 J Clin Aesthet Dermatol. 2022;15(9):30–39.
J Clin Aesthet Dermatol. 2022;15(9):30–39.
by Michael Abrouk, MD; Chloe Gianatasio, MS; Yumeng Li, MD; Jon Holmes, PhD; Joanna Dong, MD; Rebecca L. Quiñonez, MD, MS; and Jill S Waibel, MD
Dr. Abrouk, Dr. Li, and Dr. Dong are with the Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery at the University of Miami Miller School of Medicine in Miami, Florida. Ms. Gianatasio, Dr. Holmes, Dr. Quinoñez, and Dr. Waibel are with the Miami Dermatology and Laser Institute in Miami, Florida.
ABSTRACT: Objective. When using laser therapy to effectively treat scars, the choice of treatment parameters depends on the knowledge accuracy of the underlying scar pathology, which is often difficult to judge by gross physical exam. As such, more quantitative measures are needed. In recent years, optical coherencetomography (OCT) has shown promise as a real-time imaging technology of skin microstructure. A key step in developing a methodology for utilizing OCT to develop a comprehensive ‘atlas’ of OCT characteristics of a wide variety of scar types. This atlas may then be used as a tool for selecting the optimal treatment modality and parameters for each scar type.
Methods. One hundred and fifty scars of a wide range of anatomical locations were imaged using OCT, capturing both vascular and structural data. A variety of scar etiologies (e.g. burn, surgical, traumatic) and types (e.g. hypertrophic, keloidal, atrophic) were included. Comparator scans were also taken from normal, unscarred skin.
Results. OCT revealed morphological differences in the epidermis and dermis between scars and normal tissue, and between scar subtypes. Features affected by scar pathology included epidermal thickness, skin surface texture, dermal epidermal junction rugosity, blood vessel density, vessel shape and diameter, vessel direction and vascular network, dermis scattering intensity and non-uniformity. Each scar subtype showed consistent characteristics distinct from other scar subtypes.
Limitations. This was a single-site study of a patient population in South Florida.
Conclusion. OCT is a powerful new objective tool for the clinician to utilize in the pursuit of effective laser treatment parameters by enabling personalized treatment based on individual scar characteristics in order to maximize treatment capabilities.
Keywords. OCT, optical coherence tomography, scars, laser, laser resurfacing, scar treatment, laser scar treatment, keloid, striae, hypertrophic scar, surgical scar, atrophic scar, burn scar
General clinical characteristics of scarring are well understood. The etiology of scars can broadly be grouped into acne, surgical, striae, keloid, burn, and trauma. They are morphologically classified as atrophic, hypertrophic, or keloid based on organization of the extracellular matrix.
Atrophic scars are characterized by a loss of cells in both the epidermis and dermis, presenting clinically as depressions in the skin. Histology reveals a loss of collagen, likely due to the reparative process following initial insult. The most common causes are inflammatory in nature, such as acne or varicella lesions, though atrophic scars can result from trauma as well.1
Hypertrophic scars are characterized by an overproliferation of collagen and angiogenesis, which leads to the development of firm and raised papules or plaque, classically localized within the lateral confines of the original injury. Hypertrophic scars most often appear after traumatic injuries, infection, or wounds with high tension during closure.2,3 While normal distribution of collagen fibers is interwoven into a well-coordinated mesh-like structure, histology of hypertrophic scars shows highly organized Type III collagen that is parallel to the skin surface. Partial regression is possible, but many hypertrophic scars persist or worsen.4
Keloid scars are characterized as nodular, fibroproliferative growths. Their exact pathophysiology is unclear but they are known to have roughly three times more collagen than hypertrophic scars and 20 times more than atrophic scars. The collagen in keloid scars is laid with both increased thickness and decreased cross-linking.4 Keloid scars outgrow the original boundaries of the injury both vertically and laterally, and, unlike atrophic and hypertrophic scars, keloid scars can grow over years and are often pruritic or painful. Keloid scar histology has hown Type I and III collagen growing haphazardly in all skin planes without organization.5
The use of lasers in scar treatment
Scar treatment can present considerable treatment challenges to clinicians. Even in the same individual, scars can vary drastically between locations on the body. Cutaneous injuries as small as 0.5mm can trigger excess fibrotic tissue deposition or destruction of the natural tissue scaffold.1 Scar appearance can further visibly change over time, worsened by factors such as high-tension.1 Extensive or widespread injury can induce disfiguring scarring and symptomatology, such as pain and pruritis.6
Scar treatment has improved dramatically with use of advanced laser therapy.7 Lasers alter tissue precisely through controlled neocollagenesis, vascular regrowth, and tissue remodeling.8 Pulse energies and durations are adjustable to treat different targets and depths of the skin. Several classes of lasers are utilized in scar treatment, including, but not limited to, vascular, pigment targeting, nonablative fractional (NAFL), and ablative fractional (AFL). Vasculature- and pigment-targeting lasers utilize selective photothermolysis to match the chromophore and particular vessel diameters. Clinical endpoints, such as vascular spasm or lesion graying, help to monitor response. Both AFL and NAFL deliver energy to controlled fractions of the skin through microscopic beams and are less chromophore dependent; however, they have no observable clinical endpoint. Optimal outcomes with AFL and NAFL are postulated to occur by matching the depth of the laser impact to the depth of the scar pathology and releasing the tethering fibrotic bands that underly deep scarring.9,10
Optical coherence tomography (OCT)
Currently, clinicians assess the scar depth subjectively based on clinical inspection and experience.9–11 However, blood vessels within individual scars can be difficult to identify when beyond clinical assessment methods. These clinical challenges make noninvasive imaging a particularly valuable tool in pre- and post- treatment decision-making when determining optimal laser treatment parameters. Using laser scanning technology, OCT provides high-resolution subsurface images of skin microstructure at much higher resolution than is available from high-frequency ultrasound or magnetic resonance imaging (MRI).
The OCT device used in this study (VivoSight Dx, Michelson Diagnostics Ltd., Kent, England, United Kingdom) scans a 6mm x 6mm area in 30 seconds with resolution greater than 10µm and imaging depth of up to 1.0mm. OCT provides accurate, reliable measurements of scar thickness and vascular patterns in real time with no skin preparation required. The signal intensity is correlated to the tissue optical scattering properties, which are related to tissue constituents, such as collagen density. Thus, OCT may provide an objective noninvasive measurement of scar depth. In addition, dynamic OCT (D-OCT) detects the motion of blood cells in capillaries to provide imaging of the vascular component of scars, enabling measurement of blood vessel depth and diameter directly.
This study evaluated the usefulness of OCT in assessing scar tissue, compared to healthy skin, to determine optimal laser parameters for the treatment of scars. The authors also describe the methodology for interpreting the images obtained through OCT technology.
Methods
In this prospective, observational, Institutional Review Board (IRB)-approved study, 150 scars (including acne, surgical, burn, trauma, and keloid) were scanned, and the following qualitative and quantitative OCT endpoints were analyzed: depth and density of scar tissue, vascular patterns, and optical attenuation. Adjacent or contralateral normal skin was also scanned for comparison using the same endpoints, in select patients. Collected data from OCT scar imaging were then analzed to guide laser treatment parameters.
Analysis of skin structure was done in en-face mode (that is, horizontal view as seen from above). The epidermis was assessed for thickness, surface shape, and brightness. Next, areas of heterogeneity (brightness, texture, vessels) were used to pinpoint features in each participant’s normal skin versus their respective scars (e.g., hypertrophic/atrophic/keloid). Finally, dermal features were observed. Depth of scar was determined by the depth at which the irregular features were no longer clear.
Results
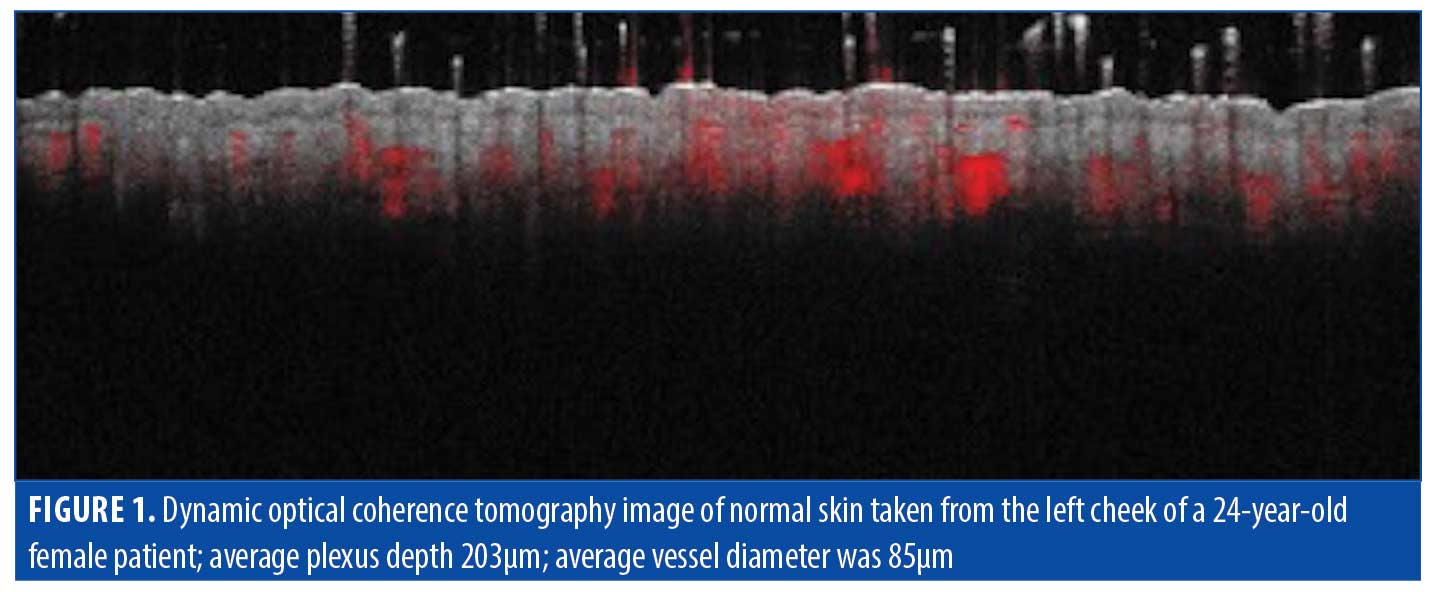
Normal skin. Understanding normal skin characteristics is important in interpretation of OCT images of scar tissue. An OCT image of normal skin for baseline assessment is shown in Figure 1. Normal skin features are outlined in Table 2.
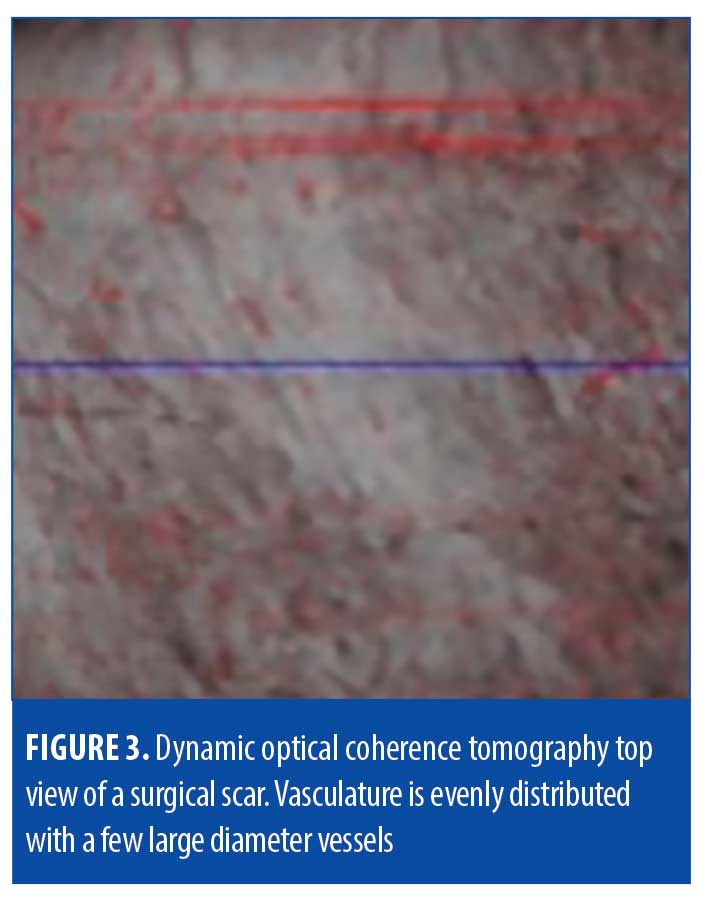
Surgical scars. Surgical scars often heal well on their own and develop minimal perceivable irregularities. Erythema, dyspigmentation, and/or textural irregularities can develop either temporarily or permanently depending on the person, depth of surgery, and technique. Analysis of surgical scar tissue through OCT revealed identifiable features in our study, but we found that surgical scars were largely comparable to that of normal skin (Table 3, Figures 2 and 3).
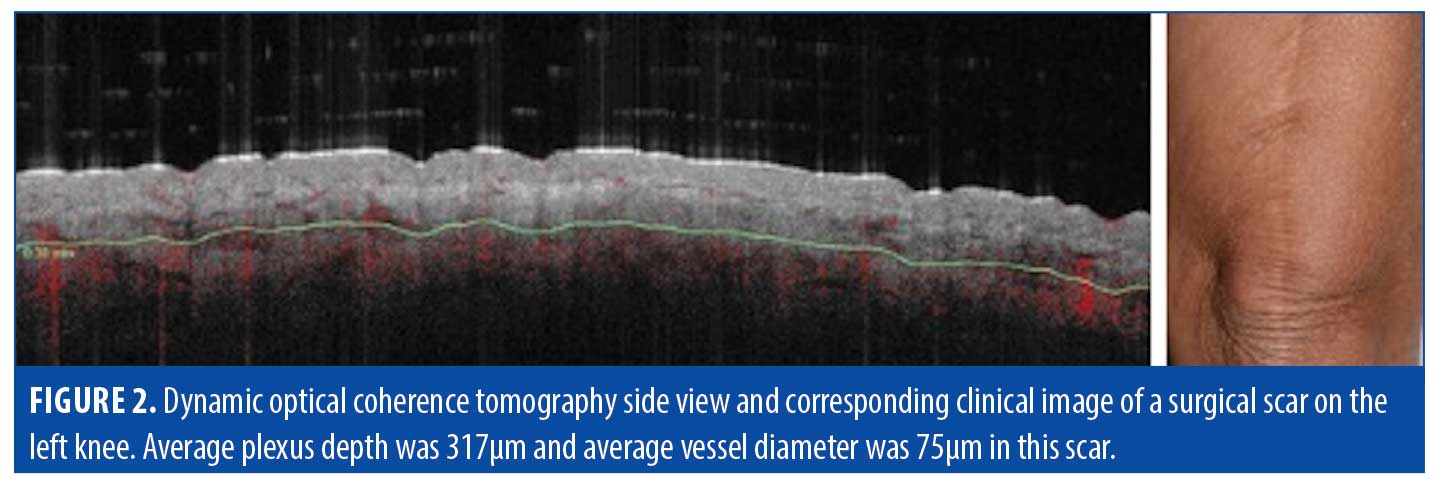
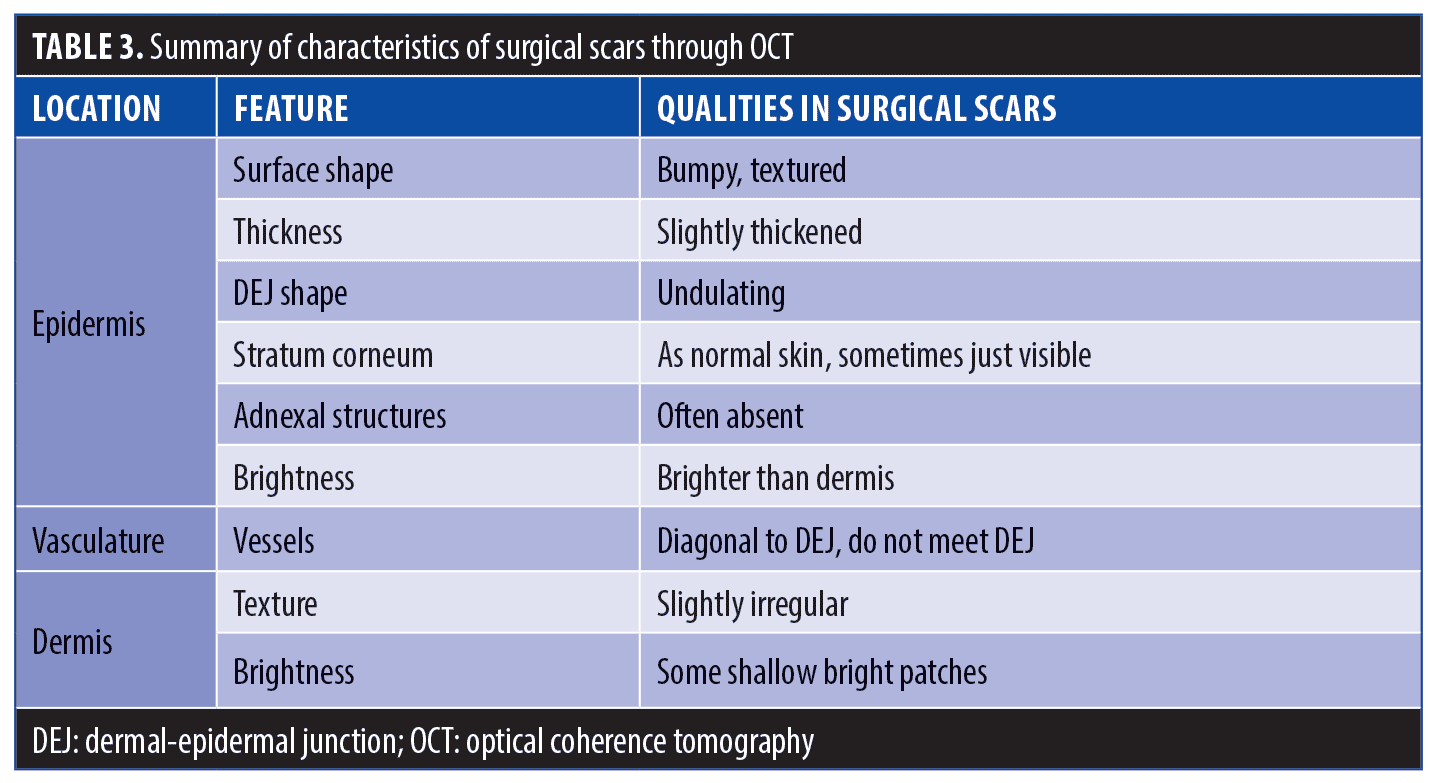
Epidermis. Analysis of surgical scar scans using OCT revealed a mildly thickened epidermis (e.g., 150µm in the example of Figure 2). The surface and dermal epidermal junction typically maintained a gentle undulation throughout, comparable to normal skin.
Vasculature. Though less thick in average diameter (75µm) compared to hypertrophic scars (195µm), the blood vessels in surgical scars were observed to still supply the epidermis diagonally, with a deep plexus at 317µm.
Dermis. The collagen distribution in surgical scarring was observed to also be less deep compared to hypertrophic scars, with bright swirling seen up to 400µm. Loss of adnexal structures in surgical scarring was also observed, compared to normal skin.
Treatment. OCT indicated that surgical scars among our patient population were typically not deep. This suggests that surgical scarring would benefit from a mid-range laser depth for target treatment settings and a vascular-targeting laser, such as pulsed dye laser, to treat the larger, irregular vessels approaching the dermal-epidermal junction (DEJ).
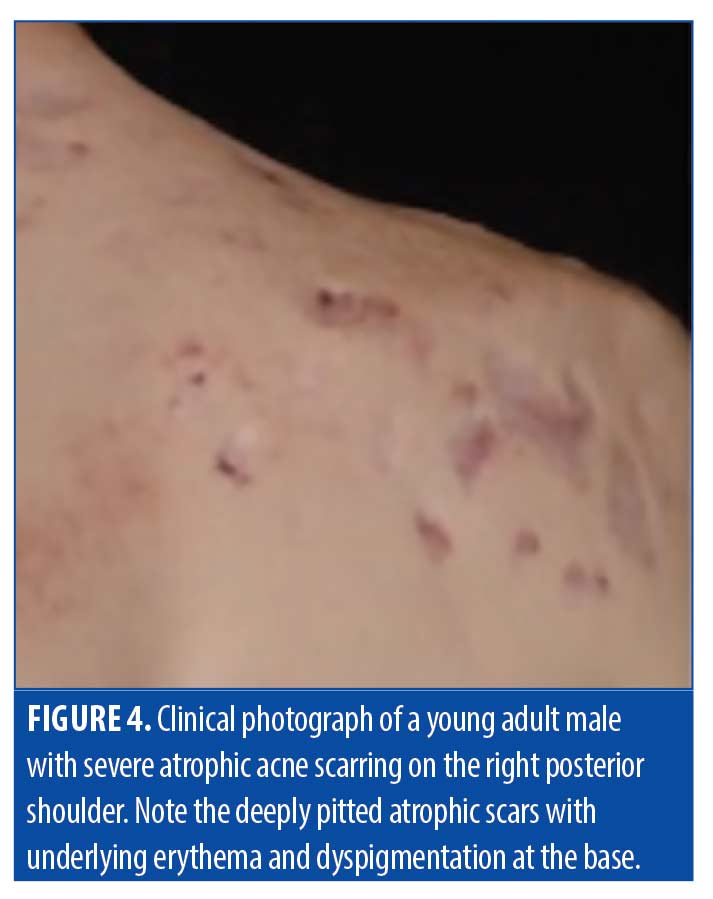
Atrophic scars. Atrophic scarring is most commonly caused by acne, as exemplified in Figure 4). Figure 5 is an OCT scan of the scarring shown in Figure 4, compared to adjacent normal skin. Figure 6 shows a typical en-face OCT scan of an atrophic acne scar of the face. In this case, the maximum depth was 220µm. Observed characteristics of atrophic scars based on OCT are summarized in Table 4.
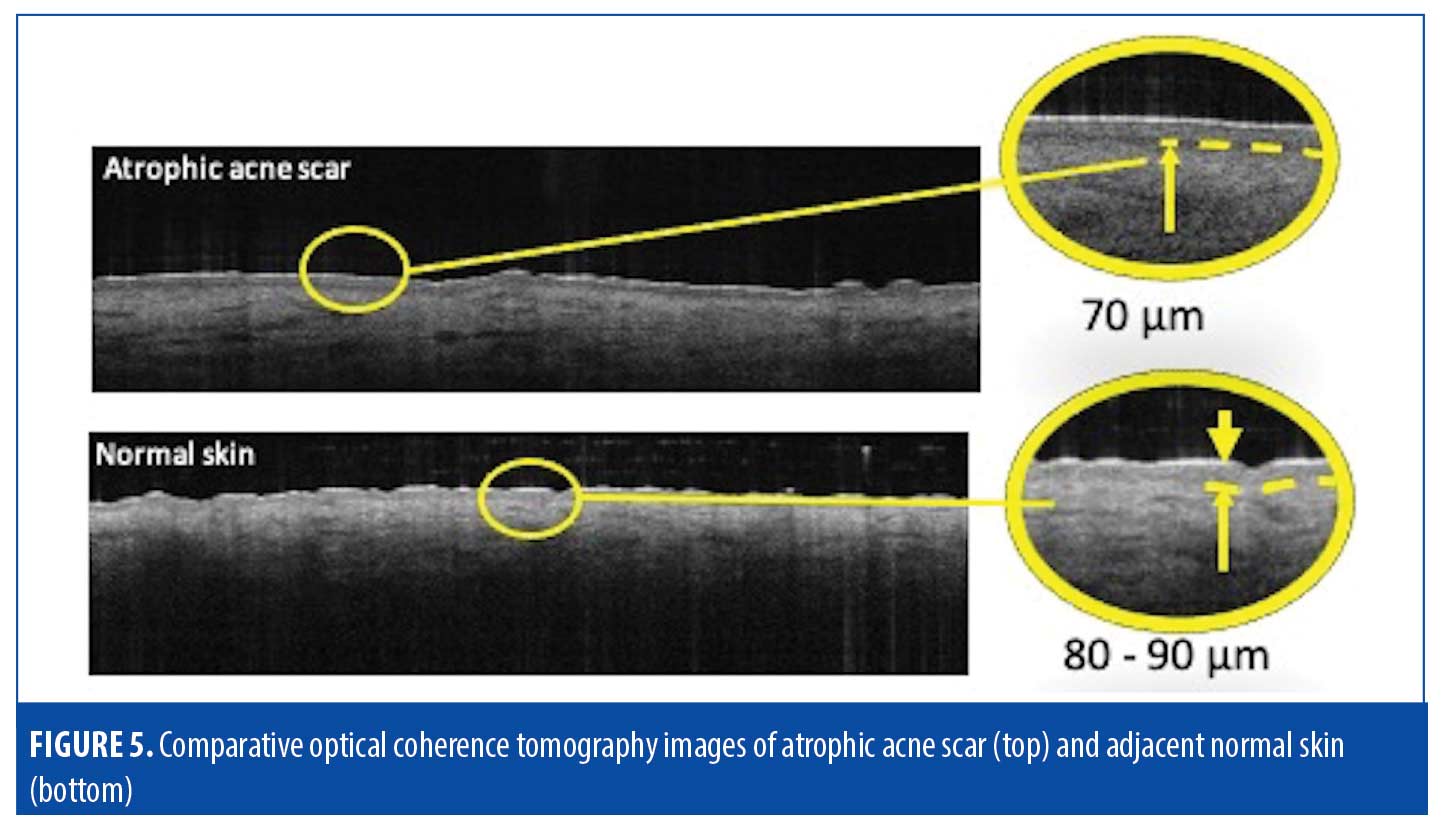
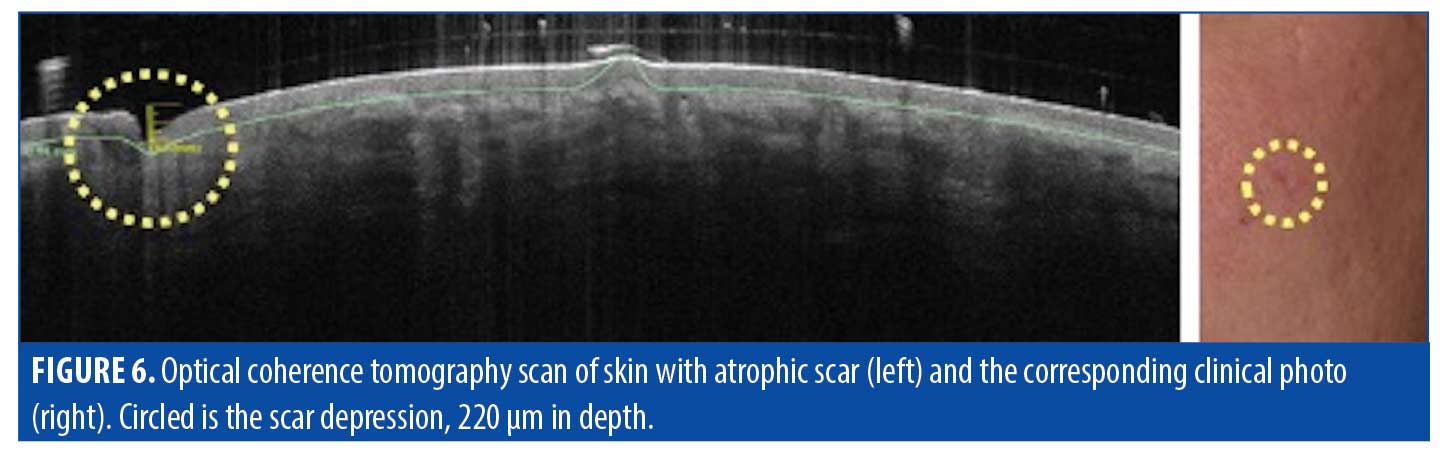
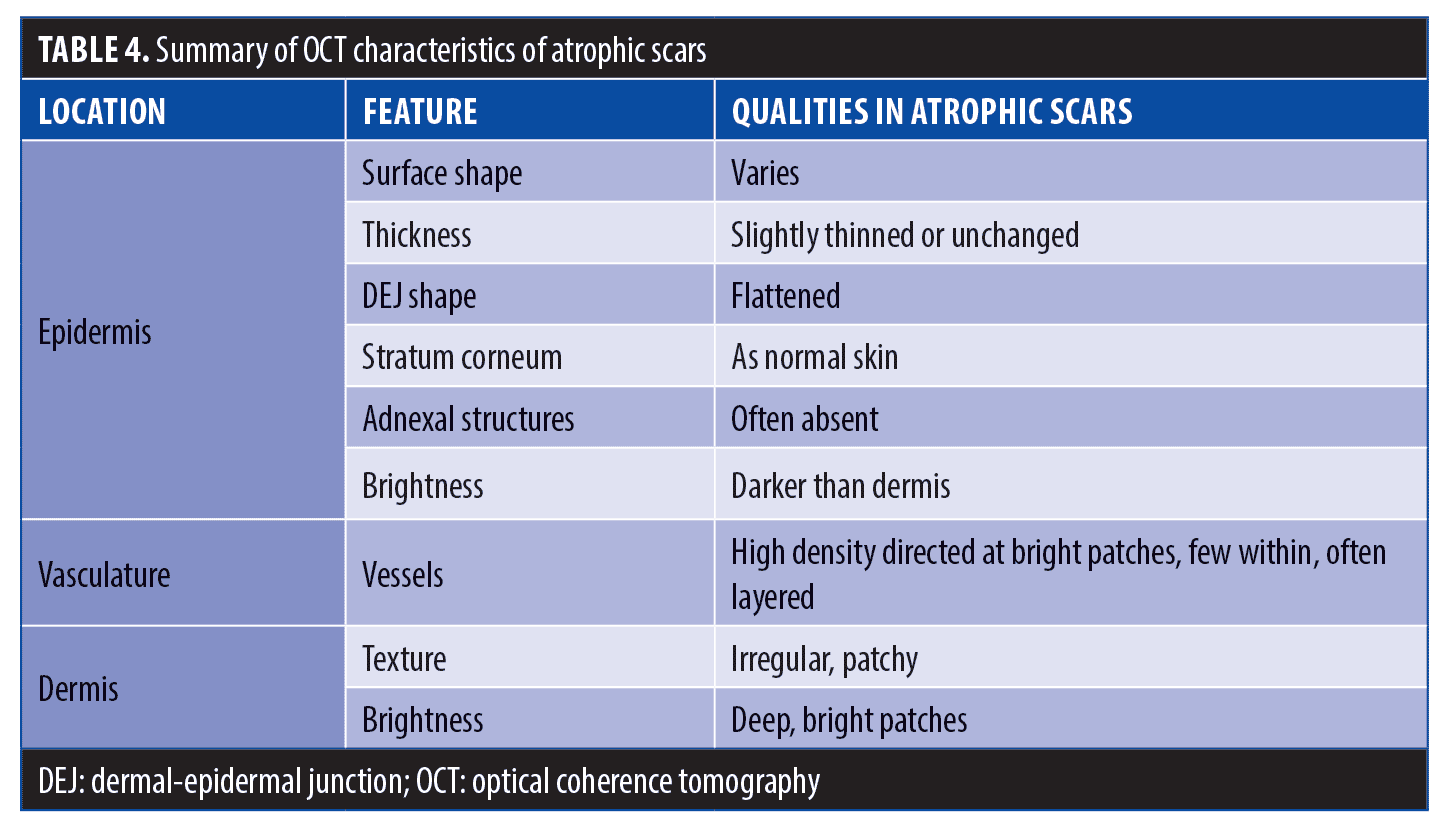
Epidermis. OCT of atrophic scars typically showed thinning and flattening of the usually undulating superficial epidermis and DEJ. The observed rigid, disorganized collagen deposition will prevent the smooth lattice network from forming properly and inhibit the the body from establishing the proper thickness or fiber organization. The epidermal layer was 10 to 20µm thinner, compared to that of normal skin, with a completely flat upper surface and DEJ. The epidermis in atropic scars also typically appeared darker than the dermis.
Vasculature. The dermis in atrophic scars typically showed scattered darkened areas intermixed with brightened, vessel-poor patches. The pattern of dark and bright patches observed via OCT suggests the presence of high-density vessels (dark patches) running toward the areas that were perceived as bright; sometimes these were distorted and appeared in layers.
Dermis. The dermis in atrophic scars, though usually lighter than the epidermis, also typically showed scattered darkened areas. Atrophic scars tend to underproduce collagen, resulting in a depressed skin surface with reduced skin flexibility. Based on OCT imaging, atrophic scar depression in our study measured an average of 400 microns in depth.
Using OCT imaging, the scanned skin surface was automatically displayed as a 3D model, enabling clear visualization of the scar depressions. This is displayed simultaneously with the en-face and camera view (Figure 7). The 3D pitting aligns with a dark area seen on the en-face view, which corresponds to reduced optical scattering attributed to decreased collagen.
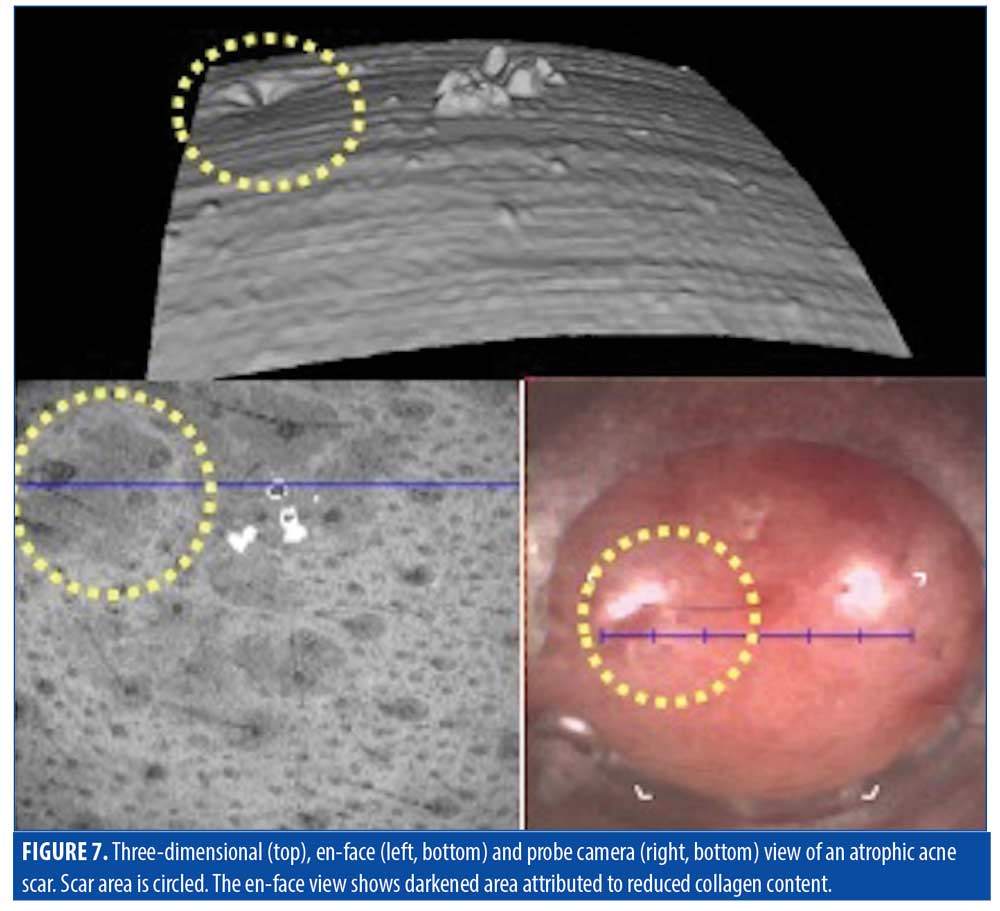
Treatment. The goal of treatment for atrophic scarring is the induction of collagen synthesis, due to the insufficiency of vasculature and collagen production in the scarred areas. Fractional lasers would be ideal to help stimulate collagen production and the formation of new vessels. Determining depth of laser treatment and monitoring treatment progress in atrophic scars can be done using OCT.
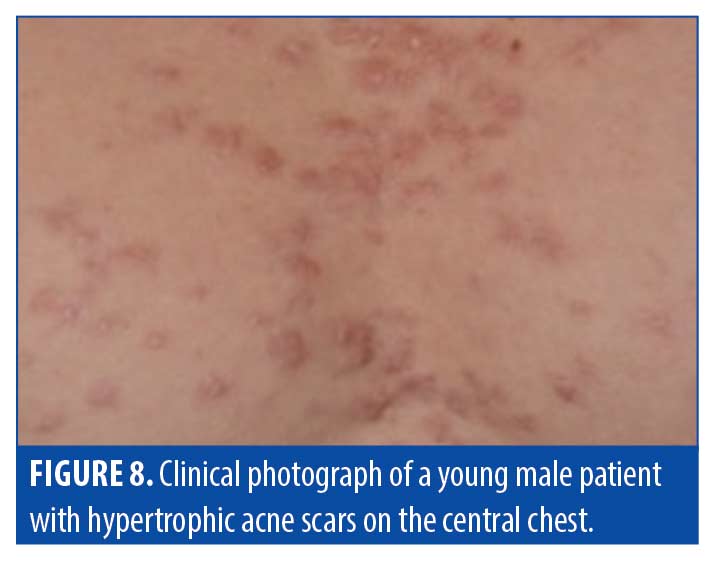
Hypertrophic scars. Hypertrophic scars are clinically characterized by their disorganized wound healing resulting from excess collagen deposition. Hypertrophic scars typically stay within the lateral confines of the original injury site (Figure 8). Table 5 provides a summary of characteristics of hypertropic scars, as observed via OCT.
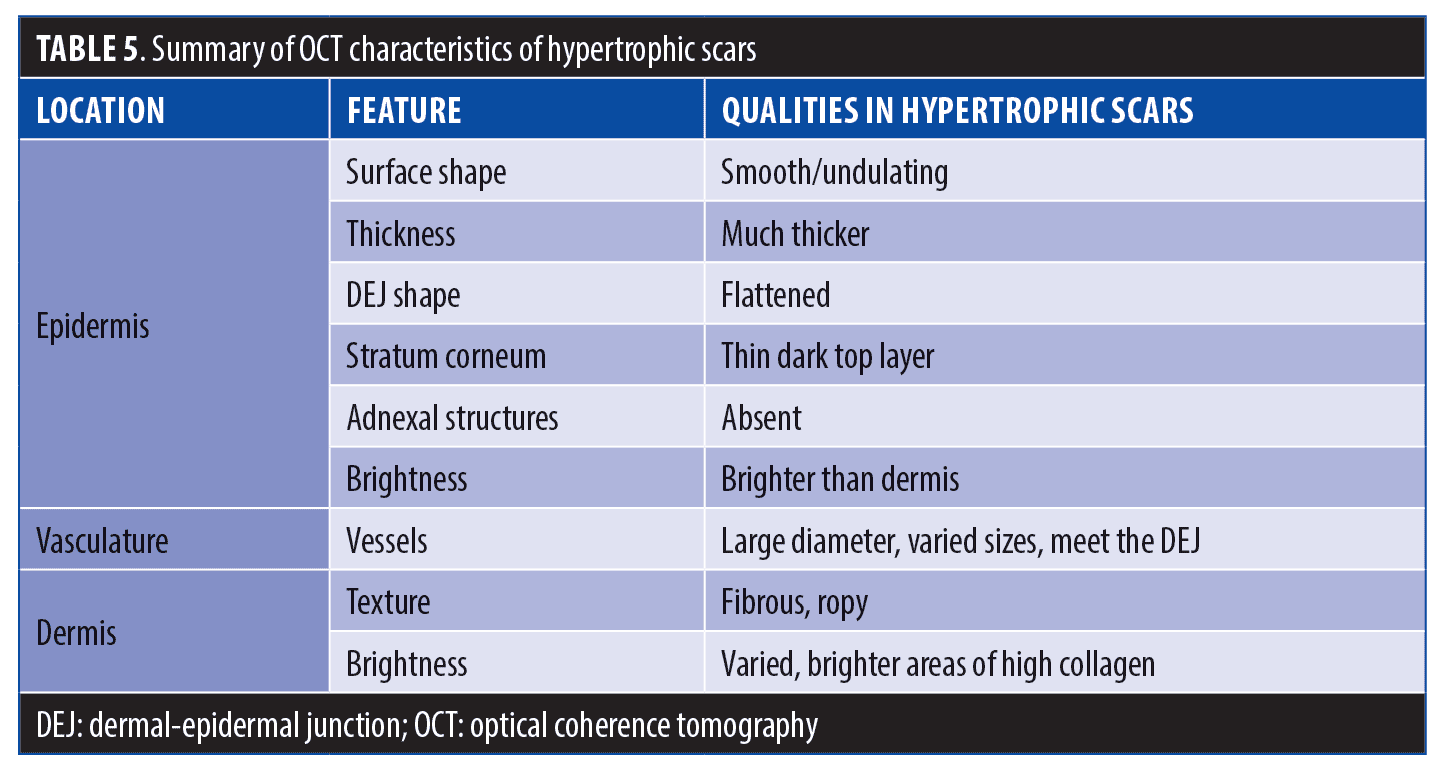
Epidermis. In our study, OCT imaging typically showed hypertrophic scars to have a thickened epidermis that was brighter than the underlying dermis, with a smooth top surface. A thin dark surface layer was often observed, corresponding to thickened stratum corneum. Some hypertrophic scars exhibited flattened DEJ. Others were heavily pitted.
Hypertrophic scars demonstrated a disorganized, heterogeneous appearance in the en-face view. Scar tissue appeared as brightened areas, corresponding to increased optical scattering from the higher collagen content. These areas often had a fibrous appearance with “swirls.” By adjusting the OCT en-face image view, it was possible to measure the depth at which the heterogeneity tapered, indicating the scar depth.
Vasculature. Under D-OCT, hypertrophic scars exhibited characteristic vascular patterns. Hypertrophic scar blood vessels typically appeared to orient from healthy skin toward the scar tissue and were usually of greater diameter than those of normal skin. Normal skin was typically observed to have a regular network of blood vessels with no preferred direction, with small vessel sizes up 70µm in diameter and depth typically 250µm or greater. In hyptertophic scars, we observed brighter patches of tissue corresponding to collagen concentrations that were often vessel-poor, and the blood vessels that were present in these areas often had a distorted serpentine shape. In nearly all hypertrophic scars, blood vessels were observed to be growing diagonally up to the DEJ—a pattern not seen in normal tissue.
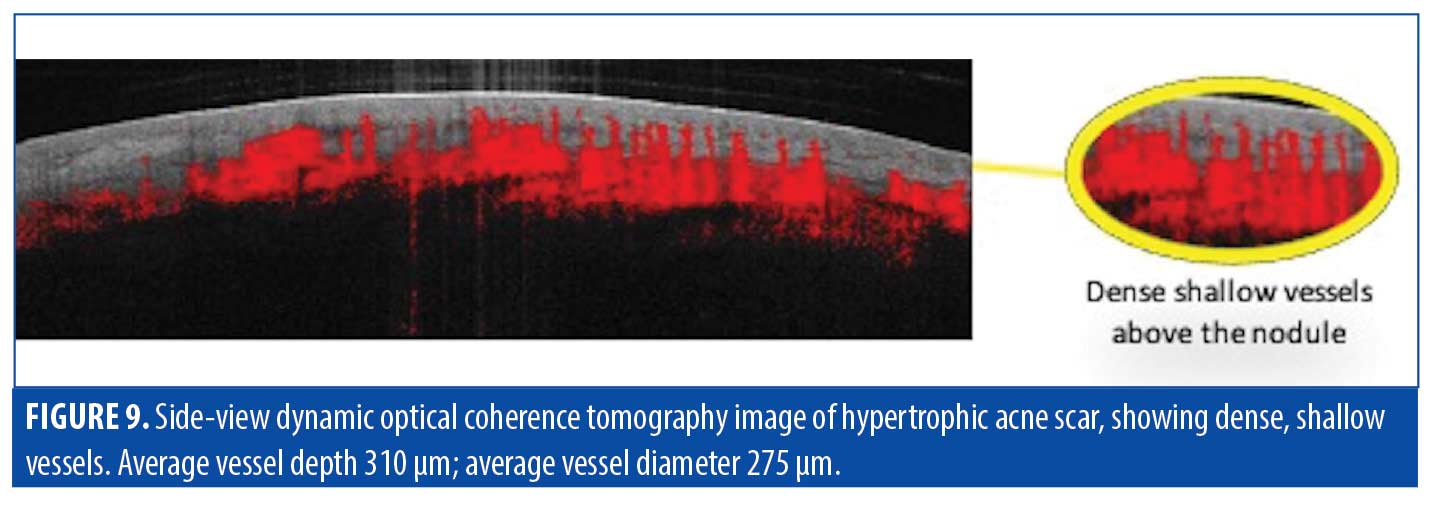
Using D-OCT, we observed a highly vascular hypertrophic scar, as would be expected for a dark pink scar (Figure 9). The vessels of this scar were shallow (200µm deep), denser than normal, more dilated than normal, and located in a dense plexus above the nodule.
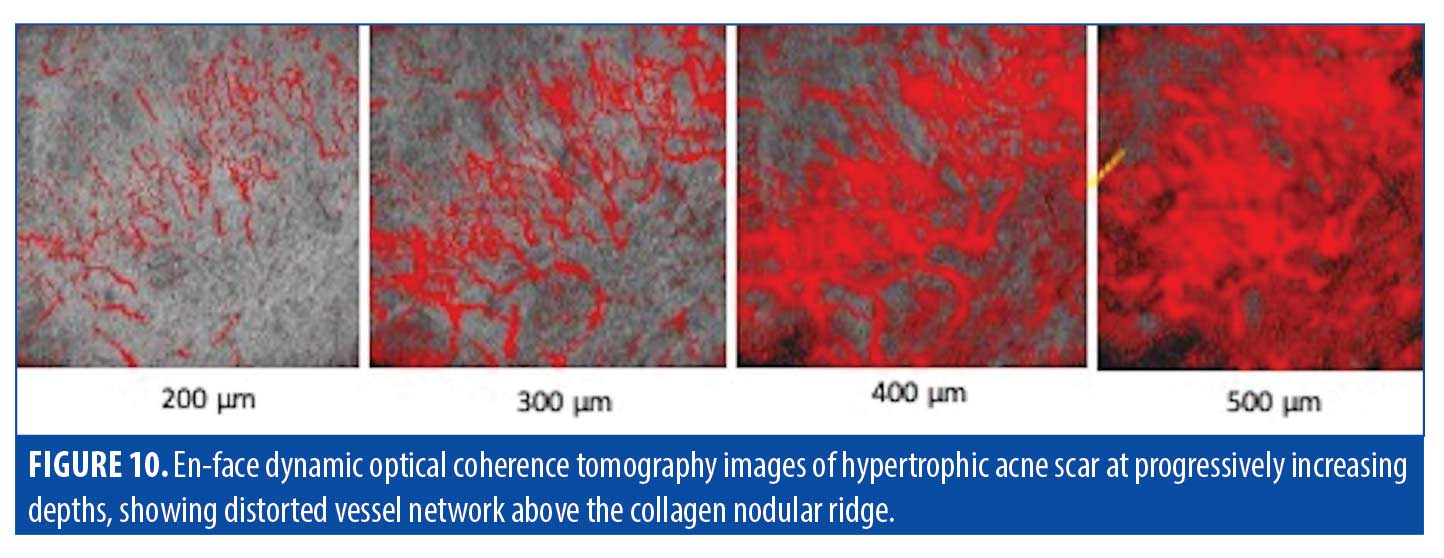
Further imaging through D-OCT can be done at incremental depths for more extensive information. At 200µm in depth, the blood vessels of the scar in Figure 10 were somewhat clustered but overall appeared normal in size and shape. At 300µm in depth, the thick,serpiginous blood vessels directed toward the area of hypertrophy become more apparent. This quality continued to become more pronounced, culminating in wildly irregular, thick, overlying vessels at the peak of the collagen nodule 500µm in depth into the skin. Dense, shallow vessels projected above the nodule as well, aiding in the common erythematous superficial appearance that is characteristic of hypertrophic scarring.
Clinically, we observed all pink scars to show increased vascularity, but the etiology was varied. In some cases, this was due to an abnormally shallow vessel plexus, while in other cases, the blood vessels were at normal depths but had increased diameters, and still in other cases, there was a mixture of both. A shallow plexus and large, deep vessels will not be treated at equal efficacy if equal laser parameters are used. OCT assists the clinician in discerning the differing pathophysiology of hypertrophic scars that may clinically appear identical. By measuring vessel depth and diameter, laser treatment parameters may be set accordingly based on Anderson and Parrish’s theory of selective photothermolysis.
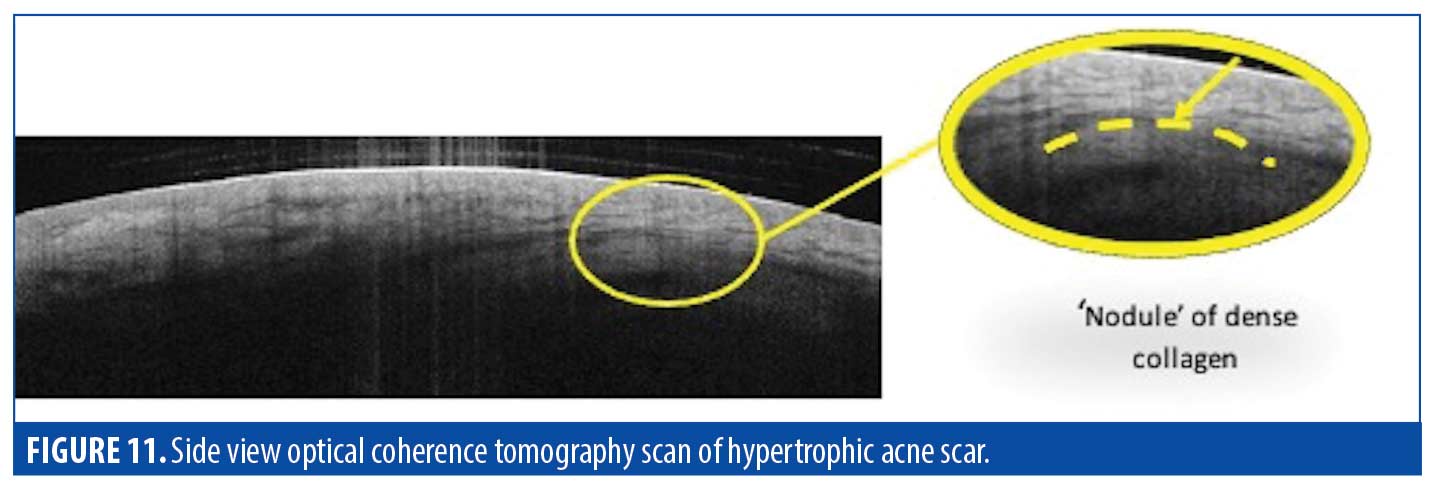
Dermis. Clinically, hypertrophic scars in our study presented as localized, raised pink areas. OCT imaging revealed that much of the excess tissue was composed of excess dermal collagen. Adnexal structures were also mostly absent in lieu of the overproliferation of surrounding tissue. See Figure 11 for the side-view OCT scan in which a portion of the dermis appeared to be compressed by a dome-shaped area of homogeneous tissue, attributed to a nodule of dense collagen.
A comparison of en-face OCT at incremental imaging depths (Figure 12) showed irregular, bright, swirling patches that began to appear at approximately 300µm depth. The brightness of these patches was attributed to increased light scatter, indicating excess collagen deposition. These patches were still visible beyond the imaging depth of OCT (up to 1mm), suggesting that the scar tissue was at least this deep.

Treatment. In our study, OCT imaging revealed hypertrophic scars to have substantially greater depth than previously understood. OCT imaging can assist the clinician in determining the optimal depth of laser treatment as well as the diameter of the individual blood vessels, for optimal results using a minimal number treatment sessions.
Keloids. Keloidal scars are characterized by an exaggerated healing response to tissue injury, with unregulated collagen deposition. Keloids can resist treatment and substantially worsen if not adequately treated (Figure 13, left photo). A summary of keloid scar characteristics observed via OCT is provided in Table 6.
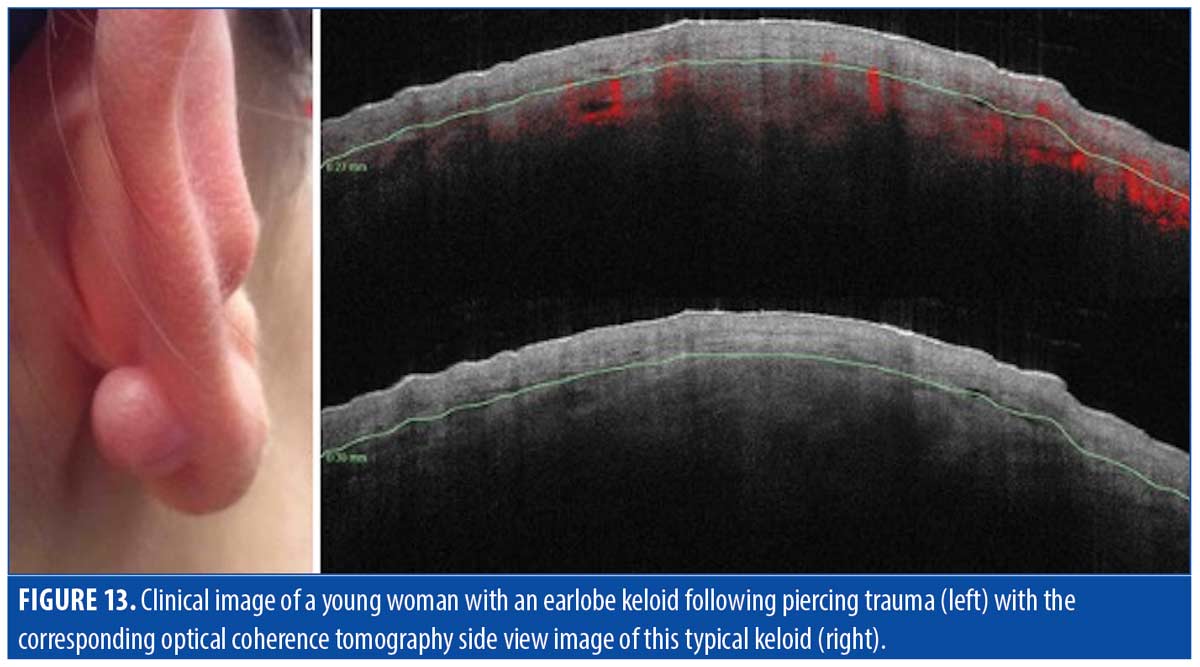
Epidermis. OCT imaging of keloid scars in our study typically revealed thickened stratum corneum and epidermis similar to those observed in hypertrophic scars— ~150µm thick, with a disorderly DEJ and dilated vessels just below the scar, as illustrated in Figure 13 (right photo).
Vasculature. Given the size and color of keloid scars coupled with their propensity for persistent growth, the likelihood of a dense vascular network seems intuitively high. However, in our study, via OCT, we observed keloids to be mostly devoid of vasculature, with the bulk of blood vessels confined to the outer layer. This suggests that keloids may grow in an outward, layered fashion, with the upper dermis fueling their growth by depositing proliferated collagen behind the scars as they continue to spread.
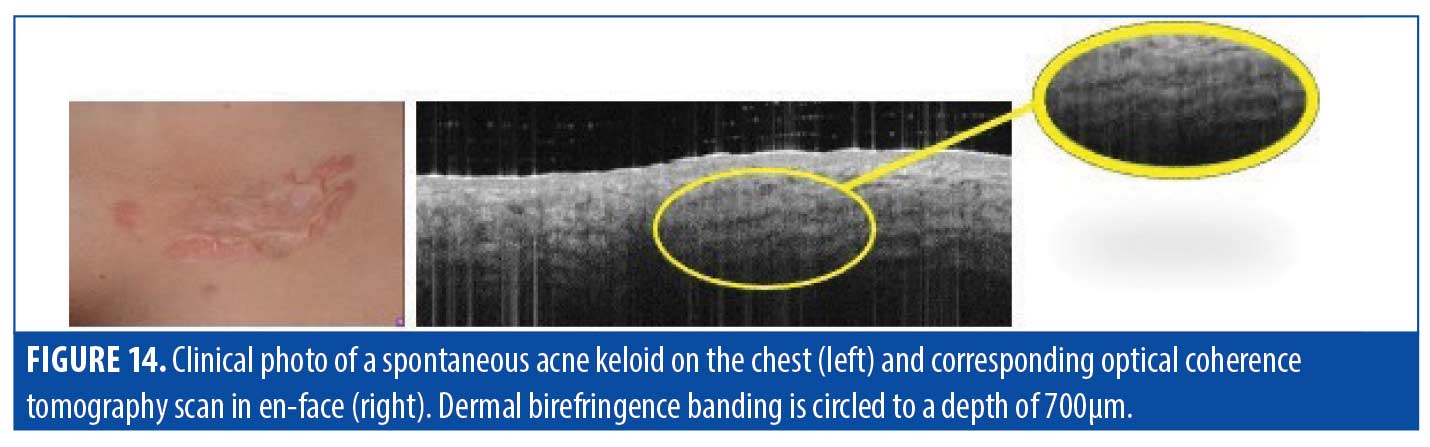
Dermis. We observed the dermis of keloid scars to be homogenous. As mentioned, the interesting aspect of a keloid is that the internal portion consists only of collagen and is not “active.” Keloids may show “banding” parallel to the surface, caused by birefringence; this suggests the presence of highly organized, linearly oriented collagen deep in the dermis (Figure 14). Though frequently compared clinically to hypertrophic scarring, the internal composition of keloid scars, such as the dense, large-diameter vascular network, is not seen in hypertrophic scars. A summary of our OCT findings for keloid scars is provided in Table 6.
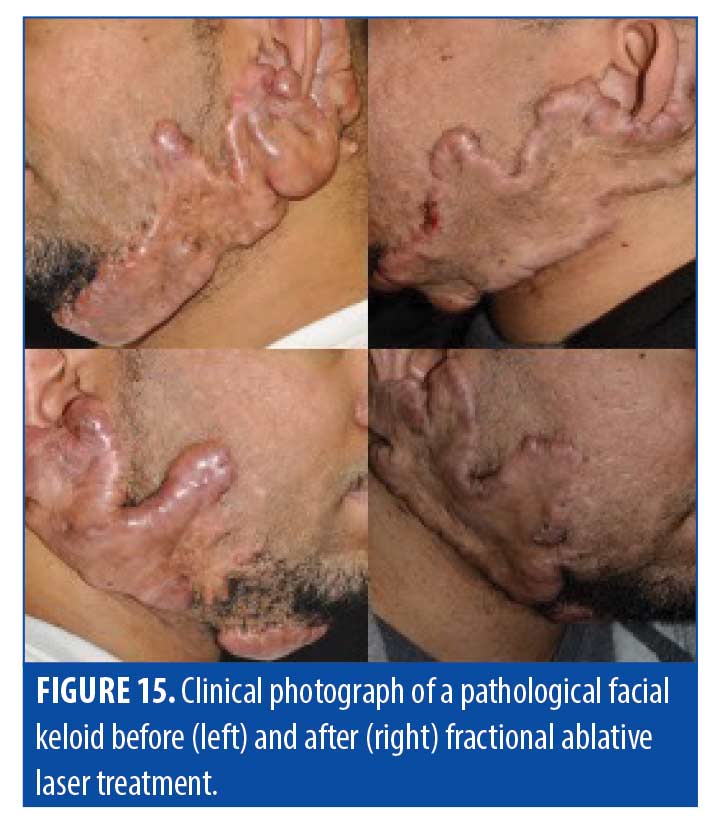
Treatment. In our observation and experience, after each laser treatment, the edge of a keloid scar tends to reactivate and continue to advance. Figure 15 illustrates this concept, with only the center successfully treated by AFL and a “leading edge” that is recalcitrate to treatment. This suggests that keloids may require a novel strategy of destroying vessels in the active keloid with long pulses of a vascular laser to help halt growth and then eliminating the excess collagen deposition with an ablative fractionated laser.
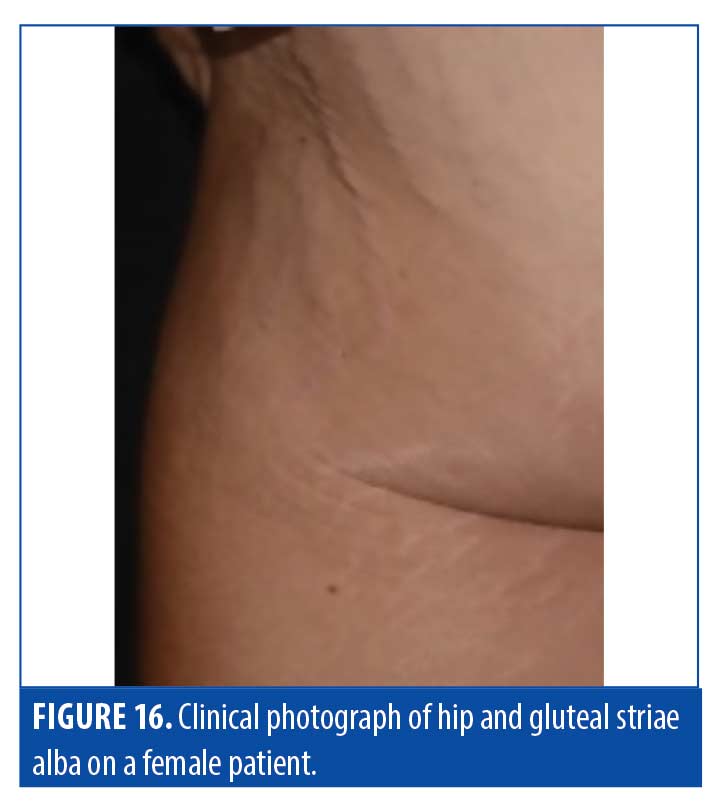
Striae distensae. The pathology of striae, classified as striae rubrae or striae alba, remains elusive, and whether they should be classified as scars is still being debated. Striae rubrae are young lesions that are characteristically red in appearance and have been shown to respond to vascular targeting lasers. Striae alba, on the other hand, are more mature lesions and can present with normal or hypopigmentation. These lesions are typically difficult to treat due to substantial textural changes, which can be resistant to standard fractional ablative CO2 lasers (Figure 16). OCT imaging of these scars sheds some light on why traditional laser treatments have had unsatisfactory results (Figure 17). A summary of striae characteristics based on OCT imaging is provided in Table 7.
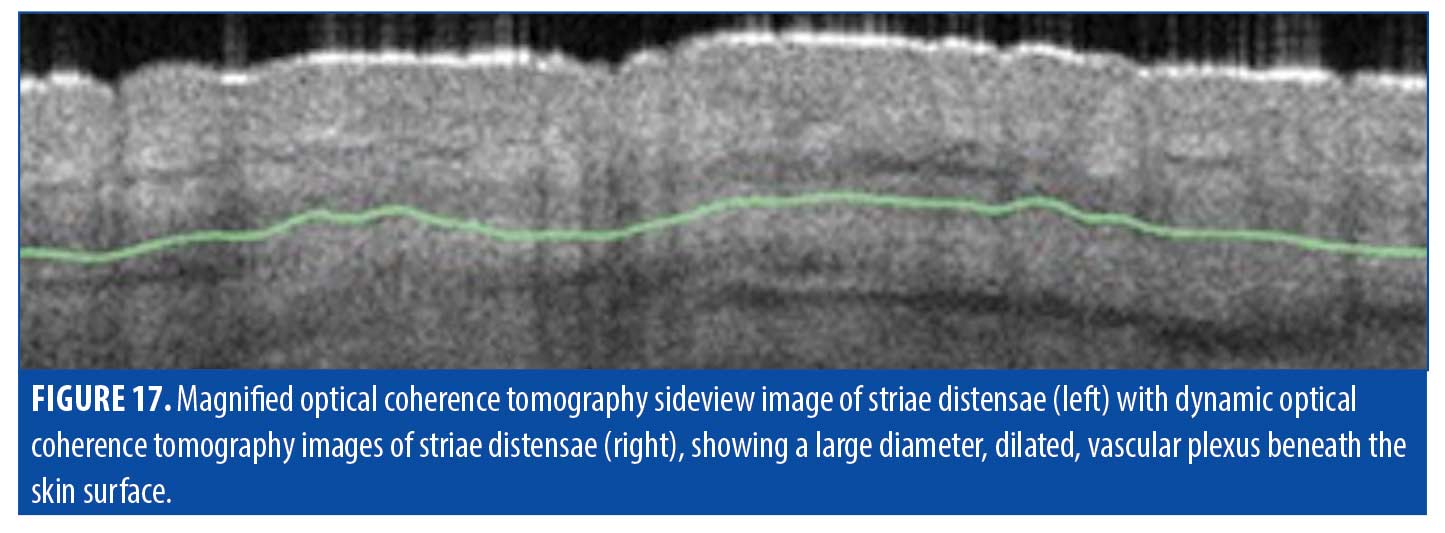

Epidermis. Based on OCT imaging in our patient sample, the epidermis of striae did not exhibit many of the features of other traditional scar types. The surface and stratum corneum of striae were not as flattened as those observed in other scar types; rather, they typically presented with more traditional undulation resembling that of normal skin. This is reasonable as striae do not begin as a superficial insult and, therefore, the barrier is not disrupted.
Vasculature. The vascular network in the imaged striae was typically observed to be large, dilated, and deep. Plexus depth was measured as 361µm, much greater than in normal tissue, and the average vessel diameter was measured to be 195µm, again much larger than normal.
Dermis. The most insightful part of our analysis of striae using OCT imaging was in observing the dermal collagen distribution in these scars. Striae had long been thought to be superficial but OCT revealed banding to a depth of at least 800µm (Figure 18). This banding may be attributed to optical birefringence due to highly organized collagen fiber bundles arranged in organized, unidirectional layers, rather than in the normal basket-weave pattern of collagen bundles in healthy skin. These observations suggest that striae are not superficial, but comprise deep layers of dense organized collagen.
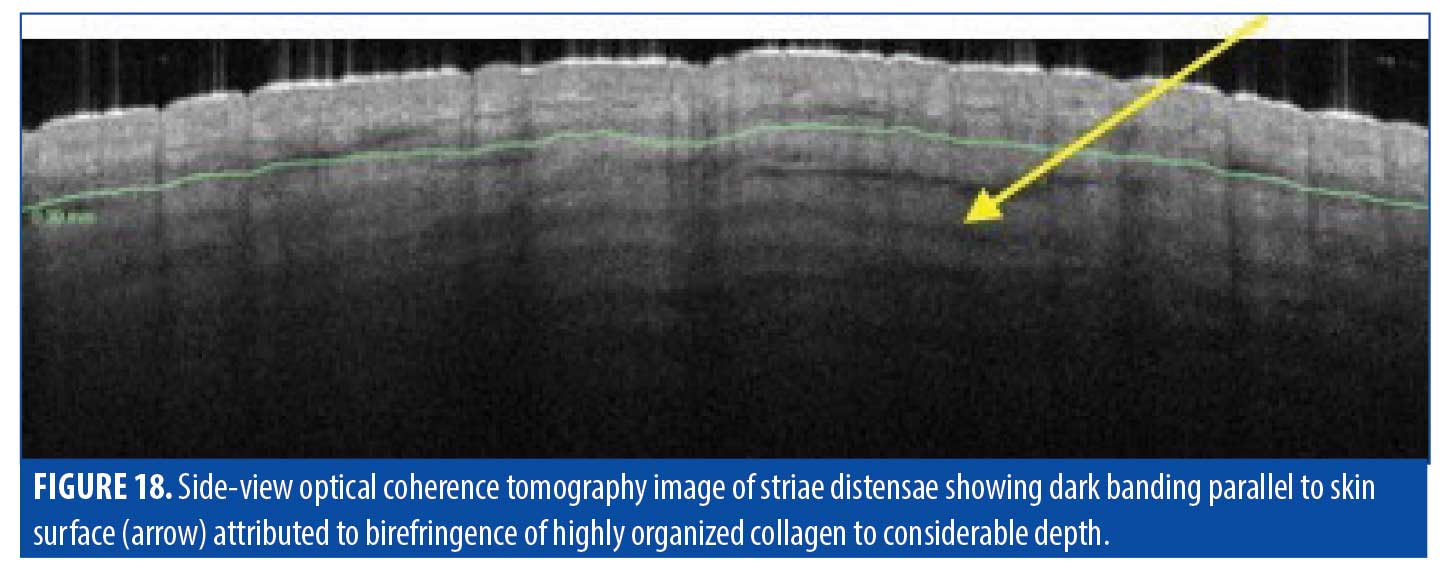
Treatment. When considering our findings based on OCT imaging of striae, we deduced that lasers used in striae treatment have not been set to penetrate the appropriate depth at which substantial effect can be achieved. Treatment of striae tailored more appropriately to match the depth of the pathology may result in better outcomes.
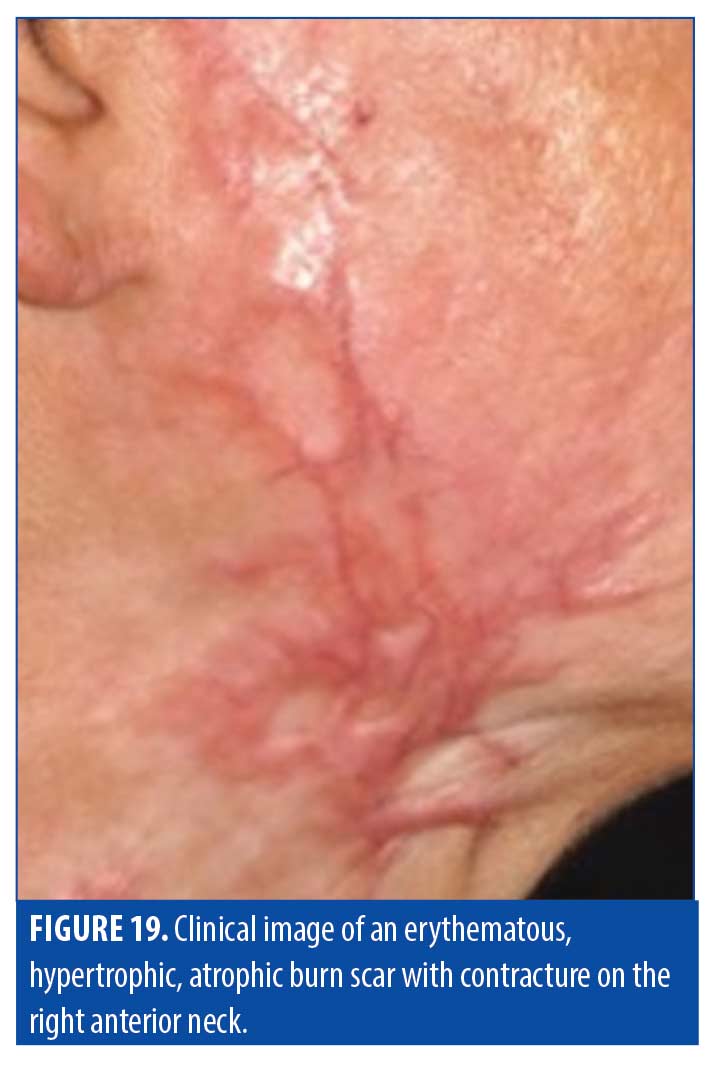
Burn scars. Burn scars are highly variable in presentation depending on source of the burn, depth of the wound, and methods of wound care used in the healing process. The extent and depth of the thermal injury can lead to varied thicknesses and subsequent attempts at wound care or skin grafting may further contribute to variability of burn scars. They are also typically heterogeneous in nature, so characterization into one category (atrophic, hypertrophic, keloid) is not generally representative of burn scars. Accordingly, OCT can be used to reveal components of individual burn scars. A summary of typical charactertistics of burn scars based on OCT imaging are provided in Table 8. Figure 19 is of a burn scar that presented with a combination of atrophy and hypertrophy with variable size and texture on the front of the neck of a patient.
Epidermis. The en-face OCT view of the scar in Figure 19 demonstrated highly irregular structural organization. The epidermis was thickened and bright, but the DEJ was blurred in contrast with the normal undulation or the commonly flattened scar appearance. Clinically, this scar rapidly alternated between hypertrophy and atrophy, which is reflected in the scan by areas of flatter epidermis alternating with areas of undulating epidermis. The epidermal layer was brighter than the dermal layer as well, consistent with all described scar types.
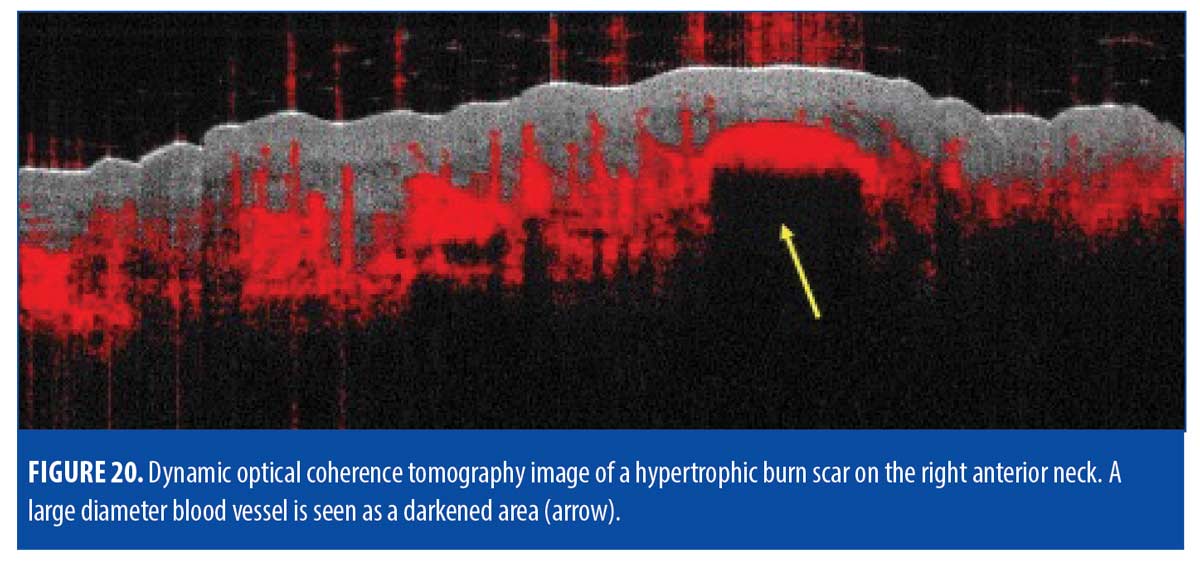
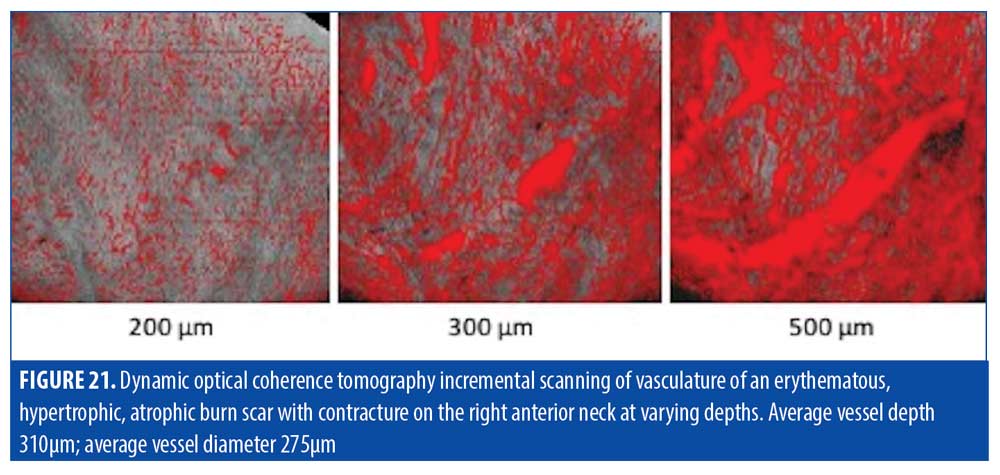
Vasculature. D-OCT imaging revealed most of the dermis to be highly vascularized with a multitude of large diameter (300-600µm) dilated vessels, corresponding with the substantial erythema seen clinically (Figure 20). The protrusion of the vessel become visible at 300µm but the bulk of the vessel along with the surrounding network of large diameter vessels became more prominent at 500µm (Figure 21).
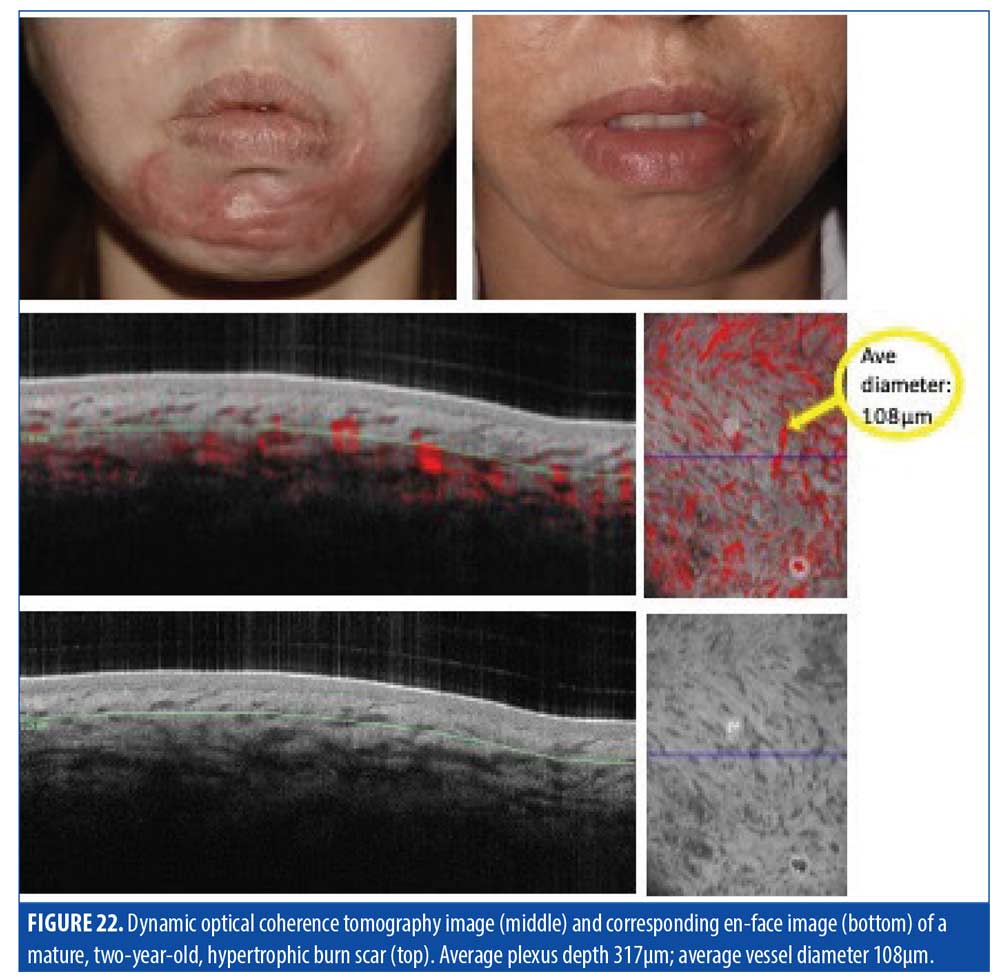
Mature hypertrophic scars (Figure 22) typically demonstrate large blood vessels near the DEJ as well, but less clustered compared to younger hypertrophic scars.
Dermis. In the right neck burn scar in Figure 19, the dermis showed a large, ovular, darkened area visible at a depth of approximately 500µm (Figure 23). As shown previously under D-OCT (Figure 21), this corresponded to an enlarged vessel. The beginning of the vessel in en-face was again visible through incremental slicing of the image, pinpointing its origin at 500µm (Figure 24).
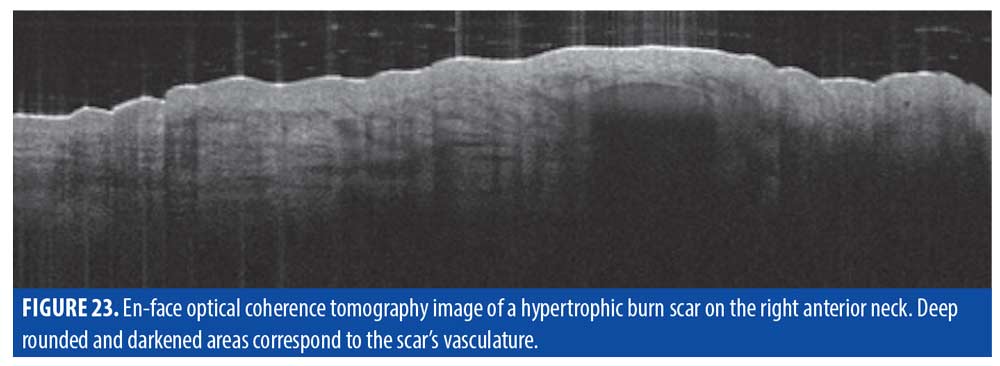

Treatment. OCT imaging suggests that burn scars are typically quite deep, which may explain why superficial treatments of pigmentation can result in suboptimal improvement. At 700µm, ablative fractional lasers would be more appropriate for depth and also for targeting the collagen bundles that can lead to cutaneous tension and textural irregularity.
Discussion
Detailed scar characteristics, such as vasculature and DEJ, can be tangibly assessed using OCT; consideration of these characteristics can then help guide the type and settings of laser treatment to optimize results. As scars are multifactorial, optimal treatment typically requires a multimodal solution involving several different treatment strategies. The scar depth and microstructure data provided by OCT imaging can assist the clinician in determining the appropriate laser treatment parameters according to the individual needs of each patient.
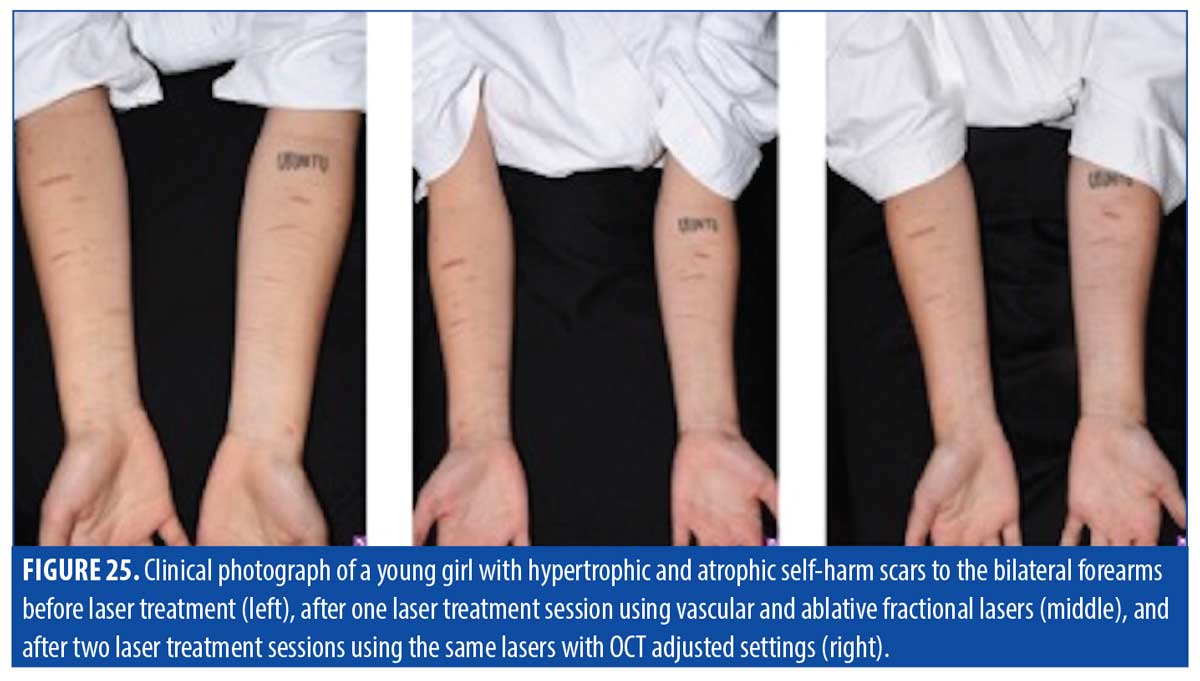
With hypertrophic scars, such as those pictured in Figure 25, OCT imaging can be used to specifically determine blood vessel depth and diameter for vascular targeting laser treatment to reduce erythema and more effectively remove vascular support from the underlying tissue. OCT can also provide a schematic of tissue depth to focus settings of ablative fractional treatment specifically at a depth that previously had been estimated based on physician experience.
Conclusion
OCT may allow the identification of the unexpected when assessing scar pathology, making it a valuable tool in the treatment of scars, particularly those that are resistant to treatment. Each scar type possesses its own tangible qualities that can be addressed uniquely for more effective treatment results. The use of OCT may help to expedite and expand scar diagnostics, which in turn may increase our understanding of scar pathology and capabilities in scar treatment.
References
- Patel L, McGrouther D, Chakrabarty K. Evaluating evidence for atrophic scarring treatment modalities. JRSM Open. 2014;5(9):2054270414540139.
- Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2010;17(1-2):113–125.
- Wheeland RG. Keloids and hypertrphic scars. In: Arndt KA, Robinson JK, Leboit PE, Wintroub BU, editors. Cutaneous Medicine and Surgery. Saunders Elsevier; Philadelphia: 1996. pp. 900–905.
- Love PB, Kundu RV. Keloids: An Update on Medical and Surgical Treatments. J Drugs Dermatol. 2013 Apr;12(4):403–409.
- Satish L, Kathju S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatol Res Pract. 2010;2010:790234.
- Marshall CD, Hu MS, Leavitt T, et al. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv Wound Care (New Rochelle). 2018;7(2):29–45.
- Issler-Fisher AC, Waibel JS, Donelan MB. Laser modulation of hypertrophic scars technique and practice. Clin Plast Surg. 2017;44:757–766.
- Anderson R, Donelan M, Hivnor C, et al. Laser Treatment of Traumatic Scars with an Emphasis on Ablative Fractional Laser Resurfacing. JAMA Dermatol. 2014;150(2):187–193.
- Waibel JS, Rudnick AC, Wulkan AJ, et al. The Diagnostic Role of Optical Coherence Tomography (OCT) in Measuring the Depth of Burn and Traumatic Scars for More Accurate Laser Dosimetry: Pilot Study. J Drugs Dermatol. 2016;15(11):1375–1380.
- Bowen R. A novel approach to ablative fractional treatment of mature thermal burn scars. J Drugs Dermatol. 2009;9:389–392
- Waibel JS, Holmes J, Rudnick A, et al. Angiographic optical coherence tomography imaging of hemangiomas and port wine birthmarks. Lasers Surg Med. 2018;50(7):718–726.

