 by William A. Hayward, PhD, MSC; Wilmer L. Sibbitt, Jr, MD; Randy R. Sibbitt, MD; Maheswari Muruganandam, MBBS; Noelle A. Rolle, MD; Monthida Fangtham, MD; N. Suzanne Emil, MD; and Scarlett K. Kettwich, MS
by William A. Hayward, PhD, MSC; Wilmer L. Sibbitt, Jr, MD; Randy R. Sibbitt, MD; Maheswari Muruganandam, MBBS; Noelle A. Rolle, MD; Monthida Fangtham, MD; N. Suzanne Emil, MD; and Scarlett K. Kettwich, MS
Dr. Hayward is with the Department of Exercise and Sport Sciences at New Mexico Highlands University in Las Vegas, New Mexico. Drs. W. Sibbitt, Muruganandam, Rolle, and Fangtham are with the Department of Internal Medicine, Division of Rheumatology and School of Medicine at University of New Mexico Health Sciences Center in Albuquerque, New Mexico. Dr. R. Sibbitt is with Montana Interventional and Diagnostic Radiology in Helena, Montana. Ms. Kettwich is with the School of Dentistry at Oregon Health & Science University in Portland, Oregon.
J Clin Aesthet Dermatol. 2018;11(5):38–42
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Objective. Benign subcutaneous lipomas can cause musculoskeletal pain and nerve impingement. We hypothesized that the potent lipolytic and atrophic effect of 40mg/mL triamcinolone acetonide would atrophy symptomatic lipomas so surgical excision could be avoided.
Design. This was a cohort study.
Setting. This study took place in an ultrasound injection clinic.
Participants. Eight subjects with painful symptomatic lipoma were included.
Measurements. Preprocedurally, the margins of the lipomas were palpated and marked with ink, then measured in centimeters (cm). Small lipomas (1–3cm) were injected with 40mg triamcinolone acetonide, while large lipomas (4–6cm) were injected with 80mg of triamcinolone acetonide. The subjects were reassessed at a four-month follow-up appointment and then again at one year and two years after the procedure.
Results. Pre-injection, all eight subjects had symptoms related to impingement or pain with compression of the lipoma. At four months post-injection, none of the patients had symptoms attributable to the lipoma (p<0.001). The mean lipoma palpable dimension was 5.0±1.2cm prior to the injection and was 2.0±1.1cm at four months after the injection, with a significant mean 3.0±0.3cm (60%) reduction in lipoma dimensions (p<0.001). Two subjects demonstrated some mild hypopigmentation of the skin at four months post-injection. Within two years, three lipomas had symptomatically recurred, one of which was removed surgically and the two of which were reinjected. There were no infections or other serious adverse reactions that occurred.
Conclusions. For individuals with painful subcutaneous lipoma, intralesional injection of 40mg/mL of triamcinolone acetonide is an effective and safe alternative to surgical excision or injection of sclerosing agents and should be considered as a reasonable therapeutic alternative in select patients.
Keywords: Lipoma, musculoskeletal, injection, corticosteroids, neurologic
Introduction
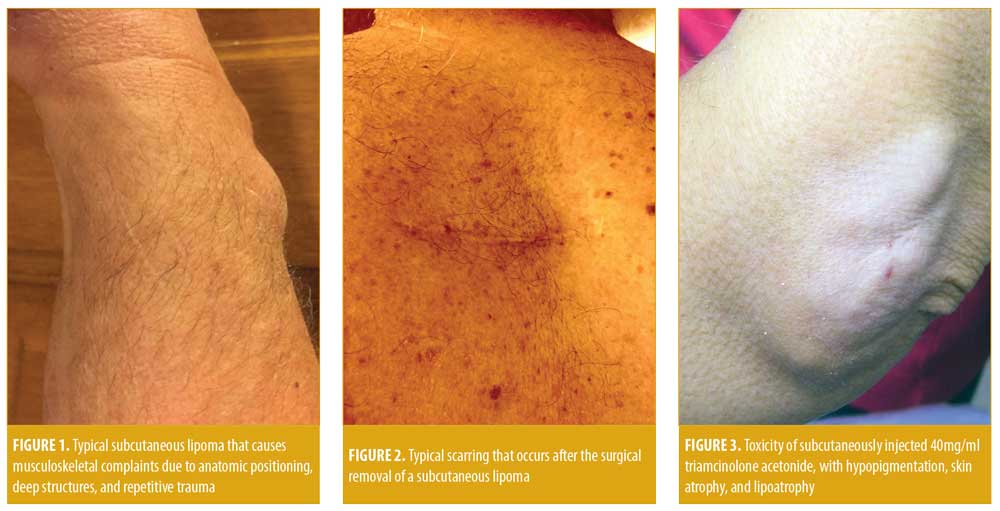
In dermatology and rheumatology practice, patients occasionally present with “lumps and bumps” that, on physical examination, turn out to be gouty tophi, rheumatoid nodules, enlarged lymph nodes, panniculitis, malignant tumor masses, or benign masses, including lipomas.1,2 Subcutaneous lipomas, although benign, can cause considerable neurologic pain through nerve impingement, musculoskeletal pain, especially on weight-bearing areas or if the lipoma is resident on the edge of a muscle or between muscles, and cosmetic disturbances (Figure 1).3–6 Symptomatic lipomas are traditionally treated with wide, deep excision, but surgical extirpation frequently results in the loss of adjacent soft tissue with permanent scarring, which can lead to adverse cosmetic outcomes (Figure 2).3
The injection of triamcinolone acetonide is typically used to locally treat inflammatory conditions of the skin, joints, and other musculoskeletal structures, but major toxicities of injection with potent corticosteroid esters include depigmentation, atrophy of the skin, and lipoatrophy (Figure 3). Thus, dermatologists tend to avoid 40mg/mL triamcinolone acetonide and instead use the 10mg/mL strength to avoid these problems.7–10 We hypothesized that the adverse effect of lipoatrophy predictably caused by 40mg/mL triamcinolone acetonide could be used beneficially to nonsurgically atrophy symptomatic subcutaneous lipomas, resulting in symptomatic improvement.
Methods
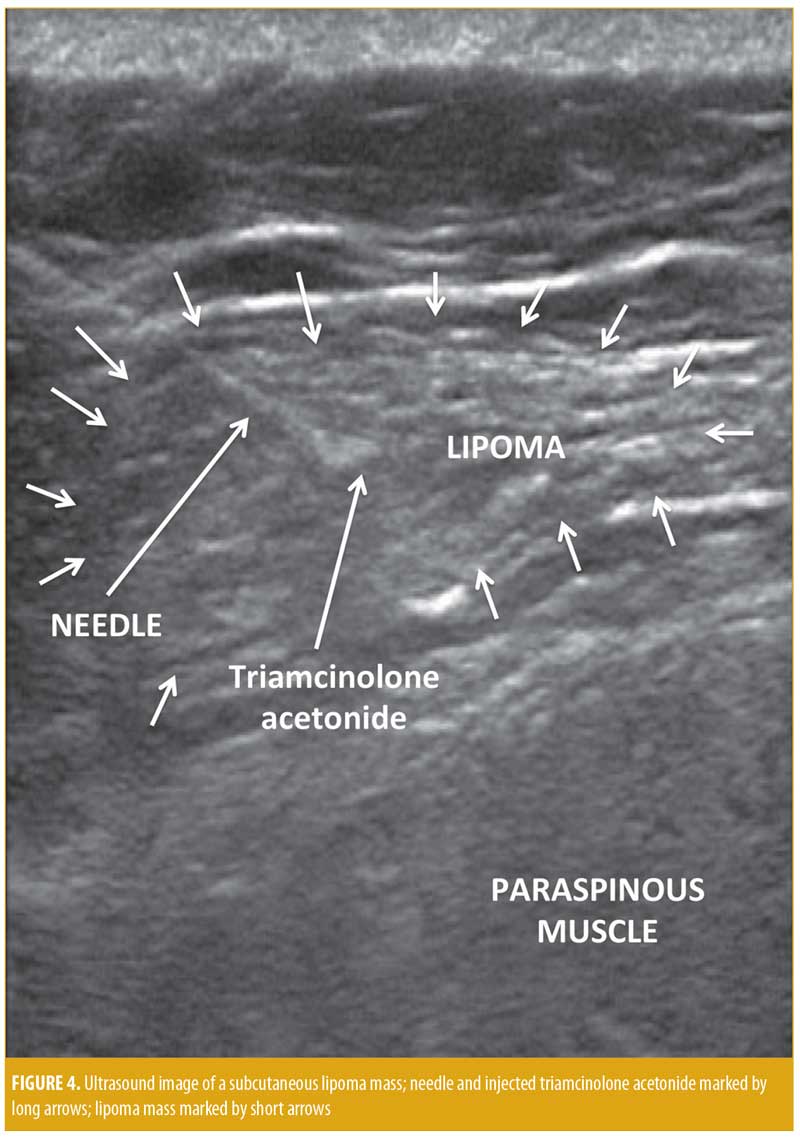
This research was approved by an institutional review board (IRB) and was in compliance with the Declaration of Helsinki and subsequent revisions. This was a cohort study consisting of a case series of eight patients with symptomatic subcutaneous lipoma who wished to avoid surgical extirpation and thus asked to have the lesions injected. Eight subjects (7 women and 1 man), each with a solitary lipoma were treated with injection therapy. Each subject provided written informed consent prior to the procedure. Data were recorded prospectively and analyzed retrospectively with IRB approval as a series of injection outcomes for quality improvement. Inclusion criteria included any patient with a painful or cosmetically deforming lipoma encountered on musculoskeletal examination in the rheumatology clinic who wanted to avoid surgical excision and preferred injection therapy. Exclusion criteria were concomitant infection or intolerance to the injected corticosteroid. Preprocedurally, the margins of the lipomas were palpated and identified by an ink marker, then measured in centimeters (cm). Small lipomas (1–3cm) were injected with 40mg triamcinolone acetonide, while large lipomas (4–6cm) were injected with 80mg of triamcinolone acetonide. Deep lipomas or lipomas in close proximity to large blood vessels, nerves, or the lungs/heart were performed under ultrasound guidance (Figure 4). For superficial lipomas, after the needle was inserted through the skin, the free hand was used to palpate the lipoma margins during the procedure to ensure the needle remained in the lipoma margins. Chorhexidine 2% was used for local antisepsis. A 22-gauge, two-inch needle (4710007050 – 22 GX2” [0.7mm × 50mm]; FINE-JECT®, Henke-Sass Wolf GmbH, Tuttlingen, Germany) was mounted on a 3mL syringe (3mL Luer-Lok™ syringe; BD, Franklin Lakes, New Jersey) filled with 40mg to 80mg of triamcinolone acetonide (maximum: 80mg) (Kenalog® 40; Bristol-Myers Squibb, New York, New York). The medication was injected deep into the center and deep areas of the lipomas in at least three separate areas, avoiding the superficial skin-side lipoma margins in order to decrease possible migration of the corticosteroid crystals via lymphatics to the skin (Figure 4).10–15 The needle was then extracted, and firm pressure was applied to the puncture sites; sterile bandage strips were subsequently applied. The subjects were assessed at four months, one year, and two years after treatment.
Results
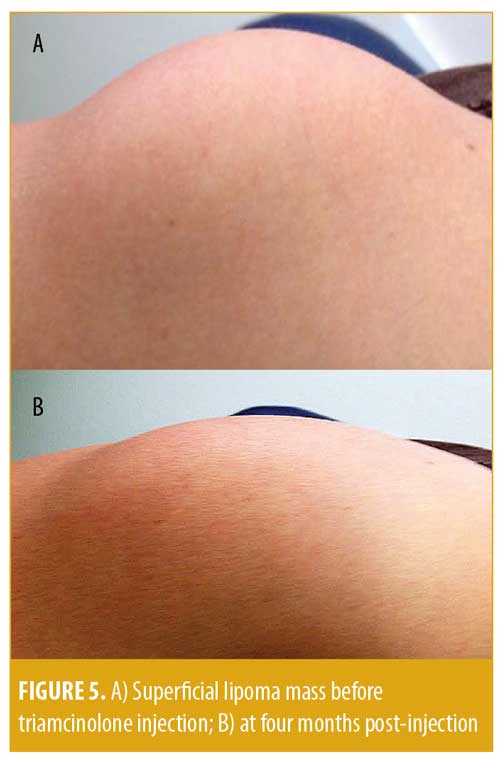
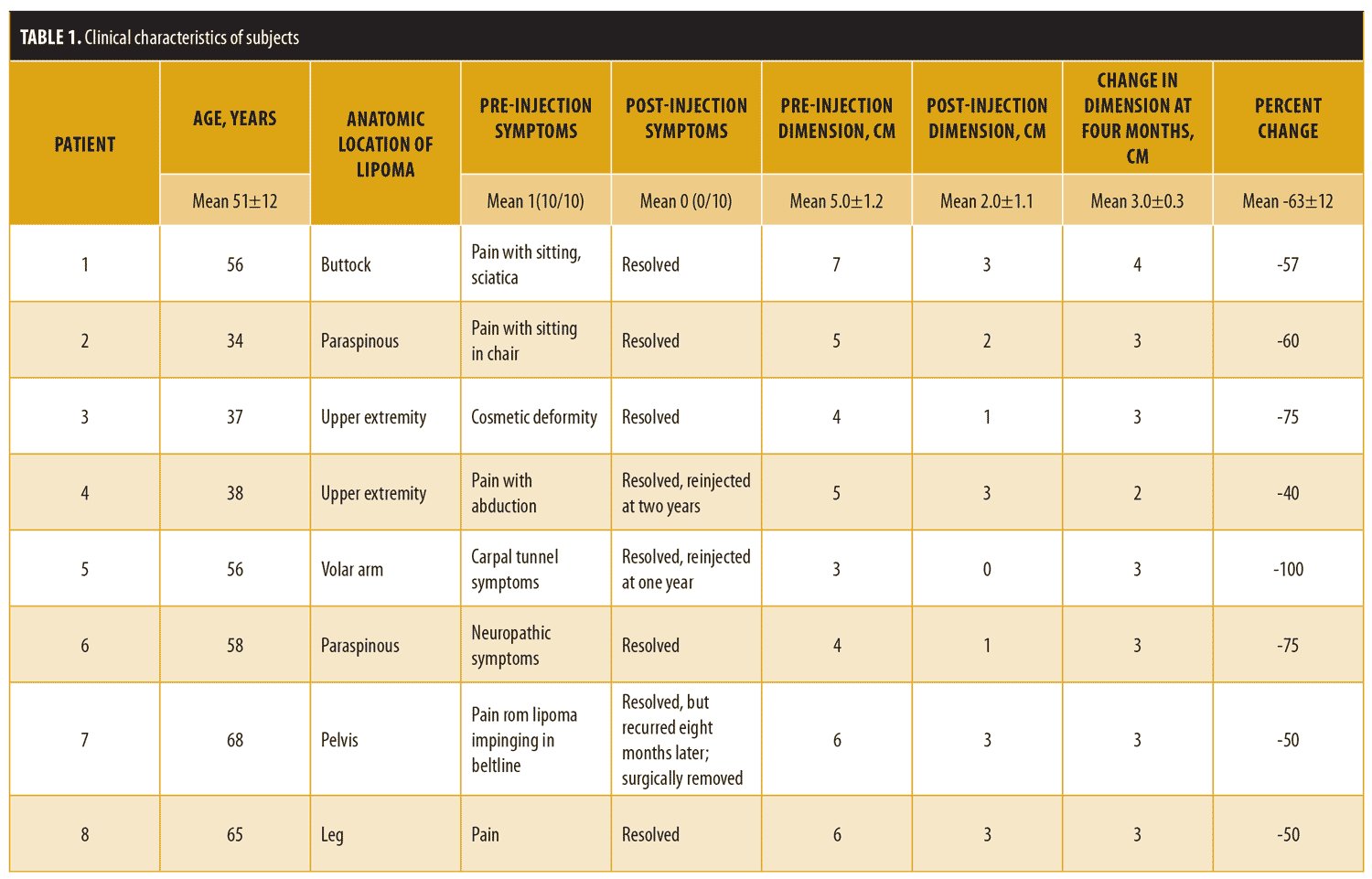
The results are shown in Table 1 and Figure 5. The mean age of the subjects at the time of injection was 51±12 years. Each subject had a symptomatic lipoma, and the location of the lipoma differed between the individual subjects (e.g., forearm, upper arm, back, buttock, pelvis, or leg) (Table 1). Prior to the procedure, all eight subjects had symptoms related to impingement or pain with compression of the lipoma. At the four-month follow-up, none of the eight patients reported symptoms attributable to the lipoma (Z value for 95% confidence interval [CI]: 1.96; Pearson’s p<0.001). The lipoma mean palpable dimension was 5.0cm±1.2cm prior to the injection and was 2.0cm±1.1cm at four months after the injection, with a mean 3.0cm±0.3cm (60%) reduction in lipoma dimension (95% CI of difference: 1.872 <3<4.128 (Wald); p<0.001). As seen in Figure 5 and Table 1, the lesions were reduced substantially in size without severe hypopigmentation or cosmetic deformity. Two subjects developed some mild hypopigmentation of the skin at the four-month follow-up visit; however, the hypopigmentation was much milder than that shown in Figure 3. Only one patient (12.5%) had complete resolution of the lipoma by palpation at the four-month follow-up visit (Table 1). Within two years, three lipomas symptomatically recurred, one of which was removed surgically and the remaining two of which were re-injected. There were no infections or other serious adverse reactions.
Discussion
Subcutaneous lipomas are benign tumors present in superficial subcutaneous adipose tissue and are especially evident in the arms, legs, neck, back, and buttocks.1–3 Although benign, subcutaneous lipomas can cause pain in weight-bearing areas, interfere with normal muscle movement, induce impingement of nerves resulting in neuropathic symptoms, and cause cosmetic concerns (Figures 1 and 5).2–6 Resultant psychological stress might also occur, which might motivate many patients to seek excisement.3 Surgical excision is the gold standard for treating lipomas. Yet, typically, large excisions are required to remove asymmetrical lipoma mass, as well as a substantial amount of normal fat, which can cause excavating deformities and surface scarring and result in cosmetic residua (Figure 2).3 Because of the scarring and deformation associated with lipoma excision, injection lipolysis has evolved as an alternative treatment, including injection of phosphatidyl choline, deoxycholate (a bile acid), and the combination of both phosphatidyl choline/sodium deoxycholate, ethanol, and other sclerosing solutions.16–21 The main problem with these sclerosing agents is that, while these solutions cause lipolysis with necrosis and involution of fat, they can be extremely irritating and destructive to tissues and can injure local nerves, arteries, and veins and cause chronic neuropathy, thrombosis, and local vascular problems.26
Corticosteroids have less potential for injury of local nerves and blood vessels, but they can also induce lipolysis and fat involution and thus have been used as a treatment of subcutaneous lipomas.16 Redman et al27 demonstrated that injection of the lipoma with 0.07mg of prednisolone a day in an intense regimen (5 out of 7 days per week for 4 weeks) resulted in a reduction in lipoma size by 50 percent. However, most lipomas recurred, requiring subsequent excision.27 Triamcinolone acetonide is a more potent and insoluble corticosteroid than prednisolone and is notorious for causing cosmetic complications, including hypopigmentation, atrophy of the skin, and lipoatrophy (Figure 3).7,28–33 Lamagna et al34 used this usually negative effect for therapeutic reasons in a much more aggressive regimen using 40mg of triamcinolone acetonide in canine lipomas, which resulted in significant atrophy of the lipomas and excellent clinical responses without complication. In our study subjects, we also used the usually deleterious atrophic effect of triamcinolone acetonide to elicit a beneficial outcome—that is, lipoatrophy—to treat lipomas by single injection. However, unlike Lamagna et al, who studied this application in canines, we used this technique in humans. We achieved a 60-percent reduction in lipoma size and a 100-percent reduction in symptomatology associated with lipoma, with few serious adverse events in our patients, confirming and extending the therapeutic results of Lamagna et al (Table 1 and Figure 5).34
The mechanisms behind lipoatrophy in a benign lipoma after a corticosteroid injection remain somewhat speculative. After injection, there is a lymphatic spread of the corticosteroid crystals along the lymphatic channels and resulting linear atrophy of the adjacent tissues (Figure 2).11–13 Lipoatrophy is characterized histologically by reduced cellularity at the site of corticosteroid injection, presence of apoptotic lipocytes, and activated perivascular macrophages engulfing segments of apoptotic adipose and stromal tissue.35–37 Corticosteroids also can cause local vasoconstriction, capillary closure, and tissue hypoxia, resulting in lipoatrophy.8 Additionally, corticosteroids can have a direct antiproliferative effect on keratinocytes and fibroblasts, accelerate catabolism of extracellular matrix proteins, cause apoptosis of fat cells, and decrease synthesis of skin lipids.8–15 All of these effects might explain the lipoma shrinkage and symptomatic improvement after corticosteroid injection.
Research has shown that there is a considerable risk of dermal atrophy and hypopigmentation when 40mg/mL of triamcinolone is injected into skin lesions instead of the dermatologic standard of triamcinolone 10mg/mL.15 Based on our study and the report by Lamagna et al, the usually toxic lipolytic effect of a 40mg/mL injection of triamcinolone acetonide was used therapeutically to atrophy symptomatic lipomas (Figure 5).15,34 Since the technique of lipoma injection keeps the triamcinolone acetonide deep into the mass of the lipoma where lymphatics were less likely to carry the crystal to the skin surface (Figure 4), skin hypopigmentation and atrophy were only a minor problem (Table 1) in comparison to superficial injections (Figure 3). Thus, triamcinolone acetonide injection of symptomatic lipomas appears to be a reasonable, viable, effective, and safe alternative to surgical excision for the treatment for lipomas.
Limitations. Limitations of this study include the relatively small number of patients and the nonrandomized, nonblinded series design, but sequential paired studies wherein patients are studied before and after an intervention is a practical trial design often used in case studies of any interventional technique. Also, it should be noted that, prior to injection, the clinician should exclude the presence of malignant lesions of the connective tissue including liposarcoma, lymphoma, osteosarcoma, and skin; metastatic malignant tumors; and other causes of soft tissue mass including infection, sebaceous cyst, foreign body granuloma, rheumatoid nodule, and tophus. These lesions usually have distinct, palpable, symptomatic, anatomic, and imaging characteristics compared to benign lipomas.1–3 If there is any question that the lesion is not a benign lipoma, excisional biopsy, needle biopsy, or additional imaging should be performed before attempting injection therapy.
Conclusion
For individuals with musculoskeletal and neurologic complaints due to superficial subcutaneous lipomas, intralesional injection of 40- to 80mg of triamcinolone acetonide appears to be an effective and safe alternative to surgical excision and injection of sclerosing agents in select patients.
References
- Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. J Int Derm. 1982;21(10):563–572.
- Nguyen T, Zuniga R. Skin conditions: benign nodular skin lesions. FP Essent. 2013;407:24–30.
- Salam GA. Lipoma excision. Am Fam Physician. 2002;65(5):901–904.
- Sbai MA, Benzarti S, Msek H, et al. Carpal tunnel syndrome caused by lipoma: a case report. Pan Afr Med J. 2015;22:51.
- Maldonado AA, Howe BM, Spinner RJ. Posterior interosseous nerve discontinuity due to compression by lipoma: report of 2 cases. J Neurosurg. 2017;126(5):1698–1701.
- McFarland GB Jr, Hoffer MM. Paralysis of the intrinsic muscles of the hand secondary to lipoma in Guyon’s tunnel. J Bone Joint Surg Am. 1971;53(2):375–376.
- Cole BJ, Schumacher HR Jr. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13(1):37–46.
- Cantürk F, Cantürk T, Aydin F, et al. Cutaneous linear atrophy following intralesional corticosteroid injection in the treatment of tendonitis. Cutis. 2004;73(3):197–198.
- Nanda V, Parwaz MA, Handa S. Linear hypopigmentation after triamcinolone injection: a rare complication of acommon procedure. Aesthetic Plast Surg. 2006;30(1):118–119.
- Okere K, Jones MC. A case of skin hypopigmentation secondary to a corticosteroid injection. South Med J. 2006; 99(12):1393–1394.
- Kikuchi I, Horikawa S. Letter: perilymphatic atrophy of the skin. Arch Dermatol. 1975;111(6):795–796.
- Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy: report of two cases and review of the literature. J Am Acad Dermatol. 1988;19(3):537–541.
- Nanda V, Parwaz MA, Handa S. Linear hypopigmentation after triamcinolone injection: a rare complication of a common procedure. Aesthetic Plast Surg. 2006;30(1):118–119.
- George WM. Linear lymphatic hypopigmentation after intralesional corticosteroid injection: report of two cases. Cutis. 1999;64(1):61–64.
- Firooz A, Tehranchi-Nia Z, Ahmed AR. Benefits and risks of intralesional corticosteroid injection in the treatment of dermatological diseases. Clin Exp Dermatol. 1995;20(5):363–370.
- Amber KT, Ovadia S, Camacho I. Injection therapy for the management of superficial subcutaneous lipomas. J Clin Aesthet Dermatol. 2014;7(6):46–48.
- Nanda S. Treatment of lipoma by injection lipolysis. J Cutan Aesthet Surg. 2011;4(2):135–137.
- Nanda S. Treatment of lipoma by injection lipolysis. J Cutan Aesthet Surg. 2011;4(2):135–137.
- Bechara FG, Sand M, Hoffmann K, et al. Fat tissue after lipolysis of lipomas: a histopathological and immunohistochemical study. J Cutan Pathol. 2007;34(7):552–557.
- Bechara FG, Sand M, Sand D, et al. Lipolysis of lipomas in patients with familial multiple lipomatosis: an ultrasonography-controlled trial. J Cutan Med Surg. 2006;10(4):155–159.
- Bechara FG, Sand M, Altmeyer P, Hoffmann K. Intralesional lipolysis with phosphatidylcholine for the treatment of lipomas: pilot study. Arch Dermatol. 2006;142(8):1069–1070.
- Kopera D, Binder B, Toplak H. Intralesional lipolysis with phosphatidylcholine for the treatment of lipomas: pilot study. Arch Dermatol. 2006;142(3):395–396.
- Rotunda AM, Ablon G, Kolodney MS. Lipomas treated with subcutaneous deoxycholate injections J Am Acad Dermatol. 2005;53(6):973–978.
- Salam GA. Lipoma excision. Am Fam Physician. 2002;65(5):901–904.
- Galaktionov VV. [Method of injection necrotherapy of benign tumors of the subcutaneous cellular tissue]. Vestn Khir Im I I Grek. 1978;121(7):46–49. (Article in Russian)
- El-Gowelli HM, El Sabaa B, Yosry E, El-Saghir H. Histopathological and ultra-structural characterization of local neuromuscular damage induced by repeated phosphatidylcholine/deoxycholate injection. Exp Toxicol Pathol. 2016;68(1):39–46.
- Redman LM, Moro C, Dobak J, et al. Association of ?-2 adrenergic agonist and corticosteroid injection in the treatment of lipomas. Diabetes Obes Metab. 2011;13(6):517–522.
- Mager DE, Moledina N, Jusko. WJ. Relative immunosuppressive potency of therapeutic corticosteroids measured by whole blood lymphocyte proliferation. J Pharm Sci. 2003;92(7):1521–1525.
- Reddy PD, Zelicof SB, Ruotolo C, Holder J. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995;(317):185–187.
- Skármeta NP, Hormazábal FA, Alvarado J, Rodriguez AM. Subcutaneous lipoatrophy and skin depigmentation secondary to TMJ intra-articular corticosteroid injection. J Oral Maxillofac Surg. 2017;75(12):2540.e1–2540.e5.
- Breit W, Frosch M, Meyer U, et al. A subgroup-specific evaluation of the efficacy of intraarticular triamcinolone hexacetonide in juvenile chronic arthritis. J Rheumatol. 2000;27(11):2696–2702.
- Jeyapalan K, Choudhary S. Ultrasound-guided injection of triamcinolone and bupivacaine in the management of De Quervain’s disease. Skeletal Radiol. 2009;38(11):1099–1103.
- Breit W, Frosch M, Meyer U, et al. A subgroup-specific evaluation of the efficacy of intraarticular triamcinolone hexacetonide in juvenile chronic arthritis. J Rheumatol. 2000;27(11):2696–2702.
- Lamagna B, Greco A, Guardascione A, et al. Canine lipomas treated with steroid injections: clinical findings. PLoS One. 2012;7(11):e50234.
- Hisamichi K, Suga Y, Hashimoto Y, et al. Two Japanese cases of localized involutional lipoatrophy. Int J Dermatol. 2002;41(3):176–177.
- Yamamoto T, Yokozeki H, Nishioka K. Localized involutional lipoatrophy: report of six cases. J Dermatol. 2002;29(10):638–643.
- Iftikhar A. Post-injection involutional lipoatrophy: ultrastructural evidence for an activated macrophage phenotype and macrophage related involution of adipocytes. Am J Dermatopathol. 2006;(4):334–337.

