 J Clin Aesthet Dermatol. 2020;13(9):33–40
J Clin Aesthet Dermatol. 2020;13(9):33–40
by Benjeev Dhillon, MD, and Tapan Patel, MBBS
Dr. Dhillon is with Define Clinic in Beaconsfield, United Kingdom. Dr. Patel is with PHI Clinic in London, United Kingdom.
FUNDING: This study was funded by Allergan.
DISCLOSURES: Dr. Dhillon is a former employee of Allergan, an Allergan stockholder, and a consultant for Allergan. Dr. Patel is a consultant for Allergan.
ABSTRACT: Objective:The Vycross™ range of hyaluronic acid (HA) fillers supports a full-face approach to facial rejuvenation. It is important to understand how much filler can be safely injected and which products to use where, particularly for injectors who are new to this range. This study assessed whether Vycross fillers can be effectively and safely used in larger quantities and tracked real-world usage across facial zones.
Methods:This was a single-center, retrospective analysis set in normal clinical practice. The study included 66 consecutive adult female patients undergoing full-face rejuvenation with Vycross fillers within a single treatment plan guided by MD Codes. Filler usage and location were noted, and any adverse events were recorded.
Results: The mean age was 48.0 years, and median time from consultation to first filler usage was 18 days. Most patients (n=40; 60.6%) had only one filler session. Forty-seven patients (71.2%) also received botulinum toxin type A. In total, 309 filler syringes were used (mean: 4.7 per patient). Sixteen (5.2%), 181 (58.6%), and 112 (36.2%) syringes were used in the upper, mid and lower face, respectively. In the upper and midface, the majority was Voluma (15/16 and 120/181 syringes, respectively). In the lower face, filler use was split across Voluma, Volift and Volbella (41, 44 and 27 syringes, respectively). Five adverse events were reported at three weeks (swelling, n=2; asymmetry, n=2; lumps, n=1). All were easily resolved. No delayed events were reported.
Conclusion: Substantial volumes of Vycross fillers can be effectively and safely used as part of a full-face approach to facial rejuvenation.
Keywords: Vycross, hyaluronic acid, dermal filler, facial rejuvenation
In recent years, improved understanding of facial anatomy and continued innovation in dermal fillers has supported rapid evolution of techniques in non-surgical facial aesthetics. In particular, Rohrich and Pessa’s1 pioneering work on the superficial and deep-fat compartments of the face and their role in facial aging has allowed for a greater appreciation of the importance of volume in the three-dimensional aging face.2–5 As a result, targeting of specific structures can be achieved via volumization and recontouring to influence both near and remote signs of aging.6
To achieve optimal outcomes with non-surgical treatments, our aesthetic toolbox must include: an ability to rapidly assess the patient’s needs (almost at first glance); awareness of the available techniques that can be used to safely target specific structures; and an understanding of the products that work best in our own hands.
Leading practitioners have developed sophisticated assessment tools and novel injecting techniques. For example, Arthur Swift has pioneered the BeautiPHIcation™ philosophy of assessment, consultation, and treatment planning,7 and Mauricio de Maio has developed the “8-point lift” and MeDical (MD) Codes™ techniques for greater standardization of treatment delivery and planning (M. De Maio, personal communication).
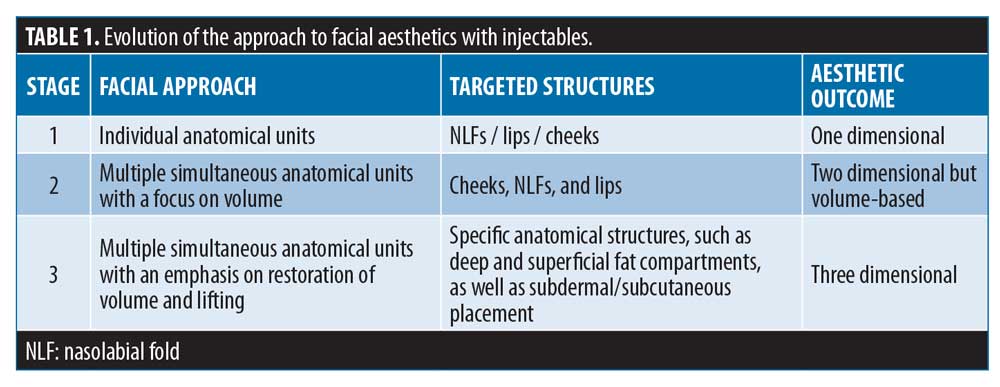
As a result, optimal practice has evolved over time, with three key identifiable stages, culminating in the contemporary focus on three-dimensional aesthetic outcomes.1 Implementing this approach requires a range of dermal fillers with varying features. Key variables that characterize the behavior of a hyaluronic acid (HA)-based dermal filler include the degree of crosslinking, HA concentration, ratio of high and low molecular weight HA, cohesivity, and gel hardness (G’).8–11
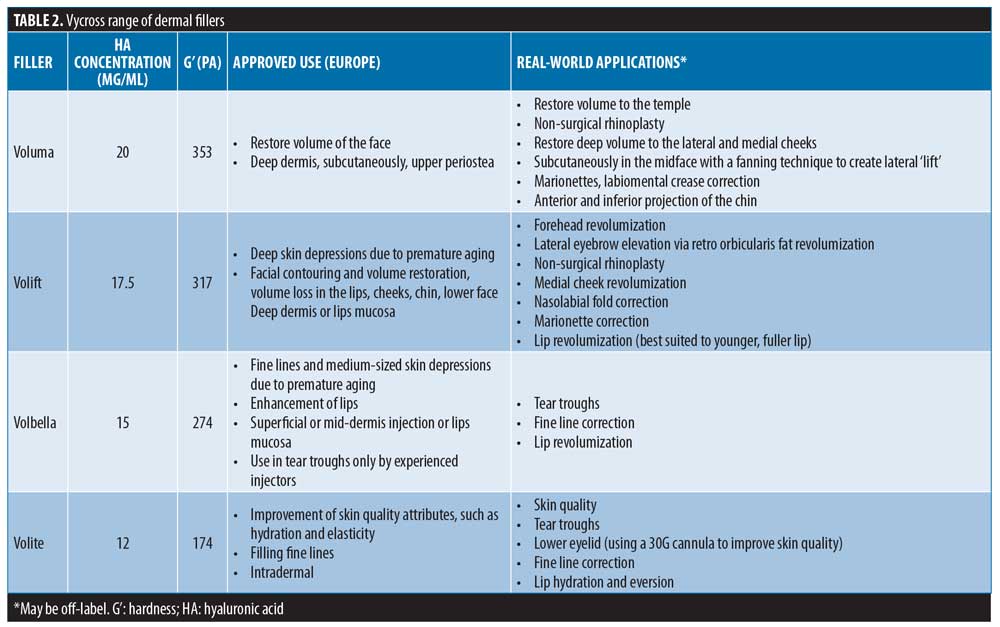
The authors favor HA-based fillers from the Vycross™ range (Voluma®, Volift®, Volbella®, and Volite®; Table 2), owing to their tailored mixtures of HA concentrations (low [<1mDa] and high molecular weight HA [>1mDa]), G’ and cohesivities.11 This has allowed an optimization of gel properties for different planes and areas of the face, allowing different results to be achieved, including volumization and lifting. The Vycross range has been approved in Europe for over five years. In the United States, Voluma has been approved (as Voluma XC) since 2013, but Volift (known as Vollure in the US) and Volbella have only recently been authorized. Hence, many practitioners in the United States are relatively new to the Vycross range, and there remains a need for greater understanding of how best to safely use these products to achieve full face harmony.
The rapid recent progression towards three-dimensional thinking and a more full-face approach has necessitated product usage outside of approved indications, often with multiple syringes being employed. This has raised questions about how much filler can be safely injected, which products should be used where, and how they can be optimally combined with botulinum toxin type A (BoNT-A) treatments. Here, we report our single-center experience of full-face treatment with dermal fillers, with the aim of both demonstrating how these products can be safely used in larger quantities and also tracking real-world product usage across facial zones.
The authors have over 20 years of combined experience with the Vycross range and its predecessor, Hylacross; BD was also part of the clinical trials that evaluated their safety and efficacy.
Methods
Study design and subjects. This was a retrospective review of data from 66 subjects within an electronic patient database. All patients underwent full-face rejuvenation using injectable dermal fillers, within a single treatment plan, at one center between October 2015 and January 2016. The study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent before treatment.
Eligible subjects were aged ?18 years and had requested full-face rejuvenation (rather than treatment for individual areas of the face) with injectables. Patients were excluded if they had undergone energy-based treatment to the face after their first session of injectables.
Techniques. All patients were treated with the Vycross™ range of HA fillers (Allergan, Dublin, Ireland), using Voluma, Volift, and Volbella. No Volite was used in this cohort. Treatment planning was guided by the use of MD Codes, but modifications were made to certain areas based on clinical judgment in individual cases (e.g. adding further points in Ck1 to provide more lateral support to the upper cheek). When indicated, patients were also treated with BoNT-A (Botox®, Allergan).
Injections were given using either the sterile needle supplied with the product (27G or 30G 1/2”) or a sterile cannula (25G, 27G or 30G). The injection techniques used (fanning vs. linear vs. small bolus vs. aliquot vs micro-aliquot) and volumes administered broadly aligned with the recommendations given in the MD Codes.
Appropriate sterile technique was maintained throughout. All patients were asked to remove make-up and the skin was prepared with chlorhexidine or Clinisept Plus (a hydrochlorous topical antiseptic).
Routine follow-up was undertaken at three weeks post-procedure, and again at three months (via telephone).
Assessments. Data collected included the total number of filler sessions within a single treatment plan, the total number of syringes of filler used, the breakdown of products used, and the number of syringes and product types injected into specific anatomic locations in the face. In addition, treatment timelines were recorded, including the times from first consultation to first filler and BoNT-A sessions, and the time between subsequent filler sessions.
With regard to individual syringes, if the majority of the content was used in one location it was coded to that area of the face, even if small amounts were used in other locations.
Adverse events (AEs) were assessed during follow-up visits.
Statistical analysis. Descriptive statistics, including mean, median and range, are provided as appropriate.
Results
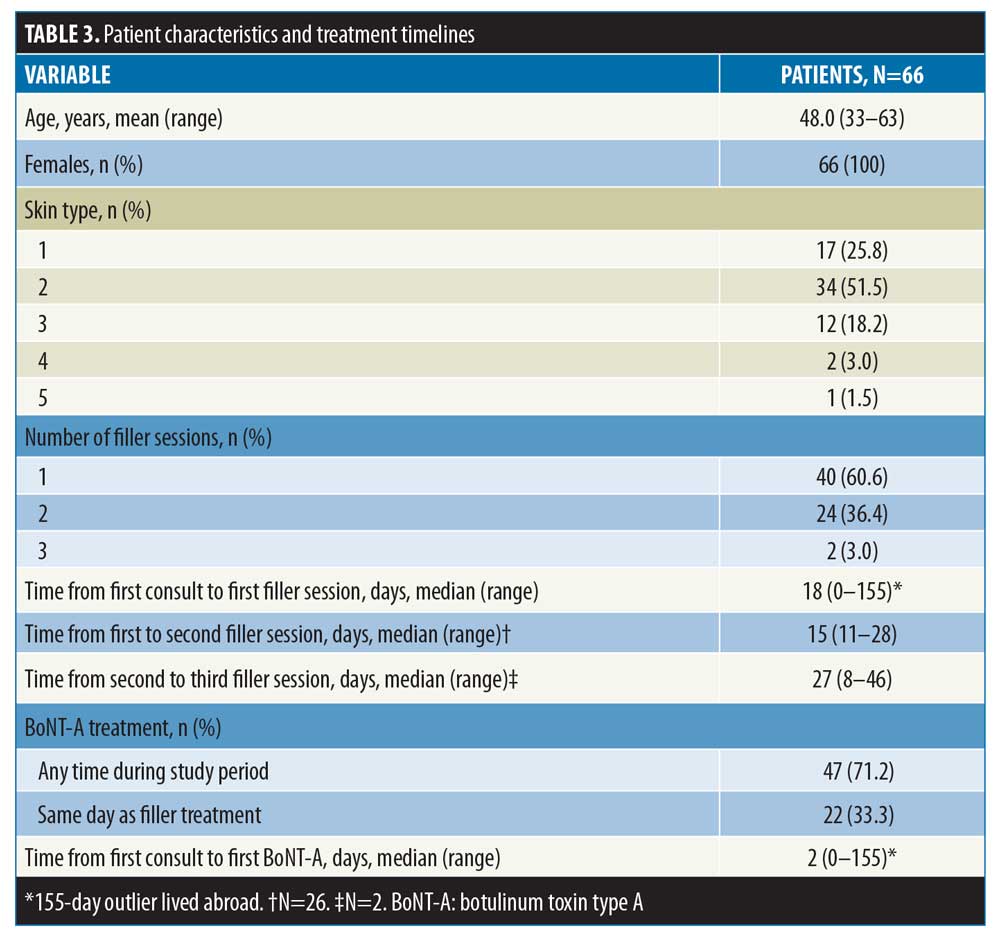
Baseline characteristics and treatment timelines. Across the study period, a total of 167 patients were treated with injectable dermal fillers. Of these, 66 met the criteria for study inclusion. The mean age was 48.0 years (range: 33–63 years), and most had skin types 1–3 (Table 3). All were female. Forty patients (60.6%) had one filler session within their treatment plan, and a further 24 had two sessions (36.4%). Two patients (3.0%) had three sessions. The median time from first consultation to first filler session was 18 days (range: 0–155 days). In patients undergoing more than one filler session, the median times between the first and second and between the second and third sessions were 15 days (range: 11–28 days) and 27 days (range: 8–46), respectively. A total of 47 patients (71.2%) also received treatment with BoNT-A, of which 22 (33.3%) received BONT-A on the same day as filler (Table 3). The median time from first consultation to the BoNT-A session was two days (range: 0–155 days).
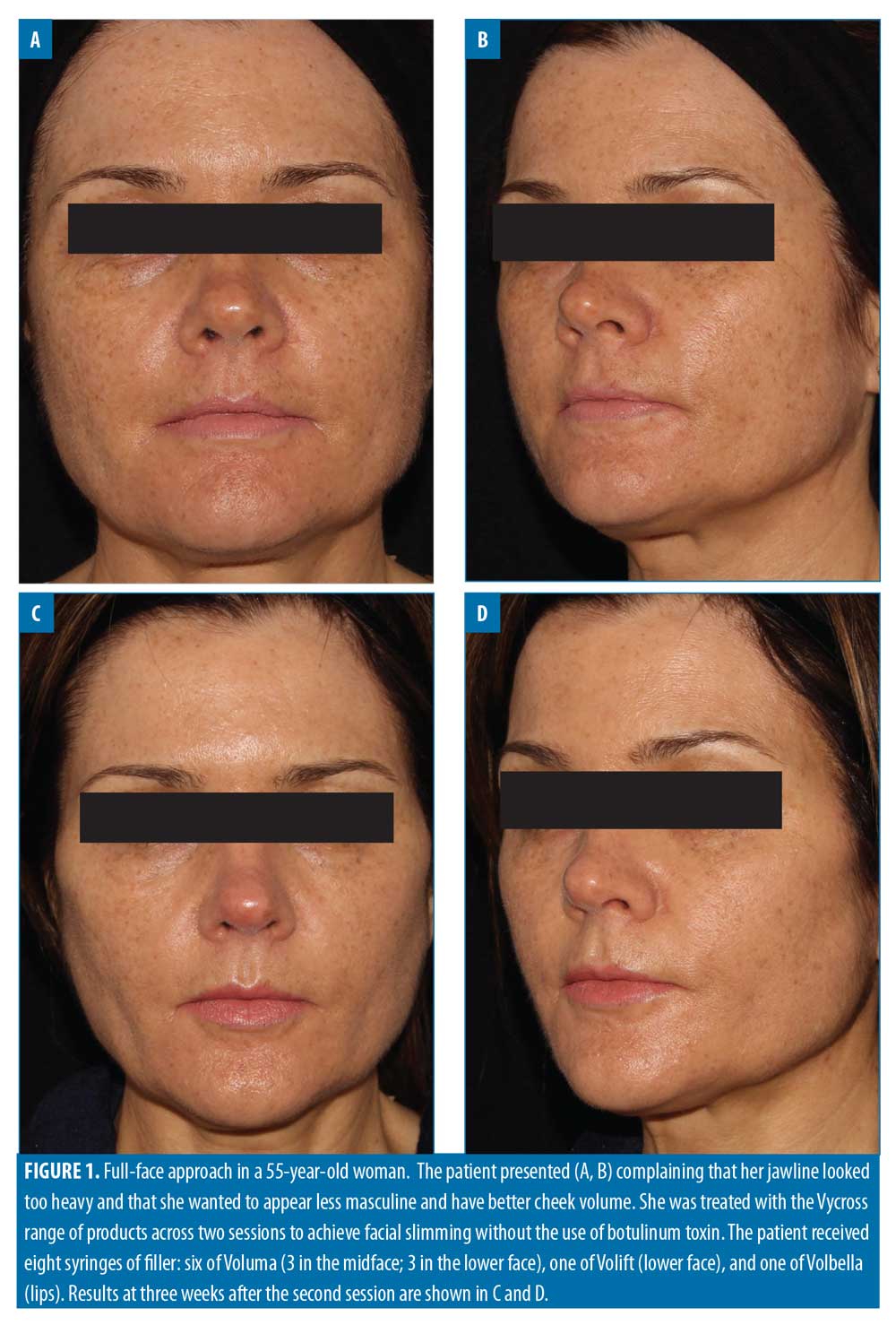
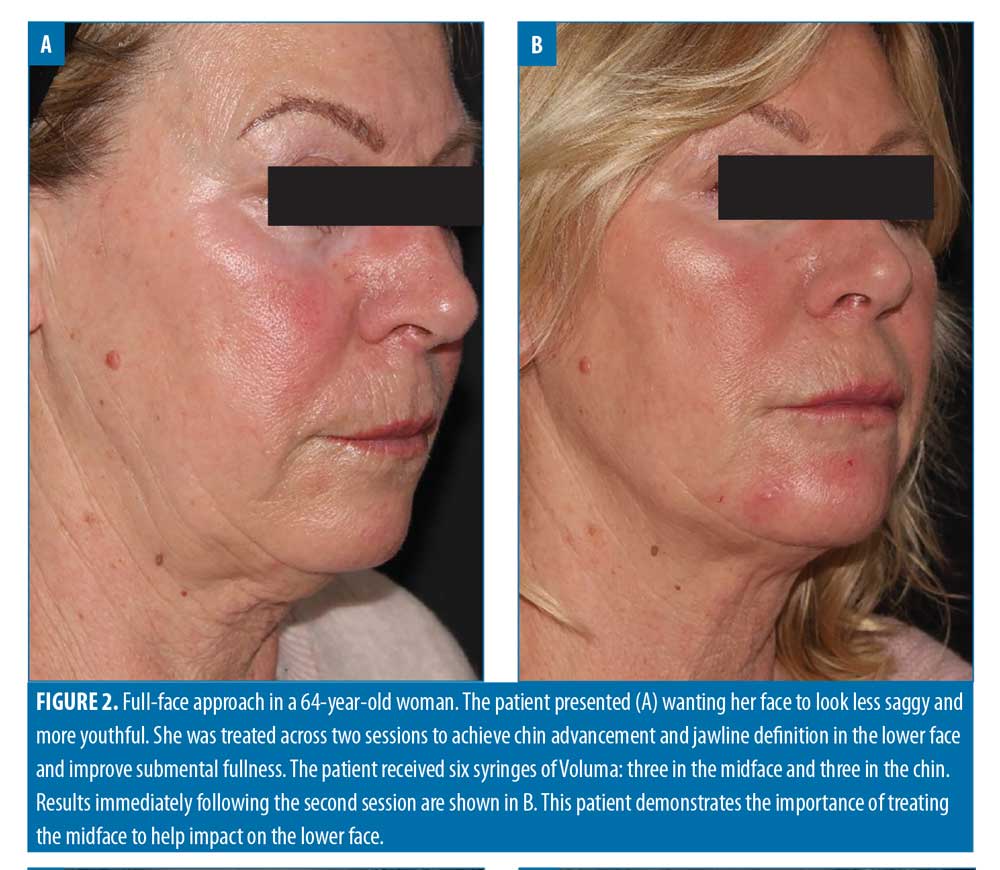
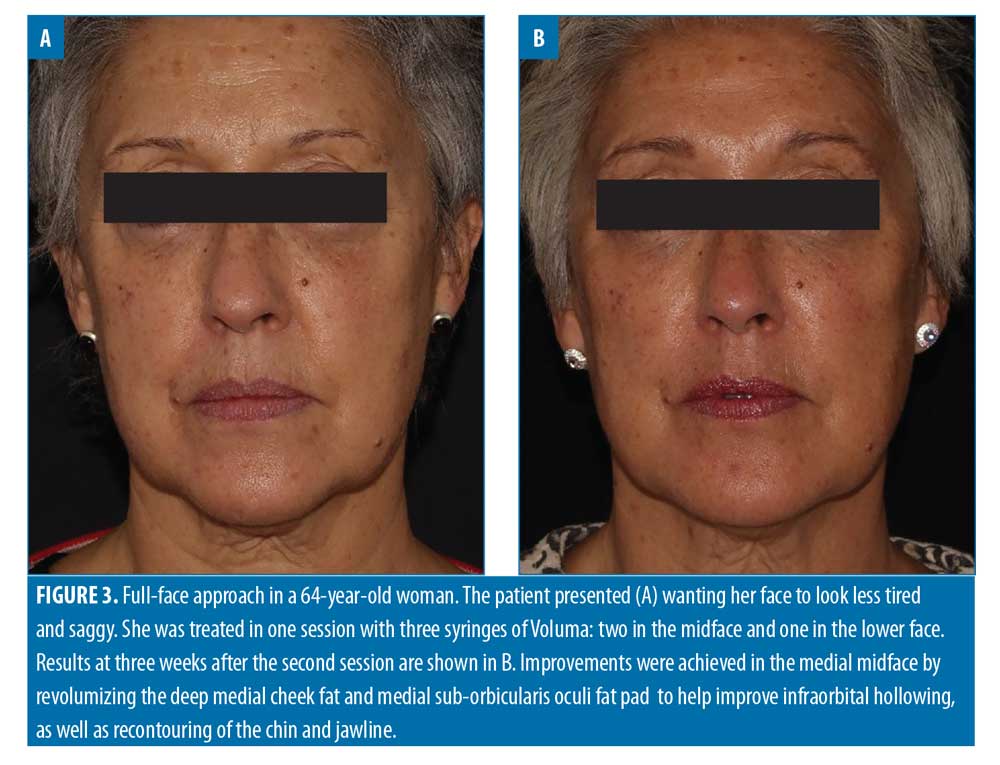
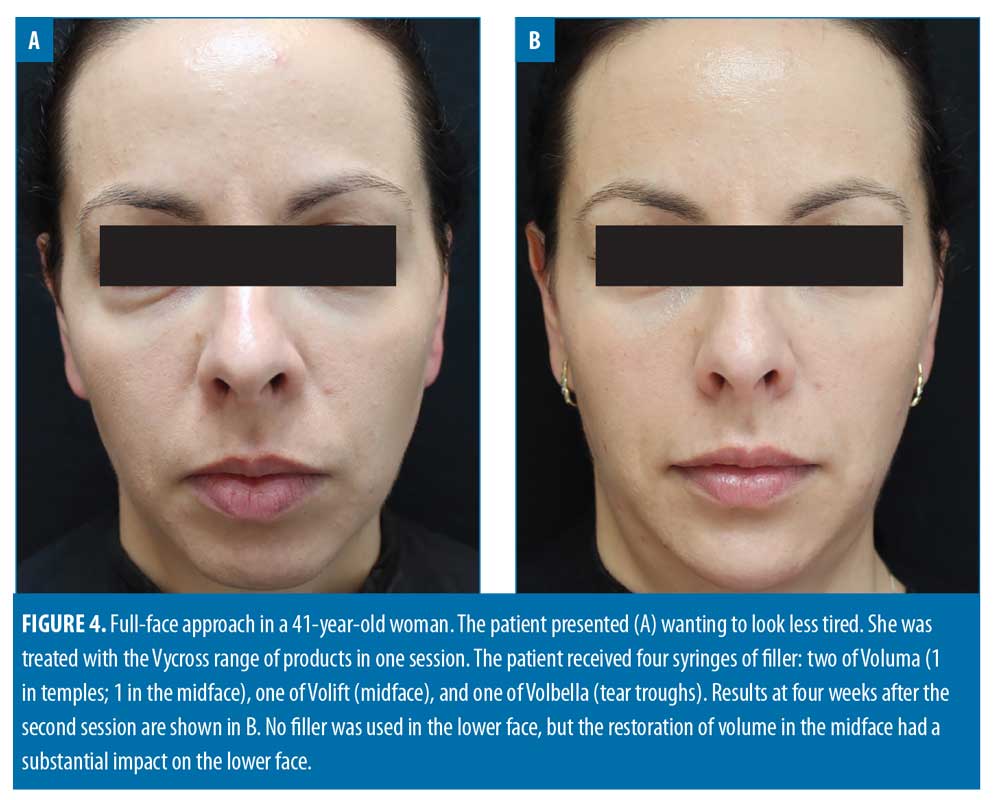
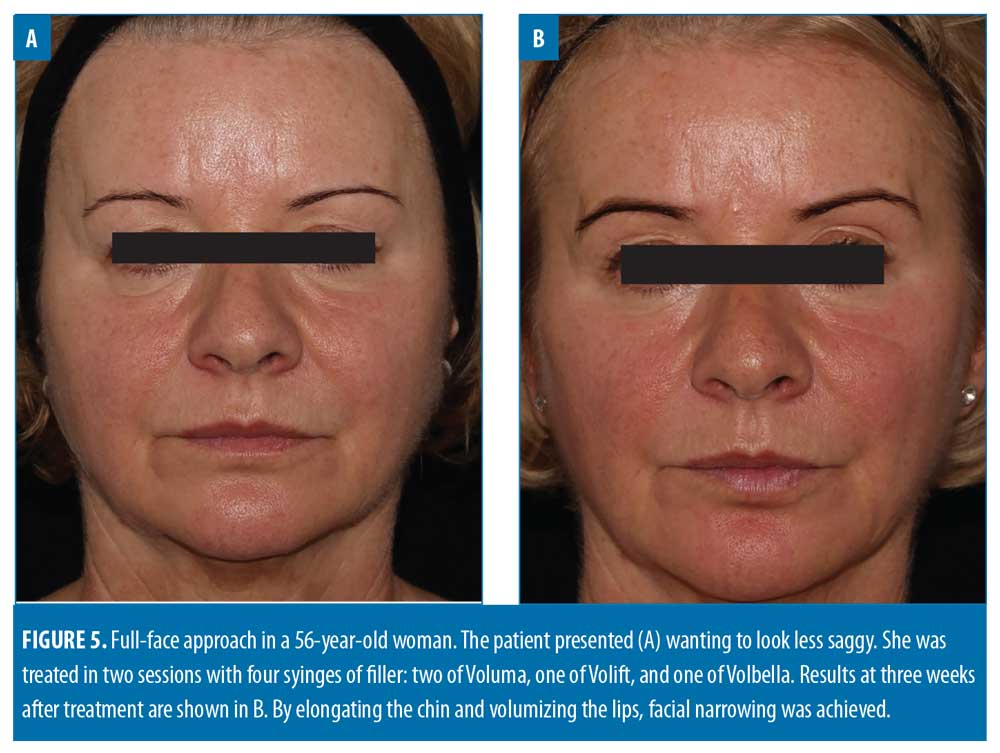
Filler usage. A total of 309 syringes of dermal filler were used, with a mean of 4.7 syringes per patient (range: 2–13). Example before-and-after images demonstrating the full-face approach are provided in Figures 1–5.
The majority of filler syringes were used during patients’ first session (201/309; 65.0%), with a mean of 3.0 syringes per patient. Fewer syringes in total were used during subsequent sessions. In the second session, 106 syringes (34.3% of the total) were used in 26 patients (mean: 4.1 syringes per patient); in the third session, two syringes (0.6% of the total) were used in two patients (mean: 1.0 syringes per patient).
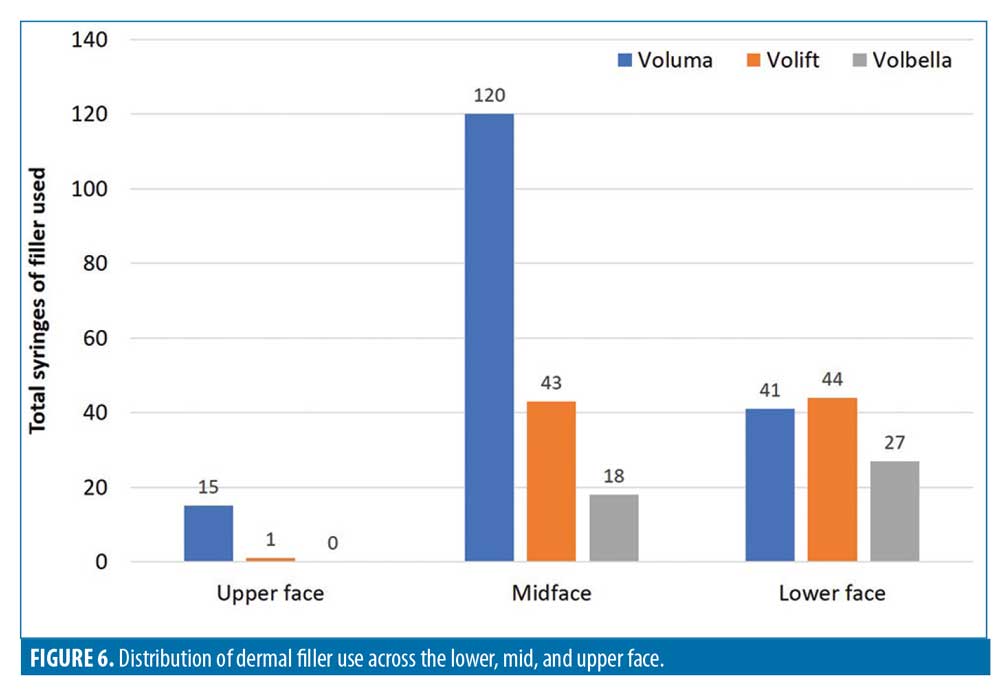
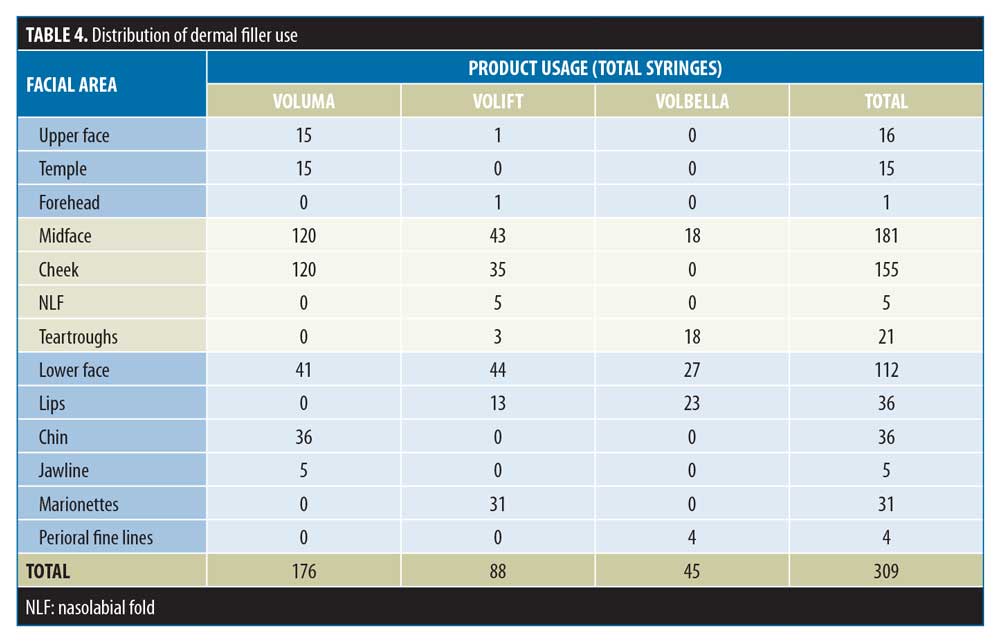
The distribution of product use is summarized in Table 4 and Figure 6. The total numbers of filler syringes used across the upper, mid and lower face were 16 (5.2%), 181 (58.6%), and 112 (36.2%), respectively. In the upper face and midface, the majority of the filler use was Voluma (15/16 and 120/181 syringes, respectively). In the lower face, filler use was split across Voluma, Volift, and Volbella (41, 44 and 27 syringes, respectively).
Adverse events (AEs). A total of five AEs were reported at three weeks: swelling, n=2; asymmetry, n=2; and product lumping, n=1. All had resolved by three months, and no new AEs were reported at that time.
The two patients who reported some swelling were the same individuals who reported asymmetry. Further examination found that the swelling only reflected minor asymmetry. In both cases, this was associated with treatment of the chin with larger volumes of product (2–4mL) to advance the chin and improve the profile. As per their original treatment plans, both patients underwent a second filler session, wherein the asymmetries were corrected. In the patient who complained of a lump, further examination revealed that some filler had taken longer than expected to integrate; this had resolved by three months.
No cases of bruising were recorded at three weeks, although this can occur in the period immediately after treatment and typically resolves rapidly.
Discussion
Here, we have described our experience of a full-face approach to facial rejuvenation, based on HA dermal fillers from the Vycross range, in a retrospective analysis of 66 patients. A mean of 4.7 syringes of filler per patient was used and more than two thirds were also treated with BoNT-A.

Only five AEs were reported at three weeks and all were easily resolved. No new AEs were reported at three months. A recent review of AEs associated with HA fillers across 55 studies found that delayed-onset complications, such as biofilms, foreign body granuloma, dyspigmentation, and scarring, can occur weeks, months or even years after the procedure.12 It is a limitation of the present study that follow up for complications did not continue beyond three months. However, across our practice, we have recorded a rate of delayed complications (6–12 months post-procedure) with HA fillers of 0.4% (Dhillon and Tapan, unpublished observation); all have been easily managed. Aseptic technique during the procedure itself might be the best prevention for delayed complications.13 This should become second nature for injectors. Some of the key elements of aseptic technique in our experience are listed in Table 5. These are particularly important with HA products that have a long duration of effect (e.g. Voluma, which can last for up to 24 months).14,15
The present data confirm the results from previous studies of full-face treatment.16–18 For example, as long ago as 2012, Rzany and colleagues17 demonstrated in 67 patients that treatment with a mean volume of 6.7mL of filler was possible without any safety concerns beyond expected injection site reactions; 80 percent of patients were satisfied or very satisfied with the durability of the results achieved, and almost two thirds felt a lot or much better than they had prior to treatment.
Optimal treatment of the aging face requires an appreciation of the interaction of various etiological factors. For instance, skeletal changes are not just due to bony atrophy, but also changes in skeletal aperture expansion as well as bone loss.2 A prime example is how the orbit increases in size with age, whereas the maxilla decreases in size, contributing to the inferior displacement of the malar fat pad and development of the nasolabial fold (NLF).3 It is our ability to influence the highly compartmentalized facial fat with dermal fillers (or fat transfer) that allows us to correct the volume losses associated with aging.1 We also now understand how these fat compartments age over time, with volume loss and inferior migration as the two main causative factors. The midface plays a particularly important role, with both deep and superficial fat compartments losing volume over time.19
However, adding volume should not be the goal of treatment.20 Instead, the aim should be to modify specific anatomic compartments, thereby restoring proportion in accordance with aesthetic ideals and removing unwanted shadows. When approaching facial treatment, practitioners should assess the facial frame, which greatly affects individual perception.20 For example, an oval face with lateral contour might be more feminine and an angular or rectangular face more masculine.20 With these ideals in mind, injectors should be able to identify target components and product of choice, based on their experience and anatomic knowledge.
The importance of product choice should not be underestimated, particularly given the frequency with which new dermal fillers come to market, with varying levels of supporting evidence. The product used can have a substantial influence on the risk of complications and on the likelihood of treatment success. The Vycross range has been well studied, with data from large trials demonstrating high levels of patient and injector satisfaction, and low rates of complications, in treating the midface and perioral areas, for example.11,21–23
More specifically, the use of a product with insufficient G’ to volumize and lift the midface can mean “wasted volume) and wasted money for patients. In the present analysis, the most highly used product was the one with the highest G’ (Voluma), which was not surprising given relatively advanced mean age of the cohort (48 years). The midface was the most treated area and might be described as the structural foundation of the face; without re-establishing volume in this zone, if indicated, improvements elsewhere are harder to achieve, particularly in the lower face.
To best appreciate the rationale for the breakdown of product use across the three zones (upper, mid, and lower face), we briefly review them below in what we believe to be the order of priority. We hope that this discussion will provide valuable assistance for injectors who are new to the concept of full-face treatment with the Vycross range, including practitioners in the United States where many of these products were only recently approved.
Midface. Strategic revolumization of deep fat compartments within the midface can influence other areas of the midface such as the NLF, as well as the lower face, particularly the jawline and perioral region.
Volumization should be prioritized within the lateral or medial cheek, specifically targeting the lateral sub-orbicularis oculi fat pad (SOOF). This is of particular importance when trying to influence a vector of pull from this point to the oral commissure. The optimal amount of filler placed in the correct anatomic location can have a substantial effect on this vector. Additionally, subcutaneous placement of a high or medium G’ filler in the pre-auricular or submalar area can help to create further lift or narrow the face.
In our practice, we use Voluma (high G’) and Volift (medium G’) for this purpose, and injection of these two products into the midface accounted for 38.8 percent (120/309 syringes) and 13.9 percent (43/309 syringes), respectively, of total filler use anywhere in the face. Furthermore, most of the Volift injected into the midface was specifically used to revolumize the medial SOOF (35/43 syringes), thereby correcting maxillary volume loss and indirectly improving the tear trough.
Only a small number of syringes (5/309; 1.6%) were used to correct the NLFs, as we prefer to make indirect changes by creating lift in the midface more laterally using a higher G’ product. When Volift was used in the NLFs, this was primarily for deep depot injection into the pyriform fossa, both to fill and to modify the lip elevators and thereby improve upper lip excursion or “gummy” smile.
When undertaking full-face rejuvenation, it is important to understand the aging changes associated with the eye. Rejuvenation of the whole face (or even just the midface) without appropriate correction of periorbital changes, if indicated, will most likely lead to a substandard result and an unsatisfied patient. The majority of treatments associated with the periorbital zone focus on the tear trough and lateral lid–cheek junction. However, revolumization of the tear troughs is generally under-performed, probably due to a lack of understanding of the etiologies associated with this area and their varying phenotypes, and/or a lack of confidence due to the proximity of the infraorbital neurovascular bundle.
We typically use a 25G 38mm blunt-tip cannula when revolumizing the tear trough. However, treatment of this area should be reserved for experienced injectors with an appreciation of both its anatomy and the appropriate injection techniques, particularly depths and volumes. Low G’ products such as Volbella are typically favored for direct treatment of the tear trough, using either a needle or cannula. In the present work, 18 of the 21 syringes injected into the tear trough were Volbella. However, a higher G’ product such as Volift might occasionally be preferred in extreme cases of poorly defined medial lid–cheek continuity, in which large volumes of Volbella would be required (and hence are best avoided). When using a higher G’ product in the tear trough, the treating physician should be particularly conservative with the injection volume. In our practice, we rarely use more than 0.5mL per tear trough to minimize the risk of complications such as the Tyndall effect or visible lumps of non-integrated filler. With the recent launch of Volite (very low G’), we have begun to use small amounts of this product, injected superficially with a 30G 25mm cannula, to improve superficial skin laxity and quality in the tear trough, as well as the lower eyelid. This technique should only be used by experienced injectors.
Lower face. In this analysis, the lower face was the second most treated zone, after the midface. The most common lower-face procedure was Voluma injection into the chin (36/112 syringes; 32.1%). No other product was used in the chin. Treatment of the chin plays a key role in full-face rejuvenation and there are several potential indications. One of the most common is Class 2 malocclusion with associated overbite or overjet.24 The importance of identifying the degree of overbite and its effect on facial aesthetics has been demonstrated, with more severe overbite coinciding with a smaller lower-face height.25 Another common complaint is a weak profile resulting from retrognathia, which can lead to superior rotation of mentalis and shortening of the lower third. However, it is possible to have a shortened lower third without retrognathia, often in the presence of a deep submental crease, requiring 1 to 2 syringes to release inferiorly. A further indication for chin revolumization is in ‘slimming’ the face. Central projection of the midface, lips and chin can all help to achieve this outcome (Figure 5).7 Hence, anterior chin projection is often coupled with a non-surgical rhinoplasty (if indicated), maxillary projection, and lip revolumization.
In the present analysis, the only other area of the lower face in which Voluma was used was along the jawline. However, this constituted only five syringes (4.5% of total filler use in the lower third). Although the jawline can be directly improved with dermal fillers, we believe this is best achieved in patients with minimal jowls and skin laxity; in individuals with heavier jowls or lax skin, the use of fillers in the jawline might have little observable effect, and can even worsen a ptotic jawline if too much product is used or placed incorrectly. We favor midface revolumization to effect superior vectors, combined with high-intensity focused ultrasound,26 to achieve skin tightening. However, patients who underwent this procedure were excluded from the present analysis.
In total, 44 of the 112 syringes of filler injected in the lower face (38.6%) were Volift, used predominantly for marionette lines or lips. Fewer syringes of Volbella were used (27 syringes; 24.1% of total product use in the lower face), mostly for the lips. Although fine lines are also a good indication for low G’ fillers like Volbella, it is rare in our practice, owing to high use of skin-resurfacing devices based on CO2 fractional or non-ablative technologies. None of the patients in this analysis received this treatment prior to fillers, but some did subsequently.
Lip treatment is a common element of full-face rejuvenation in the older demographic included in the present analysis, used either to beautify or feminize the face, or to provide anterior projection for slimming the face. In this age group, fillers are rarely injected into the lips solely to volumize but are also used to evert the red lip and hence shorten a long white roll. Low G’ products such as Volbella are often preferred (23 of 36 syringes used in the lips in the present analysis). However, in younger patients requiring lip volumization for beautification, we favor the use of a more cohesive product with a higher G’, such as Volift (13 of 36 syringes). Volift should be used with caution or not at all in individuals with a long white roll and thin lips, in whom it might contribute to increasing the length of the white roll rather than everting the lip; in these cases, Volbella might be more suitable. In almost all lip indications, fillers were used to lift the oral commisures using small volumes (around 0.025–0.05mL).
Upper face. Despite low product usage, an awareness of upper face anatomy is crucial.27 In the present analysis, only 16 syringes of filler were injected into the upper face, equivalent to 5.2 percent of total product usage. The majority of this (15 syringes) was Voluma, used to treat the temples. The remaining one syringe was Volift for improving forehead volume loss.
Although there are often indications for temple volumization, potentially leading to improved aesthetic outcomes, 7,10,11,17,28 it is not a priority for most patients nor for these authors. However, when used, temple volumization can indirectly improve lateral eyebrow ptosis, particularly in a very hollow temporal region. Elevation of the lateral eyebrow, combined with BoNT-A, can be used to improve the elevation from medial to lateral brow.7,27
Conclusions
Recent years have seen a clear paradigm shift away from using small numbers of syringes (perhaps 1–2) in most cases, towards increasing use of many syringes within each treatment plan.7,10,11,17 In this analysis, a mean of 4.7 syringes per patient was used, going as high as 13 syringes in one case. There was no evidence of increased safety concerns.
Furthermore, in the time since this population was treated, our volume use has increased, and we now recommend a mean of around eight syringes per patient for a full-face treatment. The introduction of the low G’ product, Volite, has played a role in this, enhancing our ability to treat fine lines and tear troughs through superficial injection, and allowing more product to be used.
An analogy might be drawn with fat grafting for aesthetic purposes, in which substantial quantities can now be transferred to discrete, targeted locations based on advanced techniques. Indeed, in a recent systematic review, mean volumes of fat injected included 6.5mL in the forehead, 5.9mL per side in the temples, 11.5mL per side in the mandibular area, and 6.7mL in the chin.29
With an “implant” like HA, it is essential to have expert technique, a good understanding of anatomy, an appreciation of asepsis, and high quality products, in order to reduce risk and optimize outcomes, particularly when injecting large volumes. The risk of complications with higher volumes can be further reduced by: splitting injections across more than one session; slow injection and appreciation of soft tissues; implementation of strategies to minimize the risk of bruising (e.g. the use of cannulas versus needles); and provision of appropriate post-care advice for patients.
Of course, the use of greater volumes does not necessarily equate to greater patient satisfaction. Patient education is essential. They cannot be expected to understand the rationale for using multiple syringes of product (with associated extra cost) if it is not carefully explained. The consultation is therefore key. As practitioners, we need to understand patients’ drivers but also help them to understand that a given line or wrinkle might not be their main problem. We also need to move away from syringe price as the primary factor, towards a focus on the desired outcome. All injectors will be familiar with the patient for whom little or no result is achieved because they needed more volume, but this was not possible because of price. It is our responsibility to set expectations, agree on a treatment plan, and achieve a result. Patients are willing to pay for outstanding results.
We might also need to reconsider our business models. The use of more product does not need to correlate directly with increasing cost to the patient. Instead, we should charge for a result and understand that more (or fewer) syringes than originally thought might be required.
With regard to the present analysis, we must acknowledge its limitations. Most importantly, it recruited a relatively small numbers of patients, had a retrospective design, was not specifically (prospectively) designed to monitor safety, and did not monitor long-term complications beyond three months. Several other studies have also provided evidence that full-face treatment is safe and effective.16–18 However, more well-designed, prospective, controlled studies to assess the relationship between injection volume and safety would be welcome. One other possible limitation of the present analysis was that injection strategies occasionally differed between injectors; although both follow the same MD Codes, different techniques were sometimes used, in treating the chin with either a cannula or needle, for example. Nonetheless, the study is representative of our normal practice.
In conclusion, this analysis demonstrates that substantial volumes of Vycross fillers can be safely used as part of a full-face approach to facial rejuvenation, with a primary focus on the mid and lowerface.
References
- Rohrich RJ, Pessa JE. The fat compartments of the face: Anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219–2231.
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropomorphic and histologic analysis. Plast Reconstr Surg. 1992;90:592–600.
- Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthetic Surg J. 2006;26:S4–S9.
- Forte AJ, Andrew TW, Colasante C, Persing JA. Perception of age, attractiveness, and tiredness after isolated and combined facial subunit aging. Aesth Plast Surg. 2015;39:856–869.
- Tan SL, Brandt MG, Yeung JC, et al. The aesthetic unit principle of facial aging. JAMA Facial Plast Surg. 2015;17:33–38.
- Carruthers J, Rzany B, Sattler G, Carruthers A. Anatomic guidelines for augmentation of the cheek and infraorbital hollow. Dermatol Surg. 2012;38:1223–1233.
- Swift A, Remington K. BeautiPHIcation™: A global approach to facial beauty. Clin Plast Surg. 2011;38: 347–377.
- Tezel A, Fredrickson GH. The science of hyaluronic acid dermal fillers. J Cosmet Laser Ther. 2008;10:35–42.
- Borrell M, Leslie DB, Tezel A. Lift capabilities of hyaluronic acid fillers. J Cosmet Laser Ther. 2011;13:21–27.
- Muhn C, Rosen N, Solish N, et al. The evolving role of hyaluronic acid fillers for facial volume restoration and contouring a Canadian overview. Clin Cosmet Investig Dermatol. 2012;5:147–158.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136:139S–148S.
- Abduljabbar MH, Basendwh MA. Complications of hyaluronic acid fillers and their managements. J Dermatol Derm Surg. 2016;20:100–106.
- Cassuto D, Sundaram H. A problem-oriented approach to nodular complications from hyaluronic acid and calcium hydroxylapatite fillers: classification and recommendations for treatment. Plast Reconstr Surg. 2013;132(Suppl 2):48S–58S.
- Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24 month study. Clin Cosmet Investig Dermatol. 2013;6:81–89.
- Glaser DA, Kenkel JM, Paradkar-Mitragotri D, et al. Duration of effect by injection volume and facial subregion for a volumizing hyaluronic acid filler in treating midface volume deficit. Dermatol Surg. 2015;41:942–949.
- Taub AF, Sarnoff D, Gold M, Jacob C. Effect of multisyringe hyaluronic acid facial rejuvenation on perceived age. Dermatol Surg. 2010;36:322–328.
- Rzany B, Cartier H, Kestemont P, et al. Full-face rejuvenation using a range of hyaluronic acid fillers: efficacy, safety, and patient satisfaction over 6 months. Dermatol Surg. 2012;38:1153–1161.
- Talarico S, Meski AP, Buratini L, et al. High patient satisfaction of a hyaluronic acid filler producing enduring full-facial volume restoration: An 18-month open multicenter study. Dermatol Surg. 2015;41:1361–1369.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconstr Surg. 2012;129:263–273.
- Lam SM, Glasgold R, Glasgold M. Analysis of facial aesthetics as applied to injectables. Plast Reconstr Surg. 2015;136(Suppl):11S–21S.
- Philipp-Dormston WG, Eccleston D, De Boulle K, et al. A prospective, observational study of the volumizing effect of open-label aesthetic use of Juvéderm® VOLUMA® with Lidocaine in mid-face area. J Cosmet Laser Ther. 2014;16:171–179.
- Philipp-Dormston WG, Hilton S, Nathan M. A prospective, open-label, multicenter, observational, postmarket study of the use of a 15 mg/mL hyaluronic acid dermal filler in the lips. J Cosmet Dermatol. 2014;13:125–134.
- Calvisi L, Gilbert E, Tonini D. Rejuvenation of the perioral and lip regions with two new dermal fillers: The Italian experience with Vycross™ Technology. J Cosmet Laser Ther. 2017;19:54–58.
- Kim TW, Little RM. Postretention assessment of deep overbite correction in Class II Division 2 malocclusion. Angle Orthod. 1999;69:175–186.
- Beckmann SH, Kuitert RB, Prahl-Andersen B, et al. Alveolar and skeletal dimensions associated with overbite. Am J Orthod Dentofacial Orthop. 1998;113:443–452.
- Fabi SG, Goldman MP, Mills DC, et al. Combining microfocused ultrasound with botulinum toxin and temporary and semi-permanent dermal fillers: Safety and current use. Dermatol Surg. 2016;42(Suppl 2):S168–S176.
- Sykes JM, Cotofana S, Trevidic P, et al. Upper face: Clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(Suppl):204S–218S.
- Tansatit T, Apinuntrum P, Phetudom T. An anatomical study of the middle temporal vein and the drainage vascular networks to assess the potential complications and the preventive maneuver during temporal augmentation using both anterograde and retrograde injections. Aesthetic Plast Surg. 2015;39:791–799.
- Shue S, Kurlander DE, Guyuron B. Fat injection: A systematic review of injection volumes by facial subunit. Aesthetic Plast Surg. 2018;42:1261–1270.

