 J Clin Aesthet Dermatol. 2020;13(11):22–27
J Clin Aesthet Dermatol. 2020;13(11):22–27
by Miesha Merati, DO; Christi Woods, DO; Nina Reznik; and Lydia Parker, MD
Drs. Merati, Woods, and Parker and Ms. Reznik are with The Parker Skin and Aesthetic Clinic in Cleveland, Ohio.
FUNDING: Funding for this study was provided by SkinGen.
DISCLOSURES: Dr. Lydia Parker was sponsored by SkinGen Inc (Toronto, Ontario), manufacturer of the novel human recombinant growth factor regenerative complex. Dr. Miesha Merati, Dr. Christi Woods, and Nina Reznik have no potential conflicts of interest to declare.
ABSTRACT: Background. The study of the use of growth factors in conjunction with microneedling is limited. It is hypothesized that the use of human growth factors will decrease recovery time by downregulating inflammation and will continue stimulating collagen synthesis initiated by microneedling.
Objective. We sought to evaluate the safety and efficacy of microneedling in combination with a novel human recombinant growth factor regenerative complex (PolyGF) for skin improvement.
Methods. This study was a randomized controlled trial evaluating changes in multiple skin parameters via provider, imaging, and patient self-assessments after four microneedling treatments, each spaced one month apart, with or without a novel growth factor. Twenty female patients aged 35 to 60 years were included. Evaluation parameters included fine lines and wrinkles, evenness of skin tone, skin clarity, age spots, skin smoothness, skin firmness, and hydration of skin via dermatologic Canfield VISIA (Fairfield, New Jersey) imaging, and patient self-assessment.
Results. Significant improvement in skin texture was reported to higher degrees for the PolyGF group by VISIA imaging assessments than in the control group. Subjective assessments in skin smoothness and hydration were significantly improved in the Poly GF groups. Hydration improvements were seen to a lesser degree in the control group. Improvements in firmness and texture were seen in both the PolyGF and control groups by subjective assessment. Significantly improved subjective assessments of skin tone and melanin index were reported only in the control groups.
Conclusion. While microneedling improved skin in several parameters, the addition of the novel growth factor to microneedling helped improve skin texture and hydration to a higher degree. Further studies are needed to characterize the effects of growth factor serum in conjunction with microneedling.
KEYWORDS: Microneedling, growth factors
Clinical manifestations of skin aging are a result of intrinsic (e.g., gene mutations, hormones, metabolism) and extrinsic (e.g., pollutants, ultraviolet light, and ionizing radiation) factors.1 Chronologic aging due to the passage of time, and photoaging, due to environmental factors, include alterations in pigment, loss of elasticity, fine lines and wrinkles, loss of hydration, telangiectasias, and increased risk of skin cancer.2 Intrinsically-aged skin by atrophy of the extracellular matrix and degradation of collagen and elastic fibers manifests as thinning of the epidermis with flattening of the dermo-epidermal junction.1
Photoaged skin is characterized by accumulation of abnormal elastic tissue, termed solar elastosis. This abnormal elastic tissue might replace the normal matrix composed mainly of collagen. Glycosaminoglycans, which are normally distributed between collagen bundles, serve to bind water and increase hydration of the skin. They are increased in photoaged skin; however, their function is altered due to association with abnormal elastic fibers.1
Collagen, a main building block of human skin, is synthesized by procollagen, which is derived from dermal fibroblasts. Transforming growth factor-B is a cytokine that regulates dermal fibroblasts and might promote collagen breakdown by upregulating matrix metalloproteinases (MMPs). Extrinsic factors promote reactive oxygen species (ROS) which enhance MMP breakdown of collagen and increase inflammation by the NF-KB pathway. Ultraviolet (UV) light might also directly cause DNA alterations and cross-link and modify structural proteins.2,3
Topical application of growth factors has been studied for skin rejuvenation and wound healing. Growth factors are proteins produced by keratinocytes, fibrocytes, and melanocytes that alter collagen biosynthesis; they include cytokines, platelet-derived growth factor, epidermal growth factor (EGF), granulocyte-colony stimulating factor, vascular endothelial growth factor (VEGF), keratinocyte growth factor, and hepatocyte growth factor.3,4,5,6,7 Topical growth factors have a molecular size >15,000 kilodalton (kDA), which limits their ability to penetrate the skin. In general, molecules larger than 500kDA cannot enter the stratum corneum unless there is a route of entry, for example, with microneedling.3,8,9
Microneedling punctures the skin at a controlled depth, which causes an intrinsic wound-healing cascade promoting collagen formation, and neovascularization. Migration and proliferation of fibroblasts occurs, resulting in formation of intercellular matrix, and deposition of Collagen 3. Skin tightening and collagen formation continues for 5 to 7 years postprocedure.10 In this study, we analyze the effects of microneedling with and without a novel topically-applied growth factor. We hypothesized that the use of human growth factors will decrease recovery time by downregulating inflammation, and benefit textural improvement by continuing stimulation of collagen synthesis initiated by microneedling.
Methods
This study was a randomized controlled trial, conducted in adherence with the World Health Organization International Conference on Harmonisation Tripartite Guidelines for Good Clinical Practice, local laws, and the provisions of Declaration of Helsinski. Twenty female patients aged 35 to 60 years were included. Inclusion criteria were general good health, Fitzpatrick skin type I to IV, and signs of aging and photoaging, including fine lines, uneven skin tone, ephelides, and postinflammatory hyperpigmentation. Exclusion criteria were as follows: a known history or present allergic contact dermatitis to any cosmetic products, active skin disease that would interfere with the test readings, subjects on oral medications within the last 30 days that would compromise the study, clinical anti-aging treatments within the past four weeks, unwilling to discontinue other topical facial products, pregnancy/lactation or nursing, chronic uncontrolled medical illness that might influence the skin, and those participating in other cosmetic or therapeutic trials.
Primary objectives were to evaluate fine lines and wrinkles, evenness of skin tone, skin clarity, hyperpigmentation of any cause, smoothness, firmness, and moisturization post-procedure. The secondary objectives were to evaluate skin hydration, tolerance, and product safety. Subjects and health care professionals, including one board certified dermatologist, made clinical assessments. Scales for primary outcomes were graded from 1 to 5 and separated the left and right side of the face. Healthcare professionals also performed comparative assessments with Canfield VISIA Complexion Analysis System (Fairfield, New Jersey) images as well as standard photography taken by a professional medical photographer in a controlled setting. Standard photography was taken as a precautionary measure, and only used if VISIA imaging was not clear. Additionally, skin inflammation was measured one- and two-hours post procedure.
A medical aesthetician performed full-face microneedling with a motorized microneedling pen (Rejuvapen; Refine USA, Jacksonville, Florida) at a setting of 0.5mm for all treated areas, using a hyaluronic acid gel, every four weeks on 10 patients. Ten patients received the same treatment using the polygrowth factor serum (SkinGen Inc; Toronto, Ontario) topical instead of the hyaluronic acid gel. They were also instructed to apply the remainder of the 2mL topical regenerative complex every 30 minutes post treatment, until gone. Based on product dispensing logs, all patients were adherent. The growth factor serum (Poly Growth Factor Serum) contained human recombinant nano-encapsulated growth factors to enhance penetration, as well as the following active ingredients: butylene glycol and iris florentina root extract, tetrahexyldecyl ascorbate, retinol, alpha lipoic acid, chrondrus crispus extract, and sodium hyaluronidate. The PolyGF group also applied SkinGenuity Serum followed by the Accelerator (SkinGen Inc. in Toronto, Ontario) morning and night in between treatments. After treatments, all patients were instructed to cleanse their face with a gentle skin cleanser (Avene Tolerance Extreme Cleansing Lotion; Avene in Avene, France) and to use sunscreen daily (Cyberderm Sun Whip SPF 25; Cyberderm, Ottawa, Ontario). They were instructed to avoid using any other products on their face.
The study was conducted for 12 weeks, with five visits total; Visit 1 included enrollment and baseline assessment. Microneedling treatments were performed on the second through fourth visits, and assessments made on Visits 3, 4, and 5. Visits 1 and 2 could be combined onto the same day and each visit was conducted four weeks apart. Subjects washed their faces with Avene Tolerance Extreme Cleansing Lotion (Avene; Avene, France) prior to procedure and acclimated at a temperature of 22°C +/- 5° C and relative humidity of 50% +/- 10% for 10–15 minutes. The microneedling pen was moved over the skin for a total of one pass. To ensure adherence, subjects were expected to keep a record in a diary provided on Visit 1, which documented date and time after application of PolyGF complex and any irritation experienced. Weekly telephone follow-up was conducted to document difference in primary and secondary endpoints. Weights of test products prior to dispensing and at the end of the last visit were performed to determine amount of actual product used.
Results
Raw data were not always normally distributed by the Shapiro-Wilk test, so the data was analyzed by the non-parametric Wilcoxon Signed Rank test. Significant p-values (p<0.05) were analyzed with the Wilcoxon Signed Rank test to reveal the pair (s) of data (e.g., comparison with baseline or Visit 1) with a significant (p<0.05) difference, as highlighted in red. Correction was made from baselines values.
Melanin index. Median values at 1 hour (hr) and 2hr were compared with baseline for the left, right, and center of face at each of three treatment visits (Table 1) by the Wilcoxon Signed Rank test. Differences from baseline were significant for PolyGF (center) at 1hr on 7/18/18 and for Control (center) at both 1hr and 2hr on 8/15/18. Comparisons were not made between 1hr and 2hr but from baselines. To validate comparisons between the PolyGF and control groups, baseline data for PolyGF and Control were compared with each other at left, right, and center, and differences were not significant.
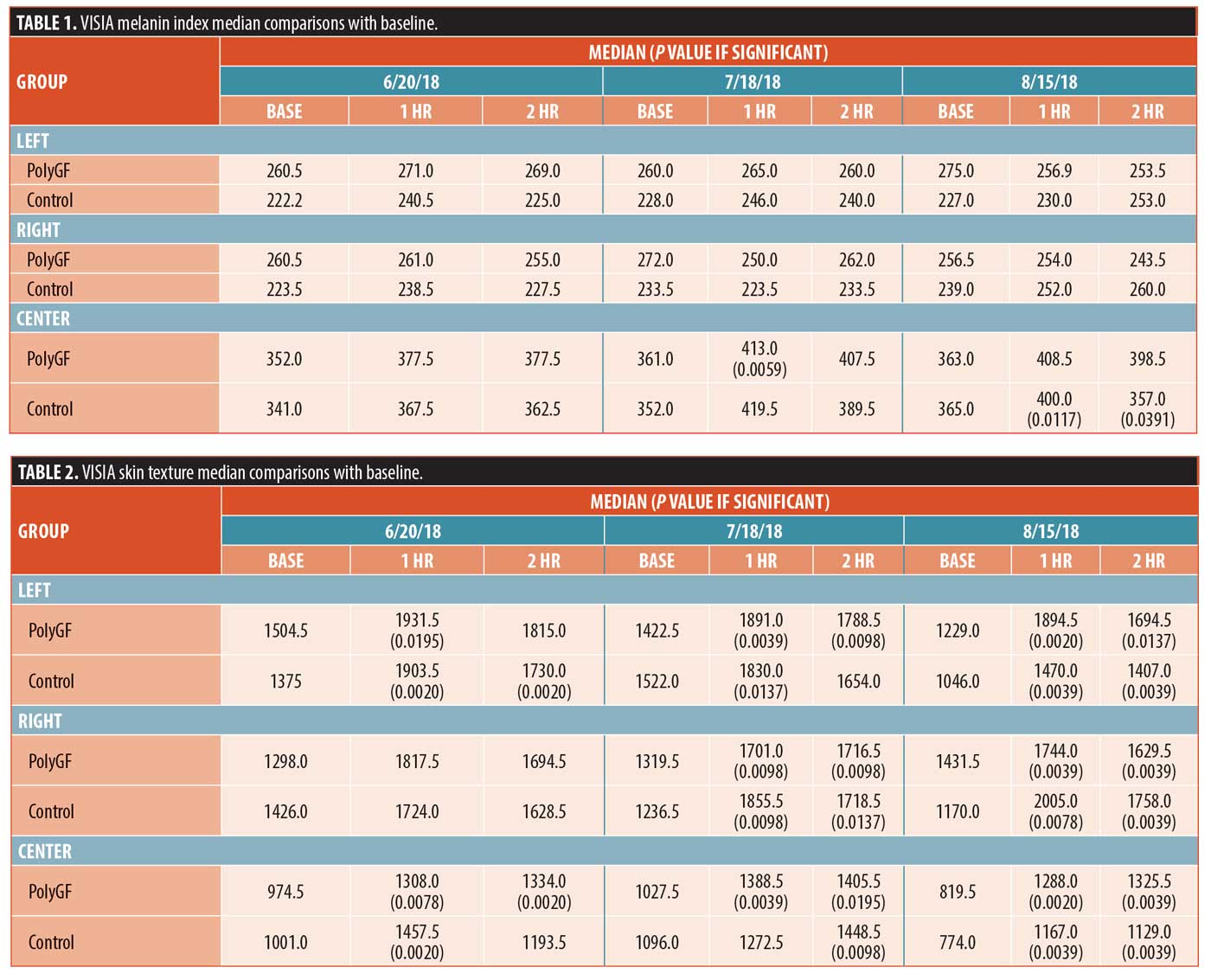
Skin texture. Significant differences in skin texture from baseline was seen in the 1hr groups for the left and center PolyGF and control groups, and the 2hr left control and center PolyGF groups on 6/20/18. On the 7/18/18 date, significant skin texture differences from baseline for all PolyGF groups, for the 1hr left control group, 1hr and 2hr right control group, and 2hr right control group. Lastly, for the 8/15/18 date, significant differences from baseline for all 1hr and 2hr left, right, and center groups were seen. Baseline data for PolyGF and control were compared with each other at left, right, and center, and differences were not significant, showing that the skin texture for the PolyGF and control groups at baseline were equivalent.
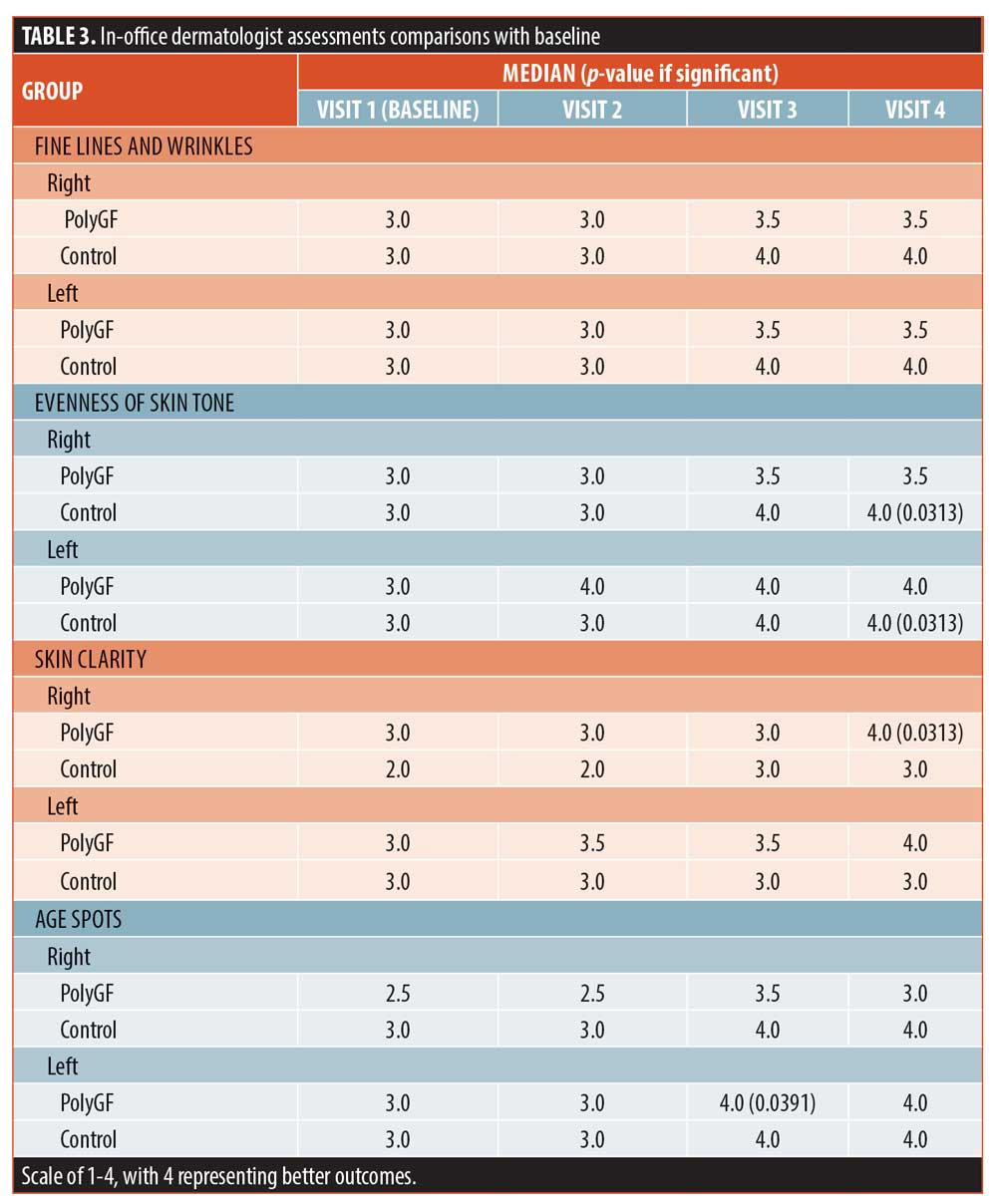
Dermatologist assessments. Provider assessments were significant for left and right controls at Visit 4 for evenness of skin tone, skin firmness, and hydration of skin. Controls were also significantly improved by provider assessment for the right facial skin firmness at Visit 3. The PolyGF group was significant for right facial skin clarity at Visit 4, left facial age spots at Visit 3, left and right facial skin smoothness at Visits 3 and 4, right facial skin firmness at Visit 4, and left and right facial skin hydration at Visits 2, 3, and 4 (Table 3). Baseline data for PolyGF and control were compared with each other at left and right for each skin attribute and differences were not significant, showing the PolyGF and Control groups at baseline were equivalent for each skin attribute.
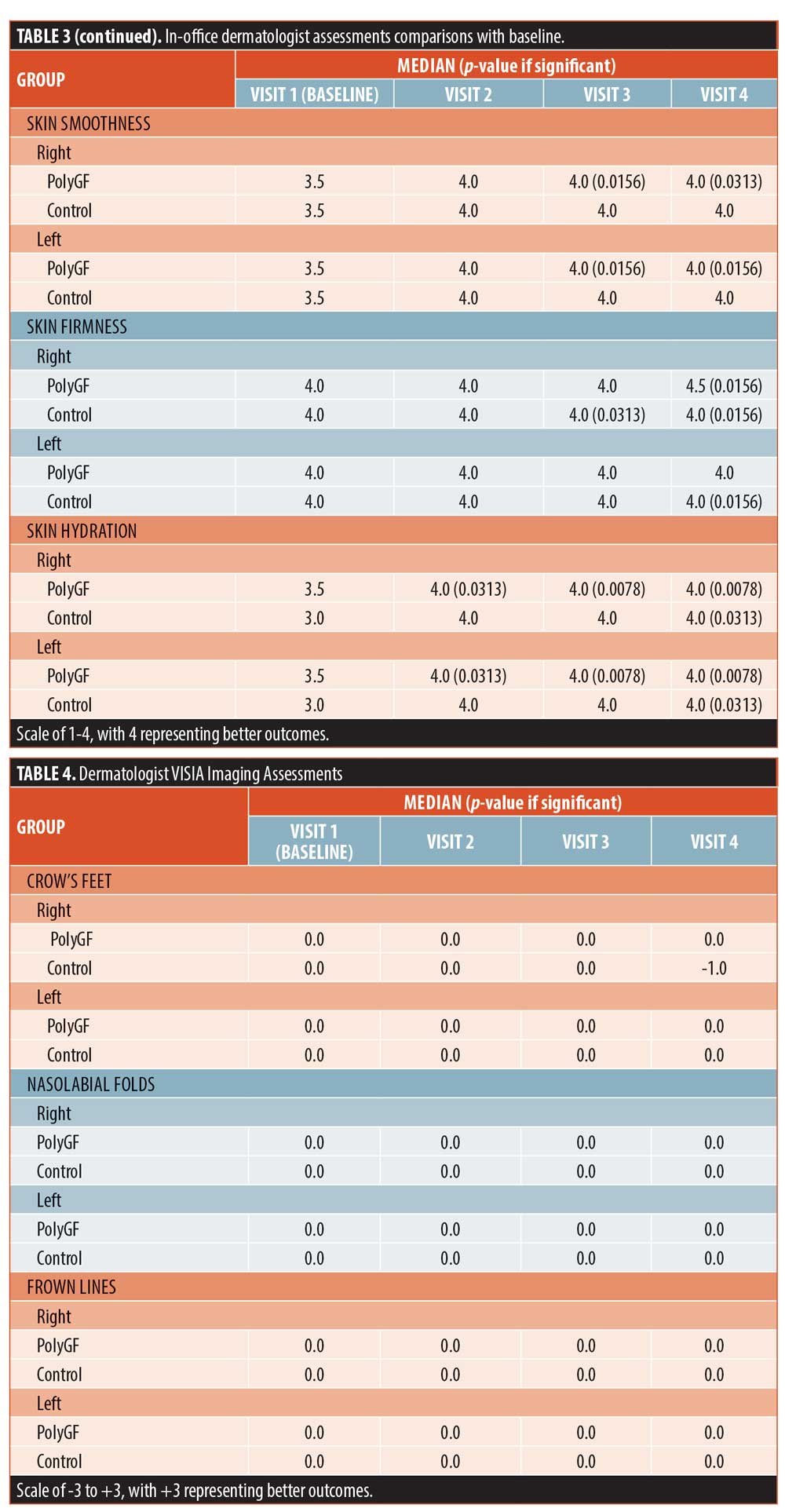
Imaging assessments. VISIA imaging analysis was only significantly changed for skin texture in the PolyGF group at Visits 2, 3, and 4 and for the left face of controls at Visit 4. Baseline data for PolyGF and control were compared with each other at left and right for each skin attribute and differences were not significant, showing the PolyGF and control groups at baseline were equivalent for each skin attribute (Table 4).
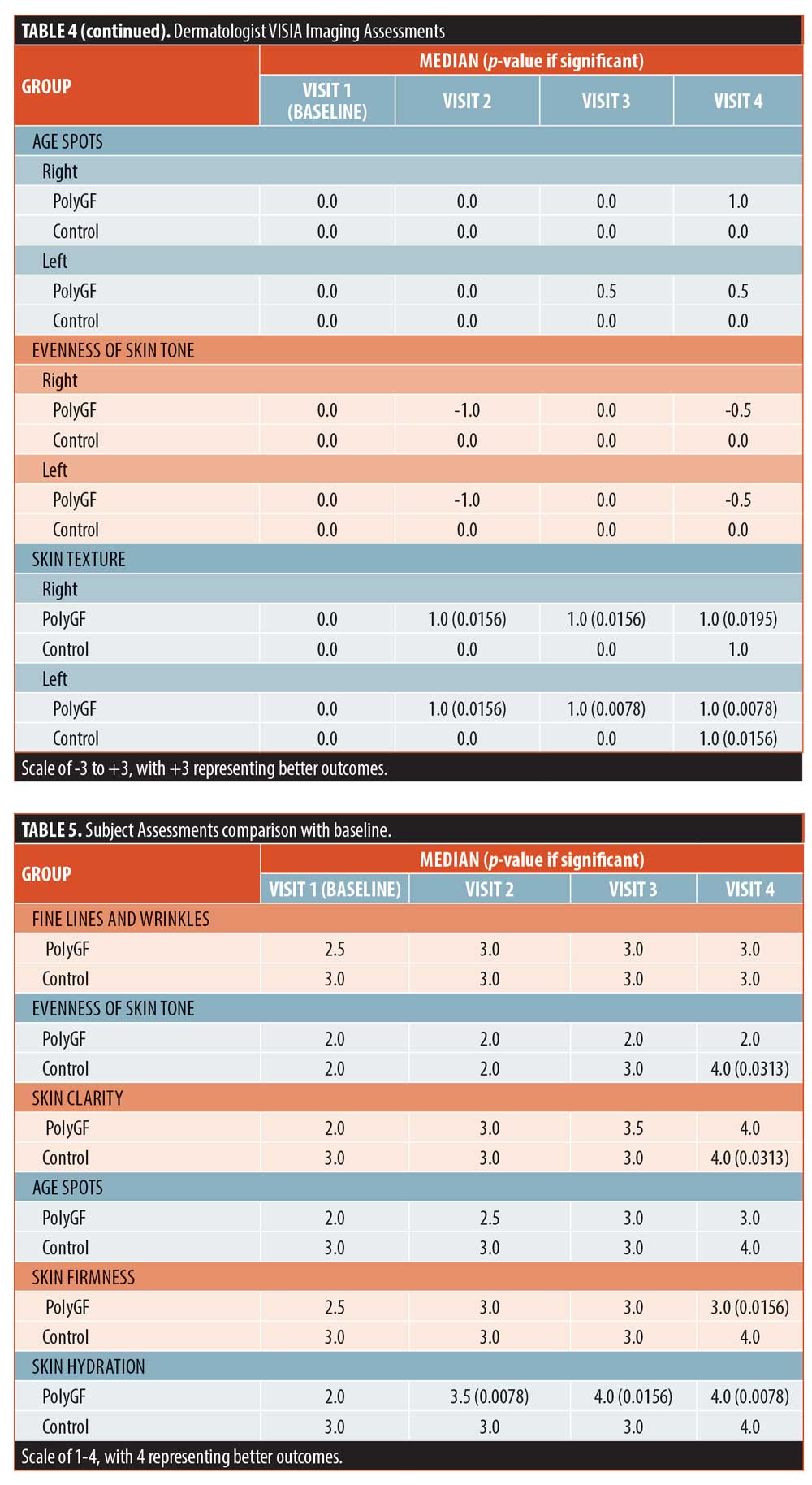
Subject assessments. Subject assessments were significant for controls in evenness of skin tone and skin clarity at Visit 4, and in the PolyGF groups for skin firmness (Visit 4) and hydration of skin at Visits 2, 3, and 4. Baseline data for PolyGF and control were compared with each other for each skin attribute and differences were not significant, showing the PolyGF and control groups at baseline were equivalent for each skin attribute. There were no adverse effects reported.
Discussion
Topical application of growth factors with microneedling has the potential to improve signs of skin aging. Clinical manifestations of intrinsic and extrinsic skin aging include pigmentary alterations, skin thinning, wrinkle and fine line formation, loss of hydration, and elasticity. Growth factors act as chemical messengers which mediate cell proliferation, reparative processes, and ECM formation,7 and microneedling creates controlled micropunctures in the skin, inducing collagen formation, neovascularization, and wound healing.10
The use of growth factors with microneedling shows encouraging results for collagen remodeling. A case series described the use of topical growth factors to photodamaged skin in eight patients after microneedling treatments spaced 10 days apart for three total treatments. The growth factor gel contained epidermal growth, fibroblast growth factor, hepatocyte growth factor, and insulin-like growth factor. Independent physician clinical evaluations via the Fitzpatrick Wrinkle Scale concluded a significant improvement in texture, fine lines, and wrinkles.7 Another study looked at 25 patients in a randomized controlled split-face study and concluded that dermaroller microneedling with growth factor resulted in statistically significant improvements in pigmentation and wrinkles.11 In a split-face trial of ten patients, El-Domyati et al12 studied manual dermarollers use with amniotic-fluid derived stem cells on acne scars. Although the serum itself did not contain growth factors, stem-cell therapies stimulate paracrine secretion of growth factors, extracellular matrix molecules, and interleukins. After five sessions that were one month apart, the authors found improvements in histological architecture of collagen and elastin, as well as statistically significant histological and clinical improvements in epidermal thickness and scar appearance.12 Another study utilizing human embryonic stem-cells and shallow microneedling (0.25mm roller) showed statistically significant improvements in pigmentation and wrinkles by physician and software-analysis after five sessions that were two weeks apart.13
Autologous platelet-rich plasma (PRP), of recent interest for collagen remodeling and hair growth, has promising results as well. Activated platelets release growth factors, promoting collagen synthesis. A split-face study of 50 patients who received three dermaroller sessions with PRP spaced one month apart, showed a 16.36-percent improvement of acne scarring on Goodman’s Quantitative Scale.14 Another split-face trial comparing PRP to vitamin C for acne scarring, with four dermaroller microneedling sessions spaced one month apart found superior results with PRP; 21 out of 27 patients treated by PRP and microneedling achieved 1–2 factor improvement on the Goodman scale, compared with 17 with vitamin C and microneedling.15 A study of 35 patients performed a split-face trial of four treatments of dermaroller microneedling spaced +/- PRP, spaced three weeks apart for acne scarring and found slightly improved results on the PRP side (Goodman and Baron mean scores decreased fom 3.2+/-0.7 to 1.8+/-0.6 on the PRP side and to 2.1+/-1.1 on the non-PRP side, with both sets of p-values being <0.001).16
Limitations. Limitations of our study included mostly subjective assessments, and a limited number of subjects. Microneedling depth was set to only 0.5mm and is considered a very superficial treatment. It is possible that the growth factors did not reach the essential depth for proper action. Additionally, factors such as evenness of skin tone and improvement of wrinkles are often time-dependent, and clinical improvements might not be apparent for several months. The study was done in the summer, when some subjects did not fully adhere to limiting their sun exposure. A final assessment several months after the study may be conducted to assess for additional improvements. Lastly, a split-face trial where the growth factor was only used on half the face might have been more appropriate to further validate the results.
Conclusions
Based on the results of our study, we conclude that growth factor in conjunction with microneedling is safe and effective for improvements in several skin parameters. The novel PolyGF serum improved subject and dermatologist assessment of hydration of skin, imaging assessment of skin texture, and dermatologist assessment of skin smoothness and texture. Dermatologist assessment improvements of evenness of skin tone, melanin index, texture, and firmness, and subject assessment of clarity was seen in the controls. Similarly, multiple studies have shown significant improvements in skin texture, fine lines, wrinkles, pigmentation, acne scarring, and histological and clinical improvements in epidermal thickness and scar appearance with microneedling and growth factors. Further studies are needed to characterize the effects of topical growth factors as adjuvants to dermatologic procedures.
Acknowledgements
We thank Fred Wilson, MS for the statistical analysis; and Paul Guilbaud and David Bryant for their guidance with the protocol.
References
- Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging, state of the art. Annals NY Acad Sci. 2005;1119:40–50.
- Mukhurgee PK, Maity N, Neema NK, Sarkar BK. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19:64–73.
- Aldag C, Teixeira DN, Leventhal PS. Skin rejuvenation using cosmetic products containing growth factors, cytokines and matrikines: A review of the literature. Clin Cosmet Investig Dermatol. 2016;9:411–419.
- Sproul EP, Argraves WS. A cytokine axis regulates elastin formation and degradation. Matrix Biol. 2013;32(2):86–94.
- Uitto J, Kouba D. Cytokine modulation of extracellular matrix gene expression: relevance to fibrotic skin diseases. J Dermatol Sci. 2000;24(Suppl 1):S60–S69.
- Verrecchia F, Mauviel A.Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007;13(22):3056–3062.
- Pamela, Ruri D. Topical growth factors for the treatment of facial photoaging: A clinical experience of eight cases. J Clin Aesthet Dermatol. 2018;11(12):28–29.
- Mehta RC, Fitzpatrick RE. Endogenous growth factors as cosmeceu- ticals. Dermatol Ther. 2007;20(5):350–359.
- Schaefer H, Lademann J. The role of follicular penetration. A differential view. Skin Pharmacol Appl Skin Physiol.
2001;14(Suppl 1):23–27. - Singh A, Yadav S. Microneedling: Advances and widening horizons. Indian Dermatol Online J. 2016;7(4):244–254.
- Lee HJ, Lee EG, Kang S, et al. Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: A randomized, controlled, blinded split-face study. Ann Dermatol.
2014;26(5):584–591. - El-Domyati M, Moftah NH, Nasif GA, et al. Amniotic fluid-derived mesenchymal stem cell products combined with microneedling for acne scars: A split-face clinical, histological, and histometric study. J Cosmet Dermatol. 2019
- Jung Lee H, Gin Lee E, Sung JH, Chung HM, Kim DH. Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: A randomized, controlled, blinded split-face study. Ann Dermatol. 2014;26(5):584–591.
- Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling with distilled water in the treatment of atrophic acne scars: A concurrent split-face study. J Cosmet Dermatol. 2016;15(4):1–10.
- Chawla, S. Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7(4):209–212.
- Ibrahim MK, Ibrahim SM, Salem AM. Skin microneedling plus platelet-rich plasma versus skin microneedling alone in the treatment of atrophic post acne scars: A split face comparative study. J Dermatolog Treat. 2018; 29(3):281–286.

