pathomechanisms involved in MDE, recent studies focused on the cellular components constituting the lesions found in patients with MDE. For example, using immunohistochemistry, we and others detected increased expression of CD4+ lymphocytes and CD34+ and CD68+ histiocytes in lesional skin of MDE patients. Moreover, matrix metalloproteinases (MMP) and its major tissue inhibitor (TIMP) expressed by CD68+ histiocytes and giant cells have been reported to play crucial role in MDE pathogenesis.1,2 Today, it is well accepted that increased expression and/or activity of elastases lead to elastin degradation in MDE and related conditions such as anetoderma. Different cells, like skin fibroblasts, neutrophil granulocytes, lymphocytes, monocytes/macrophages, and multinucleated giant cells, can release elastases, such as MMPs.1,2 Accordingly, we previously observed elevated expression of MMP-1 and MMP-12 in lesional skin accompanied by decreased expression of TIMP-1.16,43 Additionally, MMP-9 was detected by others in keratinocytes and large multinucleated giant cells in the dermis of MDE patients.14 Consequently, an abundance of CD68+ and MMP-9 expressing histiocytes and giant cells was observed in inflamed MDE lesions, whereas only few CD68+ cells were found in noninflamed wrinkled skin. Three other studies describe an increase in elastase synthesis by fibroblasts in lesional skin of MDE patients.45,46,47 To dissect the role of fibroblasts in MDE, fibroblasts obtained from patients with MDE were cultured to evaluate the activity of MMPs and their major tissue inhibitor, TIMP-1.27 Thereby, it was revealed that, at least in vitro, fibroblasts derived from patients with MDE produce lower levels of TIMP-1 than fibroblasts from control subjects.27 Elevated levels of MMP-2 and MMP-14 capable to activate in a cooperative manner pro-MMP-2 were present in MDE tissue samples.27 Furthermore, significant reactivity for MMP-1 was detected in the same MDE areas. Taken together, the data from these different studies suggest that different cell populations (i.e., inflammatory cells and fibroblasts) are altered in a manner that increases elastolytic burden, which, in turn, causes the focal loss of elastic tissue in the mid-dermis of patients with MDE. However, why this loss of elastic fibers in MDE is confined to the mid-dermis remains elusive. For example, it might be possible that particular pathogenic factors are predominantly active within the mid-dermis, such as MMP-producing macrophages or fibroblasts. For example, recent evidence indicates at least two fibroblast lineages with distinct morphology, function and possibly, tissue localization. On of these recently identified lineages is positive for the cell-surface markers Fibroblast-Activating Protein (FAP) and CD90 and usually found in the reticular dermis.48
Mutations in major structural components of elastic fibers, including elastin, fibrillins, and fibulin-5, cause severe, often life-threatening, heritable connective tissue diseases (e.g., Marfan syndrome, supravalvular aortic stenosis, and cutis laxa).30 Mutations in elastic fiber components, such as elastin, might result in abnormal proteins that potentially lead to elastic tissue degradation.30 In patients with MDE, however, evidence for familiar genetic clustering was not reported, except for one patient who also suffered from Keutel syndrome.49 Mutations in the matrix Gla protein gene, which is an ECM protein, have been observed in patients with this disease.49 Of course, the association of rare conditions such as Keutel syndrome and MDE indicates a common pathogenetic background. However, inheritance and abnormal calcification as characteristic for Keutel syndrome have not been observed in patients with MDE.1,2
Apart from studies addressing the degradation of elastic fibers due to increased elastolytic activity in MDE, other investigation focused on crucial factors in elastic tissue remodeling, including LOXL-2 and fibulins. The LOX family is a group of amine oxidases responsible for the crosslinking of collagen and elastin fibrils in the ECM. Four LOXL enzymes produced by the same gene family have been identified (LOXL-1-4), which play a major role in the remodeling of elastic tissue.10 In a pilot study, we previously detected decreased expression of LOXL-2 in lesional skin of a patient with MDE.10 In order to confirm altered LOXL-2 expression in a larger group and to identify mutations in LOXL-2, we performed an investigation in 13 patients with MDE.13 Thus, we observed decreased LOXL-2 mRNA expression in lesional skin of patients with MDE compared with healthy skin of the same patients and normal subjects.13 Compared with healthy patient skin, LOXL-2 protein expression in lesional patient skin was significantly decreased.13 Mutation analysis of the entire LOXL-2 gene was performed in five patients, all of whom were found to have at least one mutation. Together, these data suggest LOXL-2 mutations and consecutively reduced LOXL2 expression might result in a decrease in collagen and elastin remodeling and thereby contribute to the pathogenesis of MDE.13 In addition, we identified LOXL-2 promoter hypermethylation as yet another possible explanation for decreased LOXL-2 expression in MDE, suggesting that epigenetic factors might also be involved in the pathogenesis of MDE.50
Fibulin-5, a microfibril, has been identified as one of the crucial ECM proteins that forms conglomerates with elastin fibers. Kadoya et al51 showed that fibulin-5 is a good marker of skin ageing, and that the earlier loss of fibulin-5 might involve age-dependent changes in other elastic fiber components. Suda et al44 demonstrated in a case report that the reappearance of fibulin-5 in the deep dermis of the wrinkled skin and less of the erythematous skin was supposedly for elastic tissue regeneration. However, the fragmented expression of fibulin-5 was associated only with a barely incomplete reproduction of elastic fibers.44 We recently demonstrated decreased fibulin-4 and fibulin-5 expression in lesional skin of 14 patients with MDE when compared with healthy controls. In contrast, in anetoderma patients (n=9), only fibulin-4 showed decreased expression.52
Together, the aforementioned data on patients with MDE suggest that cooperation of different cell populations resident in the mid-dermis (e.g., macrophages, fibroblasts) might result in enhanced elastolytic activity due to MMP overexpression, as well as a concomitant decrease in TIMP expression. Such enhanced elastolytic activity might then produce the zonal degradation and loss of elastic tissue in the mid-dermis. The causes leading to enhanced elastolytic activity in MDE remain elusive, but are very likely multifactorial, including genetic predisposition, preceding inflammation, and (auto)immune processes. Moreover, the capacity of elastic fiber remodeling and renewal through LOXL and fibulins appears to be diminished in patients with MDE. The latter finding might be particularly important with respect to identifying treatment strategies that aim to increase endogenous elastic fiber regeneration.
Treatment Approaches for MDE
The management of MDE remains extremely challenging. The first goal in managing MDE is to diagnose the disease promptly. Unfortunately, many seek out dermatological treatment late in the disease course, as indicated by Table 1. Once elastolytic activity and consecutive loss of elastic tissue has spread, the chance of successful treatment of already wrinkled skin areas is very poor.1,2 Since the first description of MDE by Shelley and Wood in 1977,53 a variety of substances have been tested, including vitamin E, clofazimine, colchicine, and dapsone. However, no convincing benefit was reported using the aforementioned drugs for MDE treatment. Topical and systemic steroids have been administered in patients MDE, but again with no benefit.1,2 Strict UV protection is considered essential. Recently, Smithson et al54 reported a case of MDE for which novel treatment with mycophenolate mofetil was successful. Martinez-Escala et al25 reported a patient with antiphospholipid antibody syndrome who experienced cessation of progression of the skin abnormalities under the treatment with hydroxychloroquine. Altogether, however, best responses to treatment as reported in the literature included only a reduction of inflammatory lesions and/or stop of progression. Regeneration of already wrinkled skin sites has not yet been achieved and still represents the greatest challenge in MDE management.
Given the importance of elastin for the elasticity of the skin and its loss in the aging process and diseases like MDE, it is not surprising that various attempts have been made to elevate elastin levels. Therapies aiming to regenerate elastin in elastic tissues should consider all the molecules implicated in elastin fiber formation, including fibrillins, fibulins, and LOX/LOXL enzymes.28,29 Since elastic fibers consist of over 90% elastin, the integration of sufficient tropoelastin into elastin fibers appears as the obvious major target.28,29 Currently, effective therapy regimens are limited due to the obvious physical challenge of transferring materials (e.g., proteins) and/or treatments across the epidermis and into the dermis.28,29
Tretinoin is a small molecule that has been employed for many years in topical formulations to increase elastin production in skin through increased tropoelastin and fibrillin expression and secretion.55–57 Topical retinoid treatment has sporadically been reported to improve wrinkles in patients with MDE but does not appear to influence the course of the disease.1,2 Molecules, such as aldosterone and mineralocorticoid receptor antagonists, have been reported to positively impact elastin fiber deposition in skin.58 Soy and rice extracts might also increase elastin formation, as has been reported for a combination of zinc and copper.59,60 More recently, a dill extract has also been shown to have the potential to promote elastin formation by promoting LOXL synthesis and secretion into the dermis.61,62
Conclusion
MDE is a rare, female-centric skin condition that is associated with aesthetic implications and significant psychosocial impact. Current data indicate that not only elastolytic overactivity driven by dermal cells (such as macrophages and fibroblasts), but also diminished capacity of elastic fiber re-assembly, might play a significant role in the pathogenesis of MDE. Hence, effective treatment regimens need to address both pathogenetic aspects, i.e. the blockade of elastolytic overactivity as well as restoration of a normal protein/enzyme milieu in the skin required for proper elastic fiber renewal. In this regard, several interesting substances are currently under investigation in pre-clinical phases of skin aging research. However, due to the rarity of this condition, implementation of formal clinical investigations such as randomised controlled trials poses a challenge.
References
- Gambichler T. Mid-dermal elastolysis revisited. Arch Dermatol Res. 2010 Mar;302(2):85–93.
- Hardin J, Dupuis E, Haber RM. Mid-dermal elastolysis: A female-centric disease; case report and updated review of the literature. Int J Womens Dermatol. 2015 Jul 26;1(3):126–130.
- Barrado-Solis N, Rodrigo-Nicolas B, Moles-Poveda P, et al. Mid-dermal elastolysis: report of a case and literature review. Dermatol Online J. 2014;20(10):pii:13030/qt8gm489tn.
- Cruz MJ, Barros AM, Azevedo F. Generalized mid dermal elastolysis. Dermatol Reports. 2011 Dec 1;3(3):e52.
- Levy-Roy A, Tasei AM, Richard MA. [What is your diagnosis? Mid-dermal elastolysis]. Ann Dermatol Venereol. 2011;138(4):324–326.
- Sanyal S, Hejmadi R, Taibjee SM. Granuloma annulare resolving with features of mid-dermal elastolysis. Clin Exp Dermatol. 2009 Dec;34(8):e1017–1018.
- Helbig D, Schlaak M, Renner R, et al. [Mid-dermal elastolysis]. Hautarzt. 2010 Sep;61(9):779–784.
- Filatova I, Adams DR, Brod BA, Sceppa JA. Fine wrinkling of the thighs and axillae–quiz case. Middermal elastolysis. Arch Dermatol. 2010;146(10):1167–1172.
- Scola N, Goulioumis A, Gambichler T. Non-invasive imaging of mid-dermal elastolysis. Clin Exp Dermatol. 2011;36(2):155–160.
- Gambichler T, Skrygan M. Decreased lysyl oxidase-like 2 expression in mid-dermal elastolysis. Arch Dermatol Res. 2013;305(4):359–363.
- El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70(5):934–944.
- Tong PL, Qin J, Cooper CL, Lowe PM, et al. A quantitative approach to histopathological dissection of elastin-related disorders using multiphoton microscopy. Br J Dermatol. 2013;169(4):869–879.
- Gambichler T, Mahjurian-Namari M, Reininghaus L, et al. Lysyl oxidase-like-2 mutations and reduced mRNA and protein expression in mid-dermal elastolysis. Clin Exp Dermatol. 2019;44(1):47–51.
- Patroi I, Annessi G, Girolomoni G. Mid-dermal elastolysis: a clinical, histologic, and immunohistochemical study of 11 patients. J Am Acad Dermatol. 2003;48(6):846–851.
- Gambichler T, Lübbe J. Reticular variant of mid-dermal elastolysis accompanied by persistent urticarial lesions. J Dermatol. 2012;39(11):963–965.
- Gambichler T, Stücker M, Kreuter A, et al. Expression of extracellular matrix proteins in reticular variant of mid-dermal elastolysis. J Eur Acad Dermatol Venereol. 2010;24(12):1481–1484.
- Gambichler T, Breuckmann F, Kreuter A, et al. Immunohistochemical investigation of mid-dermal elastolysis. Clin Exp Dermatol. 2004;29(2):192–195.
- Smithson SL, Orchard D, Scardamaglia L. Mycophenolate mofetil to treat mid-dermal elastolysis. Pediatr Dermatol. 2018;35(4):e221–e223.
- Kottler D, Lefèvre A, Balme B, et al. [Mid-dermal elastolysis after insertion of a pacemaker]. Ann Dermatol Venereol. 2015;142(11):680–684.
- Cota C, Latini A, Lora V, Cerroni L. Mid-dermal elastolysis as a manifestation of immune reconstitution inflammatory syndrome in an HIV-infected patient. J Am Acad Dermatol. 2014;71(4):e134–135.
- Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41(5):462–468.
- Cohen PR, Tschen JA. Linear lumbar localized Lysis of elastic fibers: A distinctive clinical presentation of mid-dermal Elastolysis. J Clin Aesthet Dermatol. 2013;6(7):32–39.
- Meyer A, Aaron D, Perry A, Guill M 3rd. Erythematous reticular patches: a rare presentation of mid-dermal elastolysis. J Am Acad Dermatol. 2012;67(5): e216–217.
- Posada C, No N, De La Torre C, Flórez A. Reticular variant of mid-dermal elastolysis. Australas J Dermatol. 2013;54(1):69–71.
- Martínez-Escala ME, Rozas E, Pujol RM, Herrero-González JE. Mid-dermal elastolysis: another dermatological clue to autoimmunity? Acta Derm Venereol. 2012;92(4):434–435.
- Cutillas E, Ferrando FJ, Martí ME, et al. Reticular variant of mid-dermal elastolysis after insertion of a pacemaker. Clin Exp Dermatol. 2010;35(5):498–500.
- De Cunto G, Lamberti A, de Santi MM, et al. Middermal Elastolysis: Dermal Fibroblasts cooperate with inflammatory cells to the Elastolytic disorder. Mediators Inflamm. 2017;2017:9524594
- Vindin H, Mithieux SM, Weiss AS. Elastin architecture. Matrix Biol. 2019;84:4–16.
- Wise SG, Weiss AS. Tropoelastin. Int J Biochem Cell Biol. 2009;41(3):494–497.
- Baldwin AK, Simpson A, Steer R, Cain SA, Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2013;20(15):e8.
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115(Pt 14):2817-28.
- Yen A, Tschen J, Raimer SS. Mid-dermal elastolysis in an adolescent subsequent to lesions resembling granuloma annulare. J Am Acad Dermatol. 1997;37(5 Pt2):870–872.
- Adams BB, Mutasim DF. Colocalization of granuloma annulare and mid-dermal elastolysis. J Am Acad Dermatol. 2003;48(2 Suppl):S25–S27.
- Posada C, No N, De La Torre C, Flórez A. Reticular variant of mid-dermal elastolysis. Australas J Dermatol. 2013;54(1):69–71.
- Gambichler T, Lübbe J. Reticular variant of mid-dermal elastolysis accompanied by persistent urticarial lesions. J Dermatol. 2012;39(11):963–965.
- Gambichler T, Stücker M, Kreuter A, Matip R, Gaifullina R, Scola N, Skrygan M. Expression of extracellular matrix proteins in reticular variant of mid-dermal elastolysis. J Eur Acad Dermatol Venereol. 2010;24(12):1481–1484.
- Kottler D, Lefèvre A, Balme B, et al. [Mid-dermal elastolysis after insertion of a pacemaker]. Ann Dermatol Venereol. 2015;142(11):680–684.
- Meyer A, Aaron D, Perry A, Guill M 3rd. Erythematous reticular patches: a rare presentation of mid-dermal elastolysis. J Am Acad Dermatol. 2012;67(5): e216–267.
- Cutillas E, Ferrando FJ, Martí ME, Mateu A, Rausell N. Reticular variant of mid-dermal elastolysis after insertion of a pacemaker. Clin Exp Dermatol. 2010;35(5):498–500.
- Martin LK, Kossard S, Murrell DF. Reticular variant of mid-dermal elastolysis. Am J Dermatopathol. 2008;30(3):287–290.
- Hillen U. Reticular erythema with focal mid-dermal elastophagocytosis (REMDE). J Dtsch Dermatol Ges. 2008;6(10):857–859, 857–860.
- Bannister MJ, Rubel DM, Kossard S. Mid-dermal elastophagocytosis presenting as a persistent reticulate erythema. Australas J Dermatol. 2001;42(1):50–54.
- Gambichler T, Breuckmann F, Kreuter A, Boms S, Altmeyer P, Stücker M. Immunohistochemical investigation of mid-dermal elastolysis. Clin Exp Dermatol. 2004;29(2):192–195.
- Suda T, Hara H, Yoshitake M, Ohbayashi T, Nakamura T, Terui T. Immunohistochemical investigation of mid-dermal elastolysis with a history of erythema. Am J Dermatopathol. 2008;30(5):477–480.
- Fimiani M, Mazzatenta C, Alessandrini C, Paola M, Paola C, Andreassi L. Mid-dermal elastolysis: an ultrastructural and biochemical study. Arch Dermatol Res. 1995;287(2):152–157.
- Tajima S, Inazumi T, Kajiya H, et al. Elastin metabolism in skin fibroblasts explanted from a patient with mid-dermal elastolysis. Br J Dermatol. 1999;140(4):752–754.
- Prigent F, Baulac C, Duroselle M, Marinho E, Beranger JY, Frances C, Martinet C. [Mid dermal elastolysis: a case with a study of elastases]. Ann Dermatol Venereol. 1993;120(11):853–855.
- Korosec A, Frech S, Gesslbauer B, et al. Lineage identity and location within the dermis determine the function of Papillary and Reticular fibroblasts in human skin. J Invest Dermatol. 2019;139(2): 342–351.
- Hur DJ, Raymond GV, Kahler SG, et al. A novel MGP mutation in a consanguineous family: review of the clinical and molecular characteristics of Keutel syndrome. Am J Med Genet A. 2005;135:36–40.
- Gambichler T, Skrygan M, Reininghaus L, et al. Lysyl oxidase-like 2 promoter hypermethylation in mid-dermal elastolysis. Br J Dermatol. 2016;175(6):1354–1356.
- Kadoya K, Sasaki T, Kostka G, et al. Fibulin-5 deposition in human skin: decrease with ageing and ultraviolet B exposure and increase in solar elastosis. Br J Dermatol. 2005;153(3):607–612.
- Gambichler T, Reininghaus L, Skrygan M, et al. Fibulin protein expression in mid-dermal Elastolysis and Anetoderma: A study of 23 cases. Acta Derm Venereol. 2016;96(5):708–710.
- Shelley WB, Wood MG. Wrinkles due to idiopathic loss of mid-dermal elastic tissue. Br J Dermatol. 1977;97(4):441–445.
- Smithson SL, Orchard D, Scardamaglia L. Mycophenolate mofetil to treat mid-dermal elastolysis. Pediatr Dermatol. 2018;35(4):e221-e223.
- Bergstrom KG. Beyond tretinoin: cosmeceuticals for aging skin. J DrugsDermatol. 2009;8(7):674–677.
- Tajima S, Hayashi A, Suzuki T. Elastin expression is up-regulated by retinoic acid but not by retinol in chick embryonic skin fibroblasts. J Dermatol Sci.
1997;15(3):166–172. - Watson RE, Long SP, Bowden JJ, et al. Repair of photoaged dermal matrix by topical application of a cosmetic ‘antiageing’ product. Br J Dermatol. 2008;158(3):472–47.
- Mitts TF, Bunda S, Wang Y, Hinek A. Aldosterone and mineralocorticoid receptor antagonists modulate elastin and collagen deposition in human skin. J Invest Dermatol. 2010;130(10):2396–406.
- Zhao R, Bruning E, Rossetti D, et al. Extracts from Glycine max (soybean) induce elastin synthesis and inhibit elastase activity. Exp Dermatol. 2009;18(10):883–886.
- Mahoney MG, Brennan D, Starcher B, et al. Extracellular matrix in cutaneous ageing: the effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp Dermatol. 2009;18(3): 205–211.
- Cenizo V, André V, Reymermier C, Sommer P, Damour O, Perrier E. LOXL as a target to increase the elastin content in adult skin: a dill extract induces the LOXL gene expression. Exp Dermatol. 2006;15(8): 574–581.
- Sohm B, Cenizo V, André V, Zahouani H, et al. Evaluation of the efficacy of a dill extract in vitro and in vivo. Int J Cosmet Sci. 2011;33(2):157–163.

