 J Clin Aesthet Dermatol. 2022;15(9):25–29.
J Clin Aesthet Dermatol. 2022;15(9):25–29.
by Han Jo Kim, PhD; Ye-Jin Lee, MD; Hye-Jin Ahn, MD; Ji Hwoon Baek, PhD; Min Kyung Shin, MD, PhD; and Jae Sook Koh, PhD
Drs. Kim, Baek and Koh are with the Dermapro and Skin Research Center in Seoul, South Korea. Drs. Lee, Ahn, and Shin are with the Department of Dermatology and the School of Medicine at Kyung Hee University in Seoul, South Korea.
ABSTRACT: Background. A model for evaluating the in-vivo skin wound healing process over time is needed. Wound healing can be evaluated using reflectance confocal microscopy (RCM), which permits the dynamic characterization of the skin in a noninvasive manner.
Objective. The aim of this study was to analyze the healing process of fractionally induced microwounds using RCM.
Methods. Eight healthy volunteers had a fractional carbon dioxide (CO₂) laser applied to the healthy skin of their inner arm in a single session. The wound healing of the skin at the stratum spinosum and stratum basale layers was examined using RCM. Two dermatologists evaluated the changes in the ablative zone using a grading system (ranging from completely recovered to slightly enlarged ablative zone) at four temporal time points: 3, 7, 14, and 28 days after laser application.
Results. The ablative zone in the epidermis was 75 percent of the baseline after seven days and 25 percent of the area after 14 days compared to the baseline. The expanded ablative zones in the epidermis were observed in some subjects resulting from contraction between Day 3 and Day 7. The ablative zone completely healed 28 days after laser application in both the spinous and basal layers of the epidermis.
Conclusion. The healing process in the ablative zone of the fractional CO₂ laser-induced microwounds was observed over 2 to 4 weeks, revealing a regenerated epidermis of replaced keratinocytes from the basal layer through RCM.
Keywords: Fractional phtothermolysis, laser, laser ablation, micro-scar, thermal injury, wound healing
Ideal wound healing without scar formation is a major challenge in the medical and surgical fields. A quantitative analysis of the wound healing process could assist in the screening of pharmaceuticals and cosmeceuticals for their utility in skin regeneration. However, irregular wound contraction in animal models of wound healing could be an obstacle for evaluators attempting to measure wound repair. In addition, the ban on animal testing in the cosmetic industry has increased the need to develop innovative alternative skin models to replace the use of laboratory animals.1-3 Although an artificial skin model has been developed,4-6 it is limited in representing human skin wound healing because of the lack of a dermo-epidermal junction, skin appendage, and dermal vasculature, which are necessary in wound repair. In human skin, the process of wound healing has been assimilated by histopathological analysis conducted via an in-vivo invasive biopsy. However, multiple skin biopsies in the same wound area cannot be assessed dynamically.
Micro-wounds induced by a fractional laser are a minimally invasive wound model for evaluating the human skin wound healing process in-vivo. The concept of fractional photothermolysis involves the creation of microscopic thermal zones (MTZs) surrounded by normal skin. The surrounding viable tissue acts as a reservoir of stem cells, growth factors, and inflammatory cells that facilitate rapid wound healing.7 When visualizing the skin tissue, these normal areas outside of the MTZ can act as a normal control area. Using in-vivo human reflectance confocal microscopy (RCM), a fractional approach can help visualize the dynamic wound healing process by comparing it with the surrounding skin serving as the control site. This study was designed to analyze the healing process of fractionally induced microwounds using RCM.
Methods
Human subjects. We recruited eight healthy volunteers, who provided written informed consent. We included adults aged between 18 to 65 years, with healthy skin. The study protocol was approved by the Institutional Review Board, and participants provided written informed consent.
Laser interventions. All laser applications were performed by the same dermatologist. Each subject received a single application of fractional ablative laser on their inner arm using a fractional carbon dioxide (CO2) laser (eCO2, Lutronic, Seoul, Korea). The following parameters were used: field square-shaped optical spot size, 120μm; laser power, 10W; pulse energy, 20mJ; and density, 75 spots/cm2.
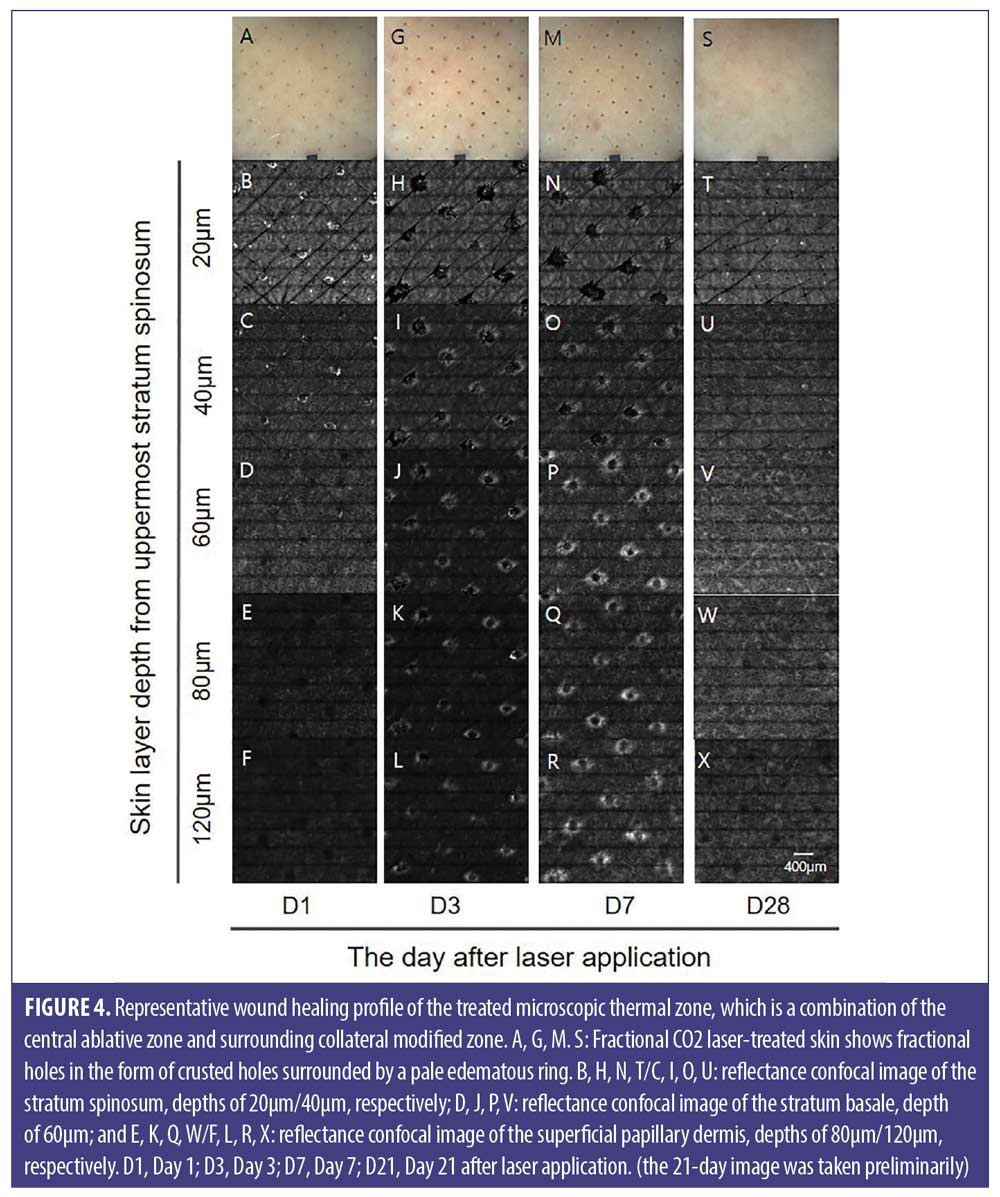
RCM. A confocal-laser microscope (VivaScope 1500, MAVIG, Munich, Germany) was used to observe the dynamic wound healing process in-vivo according to the skin depth. The deep layer of the stratum corneum is defined as the most superficial epidermal layer (0μm), which suggests that the RCM images are taken from the subcorneal region. Dermoscopic images were acquired using a VivaCam (with 35-fold magnification) to provide information on the corresponding RCM-imaged area. The analyses were conducted in a mosaic of 5×5 mm2 within each test field. The measurements were performed at four time points: Day 3 (D3), 7 (D7), 14 (D14), and 28 (D28) after laser application. The MTZ was evaluated at three depth levels: the stratum spinosum (10–30μm), stratum basale (40–70μm), and papillary dermis (90–120μm). The test areas were marked with an overhead projector film to ensure that the same area was measured during the test periods. The RCM image was taken from the stratum corneum to the papillary dermis in 10μm increments. The following intensities were used for each layer of the skin: stratum spinosum, 3mW; stratum basale, 5mW; and papillary dermis, 7mW.
We had previously identified a micro-wound in an RCM after treatment with microplasma fractional radiofrequency (RF).8 In this study, we have used the same terminology to describe the fractional CO₂ laser-induced MTZ. The MTZ comprised an ablative microchannel and a surrounding thermally modified zone, which included the coagulated and thermally denatured zones around the ablative zone. In the RCM, the “ablative zone” corresponded to empty spaces and the “thermally modified zone” corresponded to areas with a whitish ring exhibiting high reflectance.
Outcome measures. The decrements of the ablative zone were evaluated at the level of the stratum spinosum and stratum basale. Two dermatologists independently analyzed the RCM images of the ablative zone and evaluated the images from D7, D14, and D28 in comparison to the baseline (D3), according to the semi-quantitative grading system shown in Table 1. The ablative zone was evaluated using a six point scale based on the percentage change in area in proportion to the baseline (D3) (i.e., 125%, slightly worse [slightly expanded]; 100%, no change; 75%, slightly improved [slightly decreased]; 50%, moderately improved [moderately decreased]; 25%, significantly improved [significantly decreased]; and 0%, completely improved [completely decreased]). The evaluation procedure was performed twice by each dermatologist, and the mean was calculated.
Statistical analysis. A statistical analysis was performed using SPSS version 19 (IBM Corp., Armonk, New York, USA). The Wilcoxon signed-rank test was used to analyze the data between different time points. All results are expressed as the mean ± standard deviation. Statistical significance was set at P< 0.05.
Results
Eight subjects (7 female, 1 male) aged between 24 and 48 years (median, 39.62) were recruited. The subjects had Fitzpatrick Skin Types II to IV.
Dermoscopic changes. All subjects exhibited mild erythema and edema on the surface of the test skin directly after fractional CO2 laser application. All wounds were covered with lattice crusts up to D3. The crusts started to fall off around D7. By D14, all the crusts had fallen off, and the skin surface was completely healed on macroscopic examination.
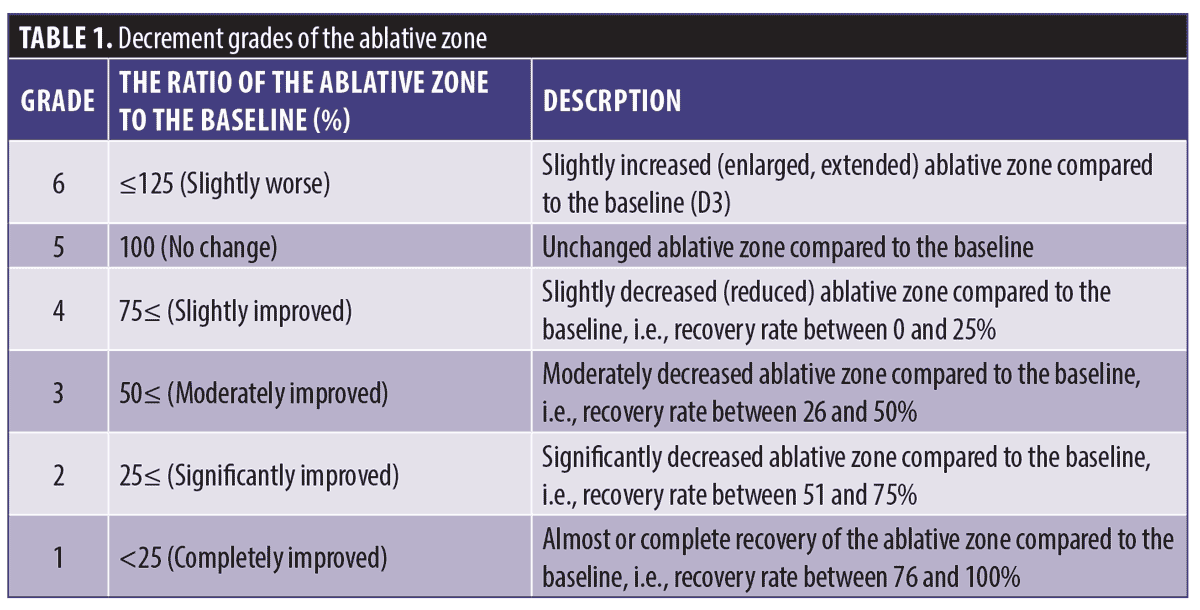
Temporal changes in the ablative zone in the stratum spinosus. For all subjects, the ablative zone was induced in a homogeneous pattern with regular distance. At D3, the ablative zone was observed as a round, dark, and empty space for denaturation. The ablative zone gradually reduced in size as a result of dynamic wound healing. However, at D7, in two subjects (25%), the ablative zone was observed to be larger than that observed at D3. At D14, the ablative zone further decreased, and was replaced by regenerated tissue. The ablative zone of the stratum spinosum showed decrement grades of 88.28±36.47 percent at D7 and 37.50±38.10 percent at D14. The ablative zone exhibited complete regeneration at D28. The changes in the ablative zone and decrement grades over time are shown in Figure 1.
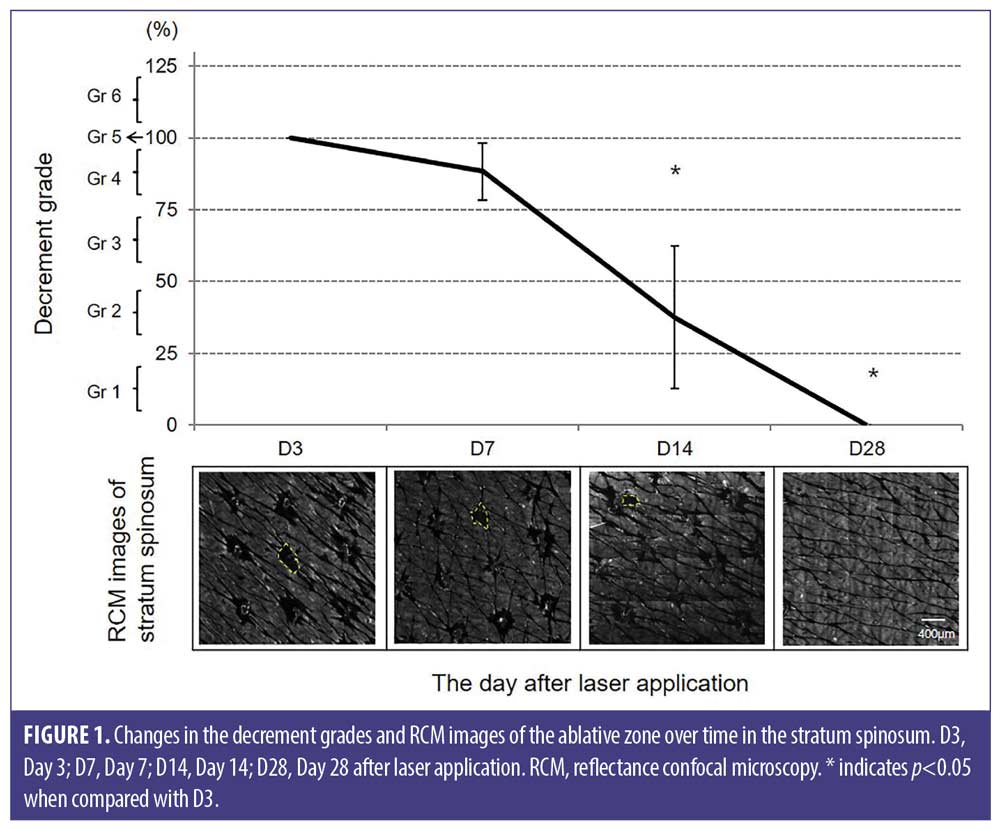
Temporal changes in the ablative zone in the stratum basale. At D3, the empty space of the ablative zone was observed to be smaller than that in the stratum spinosum. At D7, whitish areas within the ablative zone were observed, indicating its re-epithelization. The ablative zone showed decrement grades of 86.72 ± 25.39 percent at D7 and 34.38 ± 36.34 percent at D14, and complete resolution at D28. The changes in the ablative zone and decrement grades over time are shown in Figure 2.
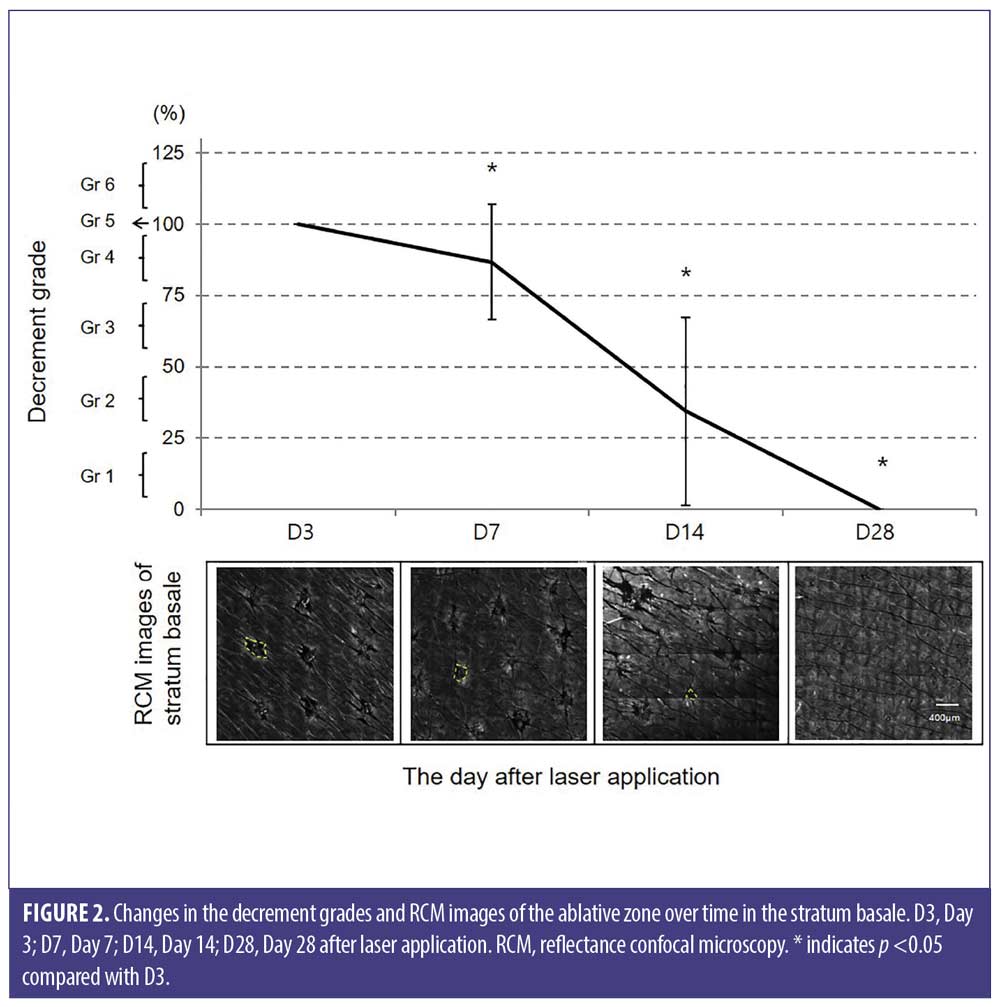
Temporal changes in the reflectance intensity of the thermally modified zone in the papillary dermis. The ablative zones and surrounding thermally modified zones were observed at different time periods. From D3 to D14, the thermally modified zone was visualized as a highly refractile ring. At D28, even after epidermal regeneration was completed, a thermally modified zone was observable in some subjects. Bright and fine fibers corresponding to newly formed collagen throughout the papillary dermis were also visible (Figure 3). Two months after laser application, a fine and bright collagen network was consistently observed.
Discussion
In this human skin in-vivo study, we demonstrated the dynamic healing process of micro-wounds induced by a fractional CO2 laser via the RCM of each skin layer (stratum spinosum, stratum basale, and papillary dermis). In the stratum spinosum and stratum basale, as the wound healing progressed, the ablative zone gradually decreased and was replaced by keratinocytes originating from the basal layer in a “bottom-up healing process.”9 At D28, the epidermal ablative zone was no longer detectable, and wound healing in the epidermis was completed. In the papillary dermis, the thermally modified zone was visualized as a high-reflectance ring (Figure 4). As wound healing progressed, the thermally modified zone was replaced by newly formed collagen fibers.
We expected the ablative zone to reduce over time as the tissues regenerated in the epidermis. Interestingly, in two of the eight subjects, the ablative zone was seen to extend in the stratum spinosum between D3 and D7. This can be attributed to contracture and necrosis of the surrounding keratinocytes, release of desmosome adhesion, and delayed apoptosis of thermally damaged keratinocytes. Therefore, when evaluating the ablative zone to assess the wound regeneration or efficacy of an ingredient for skin regeneration, measuring the size of microholes in the basal layer, followed by the spinous layer can be considered as an accurate method. To evaluate the regeneration of the ablative zone, it is also important to assess the time taken to replace the epidermal ablative zone. Sattler et al9 quantified wound healing using the diameter of the ablative zone visualized by RCM after the application of a fractional ablative laser over three weeks. They reported that, at three weeks, subepidermal skin damage was still observable in 89.5 percent of the eight W-treated areas and 100 percent of the 16 W-treated areas using RCM and optical coherence tomography. In our study, in over half the subjects, the epidermal ablative zone was closed within 1 to 4 weeks. We also confirmed that the epidermal ablative zone at the subcorneal level could exist before 28 days after laser application, when all crusts had fallen off and the surface of the skin wound had completely healed. These results suggest that, when using an energy-based device that can injure the epidermis, it is desirable to treat skin after complete recovery of the epidermal wound with an interval of longer than four weeks to minimize the side effects.
The thermally modified zone showed a high reflectance ring, which remained for a period ranging from two weeks to two months in the papillary dermis, even after complete recovery of the epidermal microwounds. We assumed that the formation of the thermally modified zone could have been attributed to the changes in reflectance because of the change in cross-linking of the dermal matrix collagen due to a thermal reaction or expression of the heat shock protein. Xu et al10 reported that heat shock protein 70 was detectable four hours after treatment with a fractional CO₂ laser, after which its levels decreased over the course of three months to a year. Further studies are needed to elucidate the mechanism of reflectance change in the dermal matrix. Longo et al evaluated dermal remodeling after applying a fractional CO₂ laser with a fluence ranging from 100 to 125mJ.11 They observed that coarse and huddled collagen was replaced by newly formed long, bright, and straight fibers between three weeks and three months after laser treatment. The long-term effect of neocollagenosis and stimulation of hyaluronic acid production takes approximately 1 to 6 months.12,13 Consistent with the results of this previous study, we observed a fine and bright matrix throughout the papillary dermis, indicating neocollagenosis at 28 days. These results warrant the study of long-term effects.
In this study, we used a fractional CO₂ laser to create microwounds. To evaluate wound healing, creation of an ablative zone using a non-ablative laser may not be clearly observable via RCM. The thermally modified zone induced by fractional radiofrequency is relatively large, but the ablative zone is superficial and broad.8 The fractional CO₂ laser can induce narrow and deep cone-like MTZ. Therefore, to create standardized microholes, a fractional CO₂ laser is considered most suitable. However, the creation of microholes can be affected by the source, pulse duration of the laser, and tissue-skin interaction. Therefore, to create a standardized ablative zone using a fractional CO₂ laser, the protocol must first be standardized.
Limitations. The limitation of our study is that our evaluation involved only one parameter. As the postfractional wound healing is affected by the percentage of treated skin and density of the ablative zones,14 to confirm the wound healing effect, a more detailed study analyzing a higher-density tissue with different parameters causing the ablative zone is needed. Additionally, our small patient sample limits our results. Larger, more detailed studies are needed to confirm our findings.
Conclusion
The ablative zone of the micro-wounds was created using a fractional CO₂ laser replacing the regenerated epidermis from the stratum basale. Considering the bottom-to-top process of epidermal regeneration, measuring the ablative zone of the basal layer enabled us to evaluate the different wound healing processes during skin regeneration.
Acknowledgments
The authors would like to express our gratitude to Dr. Chan-Yang Lee for his efforts in preparing this manuscript.
References
- Festing S, Wilkinson R. The ethics of animal research. Talking Point on the use of animals in scientific research. EMBO Rep. 2007;8:526–530.
- Halder M. Three Rs potential in the development and quality control of immunobiologicals. ALTEX. 2001;18 Suppl 1:13–47.
- Hendriksen CF. Refinement, reduction, and replacement of animal use for regulatory testing: current best scientific practices for the evaluation of safety and potency of biologicals. ILAR J. 2002;43 Suppl:S43–48.
- Netzlaff F, Lehr CM, Wertz PW, et al. The human epidermis models EpiSkin, SkinEthic and EpiDerm: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm. 2005;60:167–178.
- Ponec M. Skin constructs for replacement of skin tissues for in vitro testing. Adv Drug Deliv Rev. 2002;54 Suppl 1:S19–30.
- Supp DM, Boyce ST. Engineered skin substitutes: practices and potentials. Clin Dermatol. 2005;23:403–412.
- Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34: 426-438.
- Shin MK, Park JM, Lim HK, et al. Characterization of microthermal zones induced by fractional radiofrequency using reflectance confocal microscopy: a preliminary study. Lasers Surg Med. 2013;45:503–508.
- Sattler EC, Poloczek K, Kästle R, et al. Confocal laser scanning microscopy and optical coherence tomography for the evaluation of the kinetics and quantification of wound healing after fractional laser therapy. J Am Acad Dermatol. 2013;69:e165–173.
- Xu XG, Luo YJ, Wu Y, et al. Immunohistological evaluation of skin responses after treatment using a fractional ultrapulse carbon dioxide laser on back skin. Dermatol Surg. 2011;37:1141–1149.
- Longo C, Galimberti M, De Pace B, et al. Laser skin rejuvenation: epidermal changes and collagen remodeling evaluated by in vivo confocal microscopy. Lasers Med Sci 2013;28:769–776.
- Bogdan Allemann I, Kaufman J. Fractional photothermolysis–an update. Lasers Med Sci. 2010;25:137–144.
- Hantash BM, Bedi VP, Kapadia B, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39:96-107.
- Grunewald S, Bodendorf MO, Simon JC, et al. Update dermatologic laser therapy. J Dtsch Dermatol Ges. 2011;9:146–159.

