 J Clin Aesthet Dermatol. 2020;13(11):28–31
J Clin Aesthet Dermatol. 2020;13(11):28–31
by Dennis Niebel, MD; Sietske Poortinga, MD; and Jörg Wenzel, Prof, MD
Drs. Niebel, Poortinga, and Wenzel are with the Department of Dermatology and Allergy, University Hospital Bonn at Bonn University in Bonn, Germany.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: The development of calcium salt deposits in the skin can occur in the presence or absence of membranous ossification and are categorized into osteoma cutis (i.e., cutaneous osteoma) and calcinosis cutis. For the former, distinction into primary or secondary osteoma cutis is mainly based on clinical and histopathological parameters, as primary osteoma cutis originates without any underlying intradermal inflammatory or neoplastic process, as opposed to a far greater number of secondary osteoma cutis that occur on the grounds of inflammation or tumors. Genetic disorders might predispose a person to the formation of these overall rare tumors. However, some patients develop primary osteoma cutis in the absence of any genetic background. In pre-menopausal women with fair skin, the condition of multiple miliary osteoma cutis is a relevant differential diagnosis for solid subcutaneous facial nodules. While pathogenesis remains unclear, most affected individuals have suffered from acne vulgaris at some point. Excision might be a viable option for disturbing lesions, as are ablative lasers. Here, we discuss and review relevant causes of calcium salt deposits in the skin based on a notable case of multiple primary osteoma cutis of the face in an otherwise healthy woman.
KEYWORDS: Osteoma, osteoid, calcinosis, pseudohypoparathyroidism, acne vulgaris
Osteoma cutis (OC) is a benign condition defined as the eruption of an osseous structure in the skin, and is distinguishable from calcinosis cutis (CC).1 The former arises from membranous ossification without cartilage as a precursor; an interchangeable term is cutaneous osteoma. The latter is defined by calcium salt deposits in the absence of osteoid, which itself comprises osteoblasts, osteoclasts, and hydroxyapatite as obligatory components.
OC may be divided into a primary and secondary type, depending on the clinical and histopathological parameters. Primary OC originates without any underlying intradermal inflammatory or neoplastic process, whereas secondary OC is most often associated with chronic inflammatory diseases that lead to the degradation of collagen fibers, with other causes, including trauma and neoplasms.2,3 Traditionally, four types of primary OC have been distinguished: isolated, plaque-like (also “plate-like”), widespread, and multiple miliary osteoma cutis (MMOC).
CC can be divided into a dystrophic type, which is associated with tissue damage, and a metastatic type, which is due to elevated calcium or phosphate levels. The remaining cases are either iatrogenic or idiopathic CC, which are later discussed. The severe condition of calciphylaxis, defined by generalized calcium deposits in the small vessels, will not be further discussed in this article.
Here, we describe a rare case of multiple subcutaneous nodules on the forehead of an otherwise healthy female patient, which could be identified as symptomatic primary OC in the absence of a predisposing genetic background. Furthermore, we provide a short review of the diagnostic and therapeutic options both for OC and CC.
Case Report
History. A 39-year old Caucasian female patient (Fitzpatrick Skin Type III) sought consultation in our clinic due to the development of slowly extending solid subcutaneous nodules on her forehead. Small lesions had been noticed initially during her first pregnancy eight years earlier. There were no relevant comorbidities or comedications, nor was there any family history of dermatological diseases. During childhood, she had required sutures to the left side of her forehead, but no injury or trauma was reported apart from that. In adolescence, the patient suffered from moderate acne vulgaris, though she had received no specific treatment at that time. Continued occasional manipulation of the facial skin was reported upon questioning, which we interpreted as a mild form of excoriation disorder. However, according to the patient, this behavior did not result in any scarring skin lesions. The patient requested a therapy for the most bothersome nodules, as they were causing tenderness upon palpation and she described a feeling of disfigurement.
Laboratory investigations. Serum levels of calcium, phosphate, and parathormone were normal. There was no evidence of organ dysfunction (renal, hepatic) and the full blood count was normal. The 25-OH-vitamin D level was low: 11.9ng/ml (20 to 70ng/ml).
Clinical picture. Multiple firm subcutaneous nodules measuring 1 to 4mm in diameter were dispersed, but strictly limited to the forehead. There was no erythema or clinical clues to indicate active acne vulgaris or rosacea (Figure 1). A full body examination revealed no signs of autoimmune disorders, such as systemic sclerosis or lupus erythematodes.
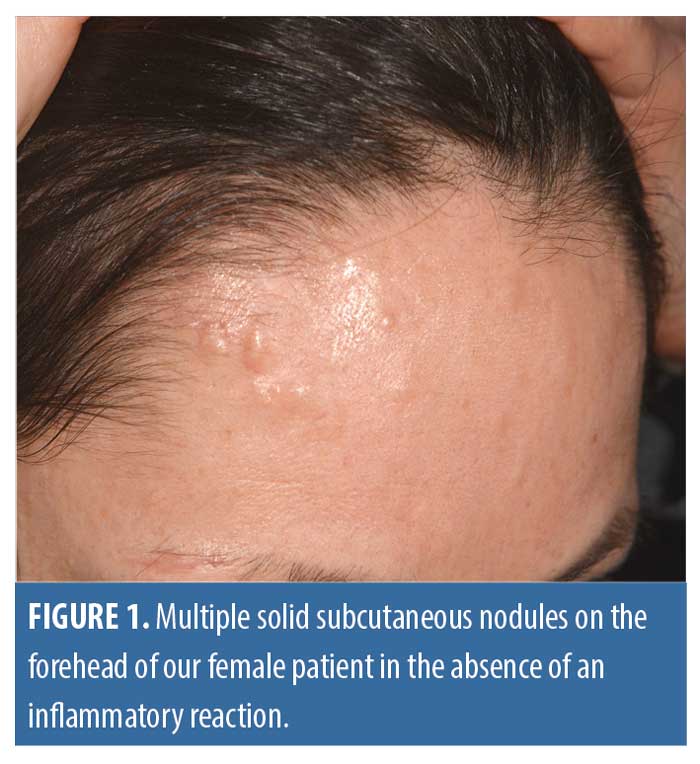
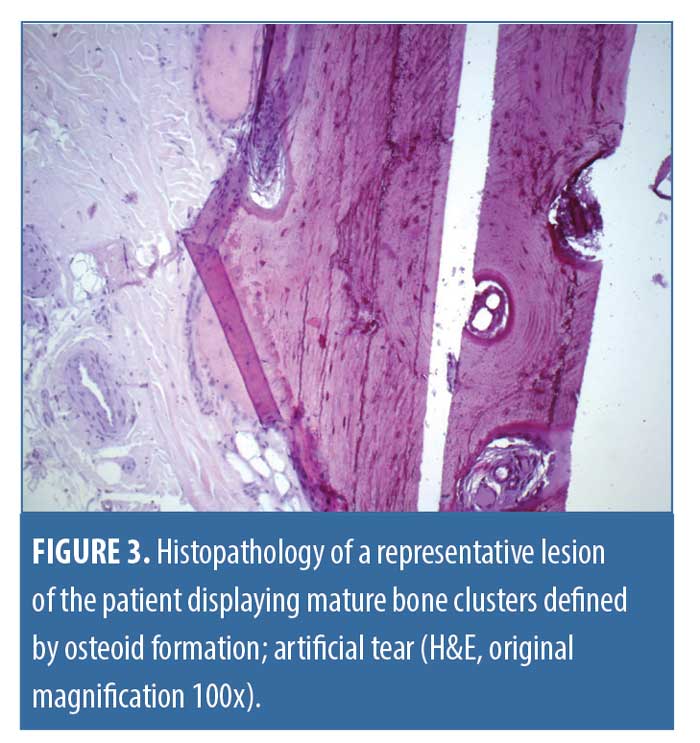
Histology. Consent was granted, and we performed a full excision of the largest lesions. Upon histological examination, there were plentiful pale eosinophil material within the cutis and subcutis, which was identified as mature osteoid due to its lamellar arrangement without any spicules or dysplastic features. Processing of the paraffin embedded sections triggered artificial tears. There were no signs of hematopoiesis (Figures 2 and 3) or any relevant inflammatory infiltrate, and, in particular, there was no chronic granulomatous foreign-body type reaction. Apart from the pronounced seborrheic glands, no other specific features or histopathologic signs of underlying chronic inflammatory skin diseases could be detected.

Outcome. The patient was satisfied with the cosmetic result. Although repeated treatment might be necessary in the future in case of extension of the remaining lesions or de novo eruption of more OC. In the absence of evidence based preventive measures, no further therapy was proposed, apart from the correction of the low Vitamin D level by supplementation. It was advised that skin picking should be ceased if possible; counseling, including behavioral treatments, could be beneficial as a supportive measure.
Discussion
OC usually appears as solid, skin-colored subcutaneous nodules of variable sizes and shapes. Signs of inflammation might also be present. The area of predilection is the face, and subcutaneous nodules of the face might resemble a multitude of different diagnoses, including benign and malign tumors and infectious or inflammatory diseases. In younger otherwise healthy patients, epidermoid cysts, fibromas, and lipomas are among the most frequent differential diagnoses. Skin-colored papules on the face, in combination with seborrhea, are likely to be closed comedones of acne vulgaris. Considering the tenderness of the lesions, rare tumors like angiolipoma, eccrine spiradenoma, neurofibroma, granular cell tumor, or leiomyoma may be taken into consideration. However, they are generally not as solid as osteomas and tend to show a reddish-brown coloration. Seldom infections, such as actinomycosis, and granulomatous diseases (such as sarcoidosis or subcutaneous granuloma annulare) might evoke quite firm lesions. On the other hand, in elderly patients, malignant epithelial tumors, like basal cell carcinoma and squamous cell carcinoma, are the most important differential diagnoses. In a relevant proportion of patients with cancer, cutaneous metastases of melanoma or other solid tumors might induce multiple subcutaneous nodules in end stage disease. Occasionally gout tophi or rheumatoid nodules might be found in acral areas of the face, such as the nose tip and ear. Finally, flat plaque-like osteoma cutis can have a similar appearance as localized scleroderma (morphea) on the head.4
Eighty-five percent of OC cases are diagnosed in relation to some benign or malign neoplastic (e.g., pilomatricoma and basal cell carcinoma), inflammatory (e.g., systemic sclerosis and acne vulgaris), or traumatic process (scars and burns), and are hence called secondary OCs (Table 1).2 Overall, primary cutaneous osteomas are rare, but they can still frequently be found in certain genetic disorders (e.g., Albright’s hereditary osteodystrophia and progressive osseous heteroplasia). As such, a thorough family history is crucial. Albright’s hereditary osteodystrophia is characterized by the dysfunction of parathormone receptors. Apart from cutaneous symptoms, patients may exhibit specific phenotypic features, such as growth retardation and cognitive disability, teeth anomalies, and brachydactylie.5 A key feature of progressive osseous heteroplasia is the very early onset of cutaneous and soft tissue ossification without dysmorphic features. In both conditions, underlying mutations impair the intracellular G-protein subunit signaling. Gardner’s disease (familial hereditary polyposis syndrome) is also associated with primary OC next to other cutaneous manifestations (e.g., abundant epidermoid cysts and desmoid tumors), which might precede the development of eponymous colorectal polyps.6 As patients bear a dramatically increased risk of up to 50 percent for colorectal carcinoma, early colonoscopy should be assigned.7 Therefore, for young patients who present with cutaneous osteomas or CCs without any inflammatory or neoplastic trigger, exclusion of genetic disorders and hyperparathyroidism must be considered.
Solitary cutaneous osteomas are rare, and they might occur congenitally or de novo (“isolated osteoma”). Plaque-like osteoma cutis is defined as a singular extensive lesion of variable localization that usually occurs early in life. Numerous lesions in a single patient might most often be found on the face (MMOC), a historical term for which is “osteosis cutis multiplex faciei”. As clinical aspects of OC vary depending on the clinical subtype, they are sometimes an accidental histological finding. A basic diagnostic work up should include a full body examination and laboratory tests of the calcium phosphate axis, including parathormone.8 In cases of clinical uncertainty, further measures may be taken to increase diagnostic accuracy. Recently, Romero et al9 described the typical dermoscopic aspects of a plaque-like cutaneous osteoma, which included concentric structures in linear arrangement with an erythematous and nacreous pink border. So far, the typical dermoscopic features of other types of primary cutaneous osteoma are not well established. Solid subcutaneous nodules may be approached by complementary high-frequency sonography and conventional radiology (X-ray / computer tomography scan). In sonography, calcium is hyperechogenic and displays an acoustic shadow. Therefore, cutaneous osteomas can be identified easily by their distinctive pattern, especially if multiple nodules are present.10 On X-ray, cutaneous calcium deposits are radiopaque and appear like adjacent bones. OC is a frequent incidental finding of dental X-rays, and CT scans are often required to further localize the lesion as two dimensional X-rays can be treacherous.11 In a larger study, up to two percent of CT scans of the maxillofacial region showed OC.12 These findings indicate that facial cutaneous osteomas might be underreported due to subclinical variants and the benign course of the disease.3
The clinical picture of CC varies depending on its underlying cause. Although, most often firm, pale lesions are found of variable dispersion. Ulceration is most often present in mechanically challenged localizations, e.g., those adjacent to the joints. CC may be painful and lead to impaired movement or paresthesia. There are helpful algorithms for diagnostic workup similar to OC.13 In addition to the priorly mentioned laboratory investigations, albumin must be measured due to the high degree of protein binding of calcium ions. Triggers for calcium depositions might be local or systemic factors, and hence they are divided into dystrophic, metastatic, idiopathic, and iatrogenic causes. Dystrophic calcinosis is related to tissue damage caused by inflammatory (autoimmune: e.g., lupus erythematodes, and autoenzymatic/digestive: e.g., acute pancreatitis, and infectious: e.g., histoplasmosis), neoplastic (e.g., atypical fibroxanthoma) or traumatic processes (e.g., posttraumatic panniculitis ossificans). CCs caused by genetic disorders are also considered dystrophic (e.g., Ehlers-Danlos and Werner Syndromes). Some conditions are defined by a predisposition to both OCs and CCs (e.g., progressive osseous heteroplasia). Benign tumors, such as trichoepitheliomas and epidermoid cysts (especially of scrotal localization), frequently develop calcification over the course of time (Figures 4 and 5). Numerous internal conditions might interfere with calcium and phosphate levels and cause metastatic calcinosis (e.g., renal failure, sarcoidosis, myeloma, and hyperparathyroidism), which will not be further discussed here. The exuberant intravenous substitution of calcium salt solutions is the main trigger of iatrogenic calcinosis, apart from a dramatic increase of calcium serum levels caused by abrupt tumor lysis. The remainder of cases are dubbed “idiopathic” calcinosis, indicating that the pathogenesis and etiology have, until now, remained unclear. Among these, subepidermal calcified nodules are mainly found on the face and scalp of children. An idiopathic calcification of the primary genital organs (penis and vulva) can be found without having caused any symptoms in a significant number of young adults. Finally, patients with organ transplants occasionally develop calcinosis cutis without any reasonable cause (“transplant-associated calcinosis cutis”). As a rule of thumb, abnormal serum levels of calcium or parathormone are indicative of an increased risk of calcium depositions even in the absence of tissue damage.13
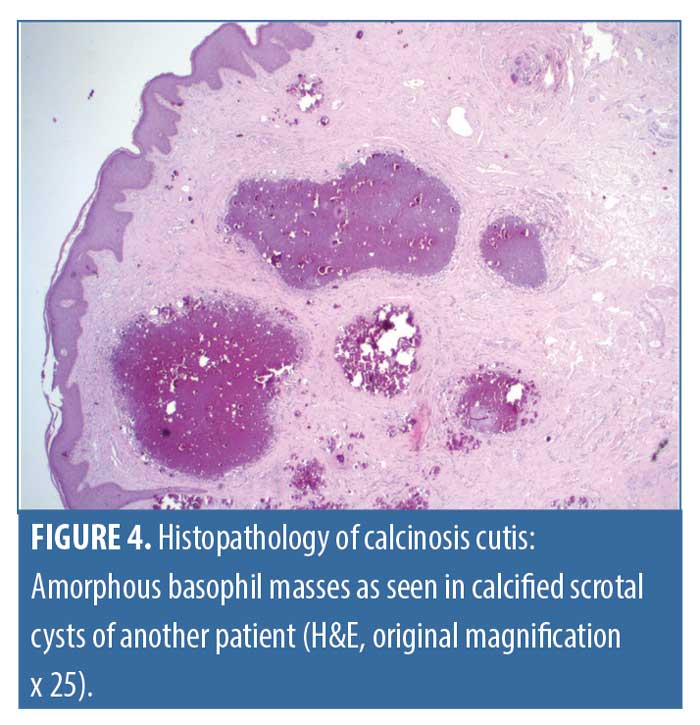
CC can be approached with the same radiological techniques as OC. Histologically, it is defined by calcium salt depositions in the absence of osteoid and metaplastic osteoblasts or osteoclasts. Heterogeneous basophil masses are a key feature that are often located in proximity to the vessels and can be accompanied by a foreign-body giant cell reaction.14
In the case of our patient, the presentation could have been seen as a typical MMOC of the face, yet the etiology of this particular type of primary OC remains unclear. Our patient did not display any sign of active acne vulgaris; therefore, we did not consider it a pivotal factor in this case. Given the frequency of acne vulgaris in the general population, there must be accompanying factors that have not yet been identified. One explanation for the development of mature bony structures in the skin is that chronic inflammation might induce metaplasia of the mesenchymal cells, such as fibroblasts converting into osteoblasts.3 Another theorem is that OC might be a result of an aberrant migration of osteoblasts into the skin. In our opinion, one can assume a pathophysiological role of expression of fibroblast growth factor and its receptor (FGF / FGFR) in primary osteoma cutis because of its pivotal function in bone turnover.15 Repeated trauma by skin picking (excoriation disorder) might lead to an abundance of epidermal growth factors that could possibly be involved in the metaplastic transformation of mesenchymal cells to osteoblasts. As of today, there has been no report of an increased risk of malignancy in primary OC of the face, and it is considered to be a benign condition.8
Treatment options for single or multiple lesions of OC include complete excision along with less traumatic techniques, such as curettage with excellent cosmetic results.16,17 Other authors have described successful treatments with ablative lasers, such as Erbium-Yag or carbon dioxide.18,19 The successful use of topical agents, such as retinoids, has been described in case reports.20 Bisphosphonates, which alter bone metabolism, have shown no beneficial effects in the past, and some authors have even identified bisphosphonates as a possible trigger of cutaneous osteoma.3
Therapeutic regimes for calcinosis cutis are not well established and depend on individual constellation. Underlying causes of hypercalcemia or hyperphosphatemia should be treated if possible. The surgical removal of calcinosis cutis bears a large risk of recurrence, but may still be considered for painful, ulcerated, or functionally impairing lesions. Dietary restrictions might be helpful in some cases, and physiotherapy and supportive measures should be assigned to the patients.
Conclusion
In summary, we have described a rare case of multiple primary osteoma cutis of the face in a 39-year-old Caucasian woman that was managed surgically by local excision to the satisfaction of the patient. In the absence of laboratory or phenotypic abnormalities and an empty family history, other relevant underlying conditions were excluded. The clinical course of idiopathic isolated facial osteoma cutis and MMOC is favorable, and predisposing factors are female sex and acne vulgaris. Symptomatic or disfiguring lesions may be managed through complete excision or ablative lasers as the most viable therapeutic options. OCs and CCs might display similarities and overlapping features; however, it is crucial to fully elucidate unclear cases and search for any underlying condition.
Acknowledgement
We thank our patient for granting permission for the use of photography.
References
- Roth S, Stowell R, Helwig E. Cutaneous ossification. Report of 120 cases and review of the literature. Arch Pathol.1963;76:44–54.
- Fazeli P, Harvell J, Jacobs M. Osteoma cutis (cutaneous ossification). West J Med. 1999;171(4):243–245.
- Duarte BM, Pinheiro RR, Cabete J. Multiple miliary osteoma cutis: A comprehensive review and update of the literature. Eur J Dermatol. 2018 Aug 1;28(4):434–439.
- Haro R, Revelles JM, Angulo J, et al. Plaque-like osteoma cutis with transepidermal elimination. Journal of Cutaneous Pathology. 2009;36(5):591–593.
- Albright F, Burnett C, Smith P, Parson W. Pseudo-hypoparathyroidism: An example of “Seabright-Bantam syndrome”. Endocrinology. 1942;30:922–930.
- Ascari-Raccagni A, Baldari U, Righini MG. Cutaneous symptoms of Gardner’s syndrome. J Eur Acad Dermatol Venereol. 1999 Jan;12(1):80–81.
- Gardner E, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet. 1953;5(2):139–147.
- Lennox L. Osteoma Cutis. 2019. Medscape. https://emedicine.medscape.com/article/1103575-overview. Accessed 22 Sept 2020.
- Romero Navarrete M, Vega Memije M-E, Arenas Guzmán R, et al. Dermoscopic and histopathological findings in osteoma cutis involving the face and scalp. Dermatol Pract Concept. 2019:31;24–27.
- Elia F, Paolino G, Donati M, Solivetti FM. Ultrasound pattern of a rare skin disease: Multiple miliary osteoma cutis. J Ultrasound. 2016;19(2):145–147.
- Alhazmi D, Badr F, Jadu F, et al. Osteoma Cutis of the Face in CBCT Images. Case Rep Dent. 2017;2017:1–4.
- Safi Y, Valizadeh S, Vasegh S, et al. Prevalence of osteoma cutis in the maxillofacial region and classification of its radiographic pattern in cone beam CT. Dermatol Online J. 2016;22(1).
- Nunley J. Calcinosis Cutis. 2018. Medscape. https://emedicine.medscape.com/article/1103137-overview. Accessed 22 Sept 2020.
- Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis. J Am Acad Dermatol. 2011;65(1):1–12.
- Su N, Jin M, Chen L. Role of FGF/FGFR signaling in skeletal development and homeostasis: Learning from mouse models. Bone Res. 2014;2(1):14003.
- Senti G, Schmid M, Burg G. Multiple miliäre Osteomata cutis. Exstirpation mittels “front-lift”-Zugang. Hautarzt. 2001;52:522–525.
- Chabra IS, Obagi S. Evaluation and management of multiple miliary osteoma cutis: Case series of 11 patients and literature review. Dermatol Surg. 2014;40(1):66–68.
- Stöckel E, Eppinger S, Stein A. Multiple miliare Osteome des Gesichts. Hautarzt. 2002;53:37–41.
- Ortiz AE, Ross VE. Letter: Successful treatment of multiple miliary osteomas of the face using an erbium-doped yttrium aluminum garnet laser. Dermatol Surg. 2011;37(10): 1548–1550.
- Cohen AD, Chetov T, Cagnano E, et al. Treatment of multiple miliary osteoma cutis of the face with local application of tretinoin (all-trans-retinoic acid): A case report and review of the literature. J Dermatol Treat. 2001;12(3):171–173.

