 J Clin Aesthet Dermatol. 2020;13(11):19–21
J Clin Aesthet Dermatol. 2020;13(11):19–21
by Michael McBride, DO; Benjamin M. Witkoff, DO; and Shannon C. Trotter, DO
Dr. McBride is a dermatology resident with HonorHealth/Affiliated Dermatology in Phoenix, Arizona. Dr. Witkoff is a resident intern physician from Larkin Community Hospital, Palm Springs Campus in Hialeah, Florida. Dr. Trotter is with Oakview Dermatology and is Associate Program Director and Clinical Instructor for the Ohio Health Dermatology Residency Program in Columbus, Ohio.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevent to the content of this article.
ABSTRACT: Hailey-Hailey (HHD), or benign familial chronic pemphigus disease, is a rare autosomal dominant blistering disorder characterized by recurrent vesicles that erode and macerate into weeping and crusting plaques. HHD has been shown to be resistant to several treatment options. Although not yet approved as a treatment for HHD, recent reports have suggested the use of low-dose naltrexone (LDN) as a successful treatment option for controlling recalcitrant HHD. We present a case of a 50-year-old woman with a 20-year history of biopsy-confirmed HHD with recurrent painful and pruritic vesicles and plaques. The patient developed significant clinical improvement of the cutaneous lesions with LDN treatment after only 26 days of treatment. It is important for dermatologists to consider LDN as a viable treatment option for HHD, especially in recalcitrant patients. We suggest this novel treatment as a rapidly effective option to resistant HHD.
KEYWORDS: Hailey-Hailey disease, recalcitrant Hailey-Hailey disease, low-dose naltrexone, benign familial chronic pemphigus disease, blistering disorders
Hailey-Hailey disease (HHD), or benign familial chronic pemphigus disease, is a rare autosomal dominant severe blistering disorder. It is caused by mutations in the ATP2C1 gene on chromosome 3, which encodes a calcium transporter protein in the Golgi apparatus.1 The resulting calcium transporter dysfunction results in the loss of epidermal keratinocytic adhesion and subsequent acantholysis. This is manifested clinically as chronic and recurrent vesicles that erode and macerate into weeping and crusting plaques.2 These lesions mostly present in the intertriginous areas.3 The vesicles and plaques subsequently become painful and pruritic with an increased risk of developing infection and possibly squamous cell carcinoma.4,5
Histologically, HHD is characterized by incomplete suprabasal acantholysis, giving the appearance of a “dilapidated brick wall” in the epidermis.6 HHD can be differentiated from other acantholytic disorders from its characteristic red dyskeratotic rim that surrounds the keratonicyte nucleus.
Traditional treatments for HHD are often unsuccessful in providing a long-term relief for majority of patients.7 Herein, we present a patient that suggests low-dose naltrexone (LDN) as a novel and reliable treatment option for a patient with recalcitrant HHD.
Case Report
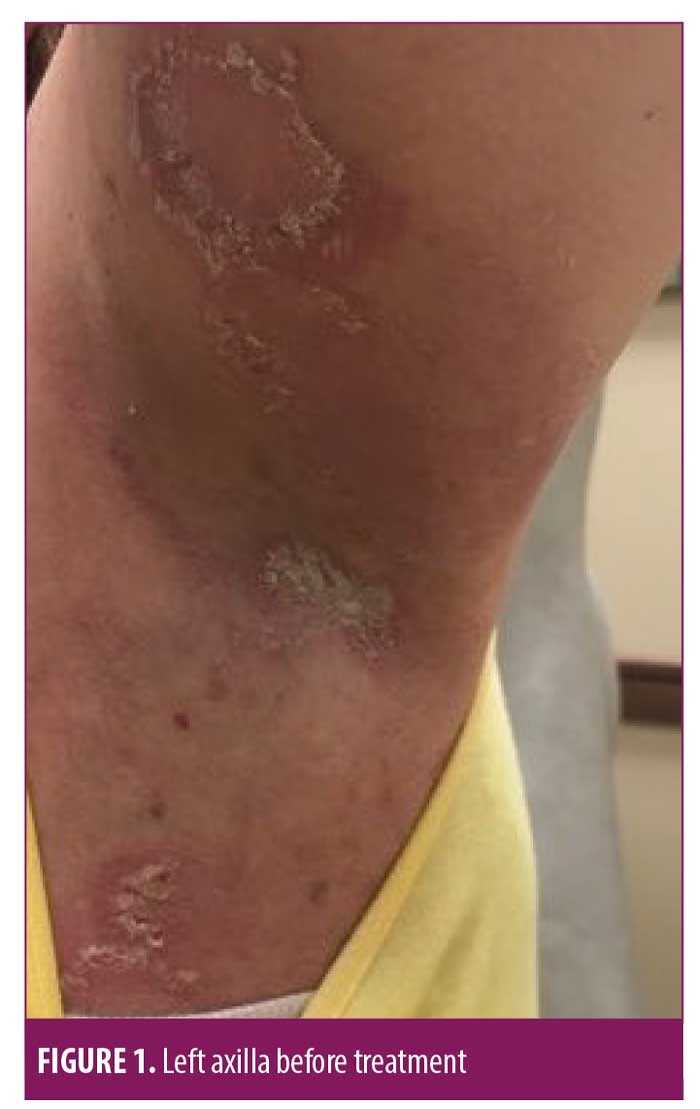
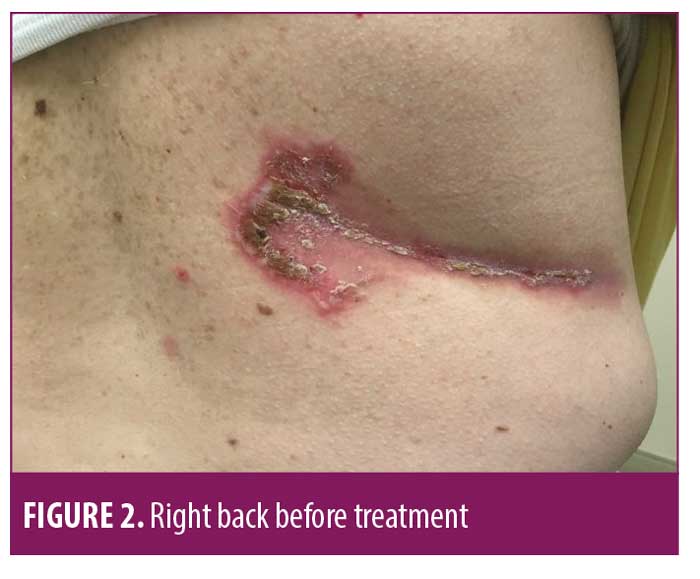
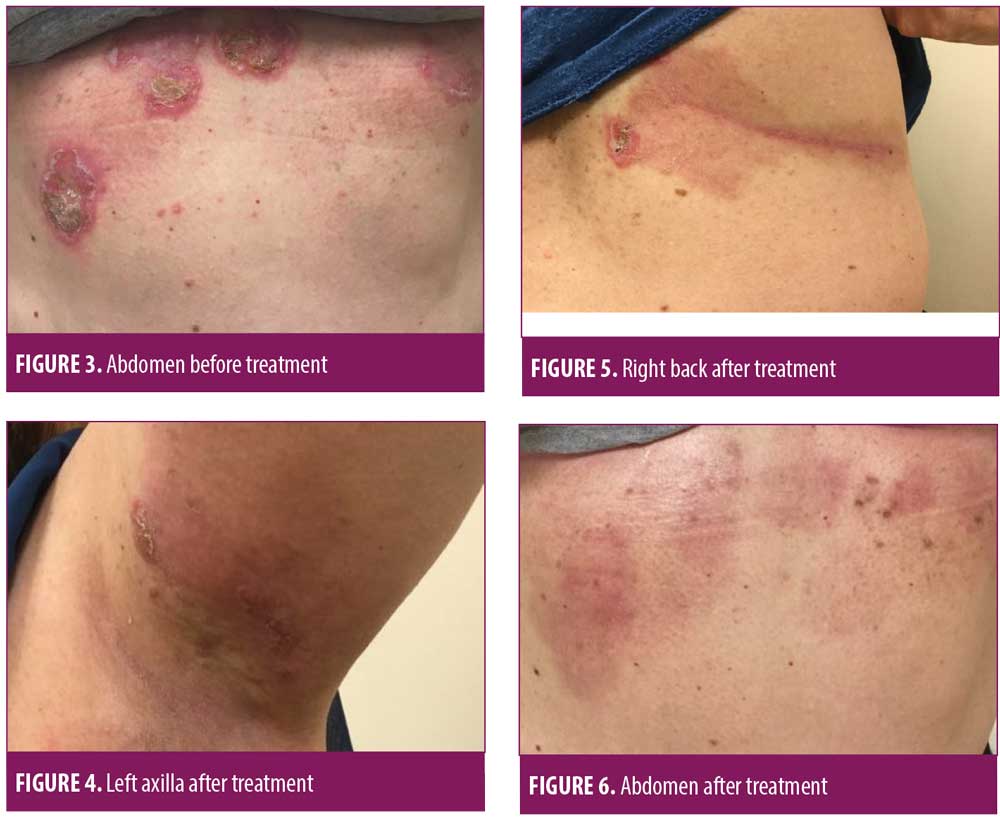
On July 6, 2018, a 50-year-old Caucasian female was referred for evaluation of recurrent flares of biopsy-confirmed HHD (biopsy completed by her previous physician). She had experienced recurrent flares of the disease for over 20 years. Upon exam, she had erosive and vesicular plaques with yellow crusting on her left and right axillae, right back, abdomen, and legs (Figures 1–3). She reported that the lesions were itchy and painful. Upon arrival, her current medications consisted of chlorhexadine wash, antiperspirant spray, triamcinolone 0.1% cream, and desoximetasone 0.5% cream. A review of her medical record showed unsuccessful treatment for the disease with recurrent flares and suprainfections dating back to September 2006 through February 2018. She had completed multiple rounds of several different topical and oral antibiotics, topical and oral antifungals, topical steroids, oral steroids, intramuscular steroid injections, glycopyrronium bromide, and antihistamines. She was instructed to discontinue the triamcinolone 0.1% cream and desoximetasone 0.5% cream, and to continue on the chlorhexadine wash and antiperspirant spray. Additionally, she was started on fluocinonide 0.1% topical cream to be applied to the lesions and mupirocin 2% topical ointment applied twice daily to impetiginous lesions. After reviewing potential new treatment options for HHD, LDN 1.5mg at bedtime was also added to her treatment regimen.
At her four-week follow-up appointment, the patient reported pink patches and crusted pink plaques with mild to moderate improvement in her lesions (Figures 4–6). She reported the pain from her lesions had significantly decreased, and she was satisfied with her improvements. She reported taking the naltrexone daily but only used the fluocinonide 0.1% cream and chlorhexadine wash a few times throughout the month. The only side effect from her treatment regimen was vivid dreams, which she said were tolerable. She was instructed to continue with her treatment regimen and was scheduled for follow up in 12 weeks.
Discussion
Traditionally, treatments for HHD include oral and topical antibiotics, oral and topical corticosteroids, cyclosporine, dapsone, and methotrexate for patients with recalcitrant disease.7 However, these agents often fail to provide long-term relief for the majority of patients.8 Recently, two published case reports suggested LDN to be a successful treatment option for patients with recalcitrant HHD.9,10
Currently, naltroxone extended release injectable suspension is approved for the treatment of alcohol dependence in patients, who are able to abstain from alcohol in an outpatient setting prior to its initiation, and opioid dependence, following opioid detoxification.11 The use of LDN for HHD is currently off-label.
Despite its off-label use, LDN has recently shown potential improvement in several different inflammatory diseases, including fibromyalgia, Crohn’s disease, regional pain syndrome, chronic pruritus, and multiple sclerosis.12 At lower dosages (1.5mg to 4.5mg), naltrexone exhibits paradoxical properties, including anti-inflammatory and analgesic actions.13-15
By acting as an antagonist, it is hypothesized that LDN exhibits a partial blockade on mu-opioid receptors causing a systemic analgesic effect.15 These opioid receptors are present in keratinocytes and act to improve wound healing.16 LDN acts on these receptors to destabilize intercellular adhesion within keratinocytes and promote the migratory keratinocyte phenotype, which is required for fast wound closure of the skin.17 It can be hypothesized that the action on the keratinocyte mu-opioid receptors within the basal layer of the epidermis causes increased cellular adhesion and healing of the skin lesions seen in our patient with HHD.9,10
LDN has also been shown to antagonize toll-like-receptor (TLR) complexes found in the body.18 TLRs are found diffusely throughout the body, including microglia in the central nervous system and keratinocytes within the epidermis. The TLR found in the microglia of the central nervous system are responsible for producing pro-inflammatory cytokines, substance P, reactive oxygen species, and excitatory amino acids.19 LDN can act to inhibit the production of these molecules and decrease systemic inflammation. Also, keratinocytes express TLR receptor complexes. When activated, the TLR initiates a rapid intracellular Ca2+ response, NF-kappaB nuclear translocation, and the production of pro-inflammatory cytokines and chemokines including IL-1alpha, IL-1beta, and IL-8, which initiate a host pro-inflammatory response similar to that described by microglia.18 By antagonizing these TLR complexes, naltrexone can be thought to decrease this downstream signaling cascade and reduce inflammation within the epidermis. This mechanism may be another reason to explain why our patient with HHD improved after taking LDN.
Although the exact mechanism leading to clinical improvement in our patient with HHD is not fully understood, recent studies explaining LDN effects on decreasing local and systemic inflammation and improving cutaneous wound healing may help explain our finding. More research must be done to determine LDN’s use for improving recalcitrant cases of HHD. However, LDN appears to be a promising treatment for HHD.
References
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24(1):61–65.
- Chiaravalloti A, Payette M. Hailey-Hailey disease and review of management. J Drugs Dermatol. 2014;13(10):1254–1257.
- Peluso AM, Bonifas JM, Ikeda S, et al. Narrowing of the Hailey-Hailey disease gene region on chromosome 3q and identification of one kindred with a deletion in this region. Genomics. 1995;30(1):77–80.
- von Felbert V, Hampl M,Talhari C, et al. Squamous cell carcinoma arising from a localized vulval lesion of Hailey-Hailey disease after tacrolimus therapy. Am J Obstet Gynecol. 2010;203(3):e5–e7.
- Holst VA, Fair KP, Wilson BB, et al. Squamous cell carcinoma arising in Hailey-Hailey disease. J Am Acad Dermatol. 2000;43(2 pt 2):368–371.
- Haber H, Russell B. Sisters with familial benign chronic pemphigus (Gougerot, Hailey and Hailey). Proc Royal Soc Med. 1950;43(7):558–560.
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76(3):551–558.e3.
- Izumi AK, Shmunes E, Wood MG. Familial benign chronic pemphigus. The role of trauma including contact sensitivity. Arch Dermatol. 1971;104:177–181.
- Ibrahahm O, Hogan SR, Vij A, et al. Low-Dose Naltrexone Treatment of Pemphigus (Hailey-Hailey Disease). JAMA Dermatol. 2017;153(10):1015–1017.
- Albers LN, Arbiser JL, Feldman RJ. Treatment of Hailey-Hailey Disease with Low-Dose Naltrexone. JAMA Dermatol. 2017;153(10):1018–1020.
- Vivitrol [package insert]. Waltham, MA. Alkermes, Inc; 2015.
- Younger J, Parkitny L, Mclain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33(4):451–459.
- Smith JP, Bingaman SI, Ruggiero F, et al. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial. Dig Dis Sci. 2011;56(7): 2088–2097.
- Cree BA, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol. 2010;68(2):145–150.
- Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009;10(4):663–672.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72(3):333–337.
- Bigliardi PL, Neumann C, Teo YL, et al. Activation of the delta-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration. Br J Pharmacol. 2015;172(2): 501–514.
- Song PI, Park YM, Abraham T, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002;119(2):424–432.
- Harry GJ, Kraft AD. Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008;4(10):1265–77.

