 by Betul Tas, MD; Aysel Çaglar, MD; and Serdar Altinay, MD
by Betul Tas, MD; Aysel Çaglar, MD; and Serdar Altinay, MD
Dr. Tas is with the Health Sciences University, Istanbul Bagcilar Research and Training Hospital, Department of Dermatology in Istanbul, Turkey. Dr. Çaglar is with the Health Sciences University, Istanbul Bagcilar Research and Training Hospital, Department of Pathology in Istanbul, Turkey. Dr. Altinay is with the Health Sciences University, Bakirkoy Sadi Konuk Research and Training Hospital, Department of Pathology, in Istanbul, Turkey.
J Clin Aesthet Dermatol. 2018;11(5):43–47
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: We present the case of a 15-year-old girl who presented with an unusual grouping of lesions on her upper left leg. The lesions had been present since birth. The patient had five different types of lesions: 1) transparent grouped or scattered yellowish vesicles; 2) keratotic-surfaced, grouped dark-yellowish papules; 3) bright-red grouped papules; 4) keratotic-surfaced grouped dark-red papules; and 5) patchy, punctate, and erythematous red macules. All of the lesions were intertwined along the lines of Blaschko and were in the form of irregular serpiginous plaques. Histopathological examinations of the lesions showed three main histological features, and diagnoses of the lesions were made as lymphangioma circumscriptum, lymphangiokeratoma, and verrucous hemangioma. To the best of our knowledge, such intertwined lesions have never been reported in the literature. Hence, we suggest that the name of this unique combination of lesions be “congenital nevoid mixed hemato-lymphangio-keratoma serpiginosum.”
Keywords: Lymphangioma, hemangioma, lymphangiokeratoma, verrucous hemangioma, angiokeratoma circumscriptum, angioma serpiginosum
Introduction
Different lymphatic and angiomatous malformations of the skin rarely co-occur, and are all complex diseases. These malformations are mostly seen at birth, and they can be of a nevoid pattern, superficial, or with deeper involvement.1 Two of the most widely recognized lesions are angioma serpiginosum (AS)2,3 and lymphangioma circumscriptum (LC).4 Occasionally, angiomatous and lymphamatous lesions overlap and are known as “ hematolymphangioma.”5 Additionally, a hyperkeratosis can develop on the surface of angiomatous and lymphomatous lesions, and are known as angiokeratoma (AK)6 or verrucous hemangioma (VH)7 for angiomatous lesions and lymphangiokeratoma (LK)8 or verrucous lymphangioma (VL)9 for lymphangiomatous lesions. However, to the best of our knowledge, an overlapping of these lesions in a patient has not been reported in the literature.
Case Presentation
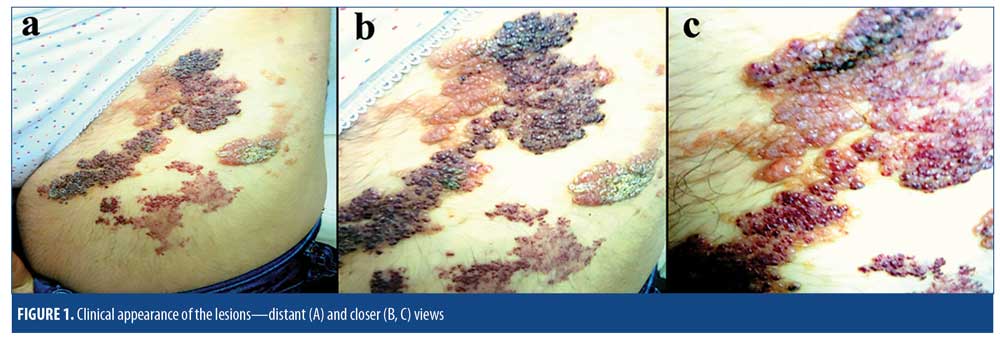
A 15-year-old girl presented to our outpatient clinic with vesicular leg lesions that had been present since birth. When the lesions were first noticed by her, they appeared only as erythematous punctate smooth patches with no vesicles or papules; however, when the patient was three years old, a few red bulges emerged and increased in number steadily as she aged. Over time, the bulges developed into bright red papules, with a few yellowish vesicles also appearing near or around the red patches. When the patient was nine years old, her parents observed some thickening and crusting in yellowish and red papules. The color of the papules also changed, with the yellowish ones turning a dirty, dull-colored yellow and the red ones turning a darker red. The lesions were serpiginous and were situated on an area with dimensions of 32cm×14cm on the upper and anterolateral part of the left leg. All of the lesions were symptomless, and the patient only complained of the cosmetic appearance of the lesions.
Upon clinical examination, five different types of lesions were detected: 1) transparent, grouped or scattered yellowish vesicles; 2) keratotic, grouped, dark-yellowish papules; 3) bright red, grouped papules; 4) keratotic surfaced, grouped dark red papules; and 5) patchy, punctate, and erythematous red macules. All of the lesions were intertwined along the lines of Blaschko and were in the form of intricate and irregular serpiginous plaques (Figures 1A–C). A systemic physical examination was performed, and routine laboratory testing included a complete blood count; platelet aggregation; prothrombin time; partial tromboblastin time; bleeding time; erythrocyte sedimentation rate; and estrogen and progesterone levels, and biochemistry panel. No pathology was detected. The patient had no neurological or additional vascular findings. A superficial tissue ultrasonography of the lesional area did not show any subcutaneous vascular involvement. The Doppler angiography of the left leg, abdominal ultrasonography, and magnetic resonance imaging of the left leg, pelvis, and abdomen showed no abnormalities. Excisional skin biopsies were taken from the lesions, and preliminary diagnoses of lymphangioma (LA), LK, hemangioma (HA), angiokeratoma circumscriptum (AC), VH, and AS were made.
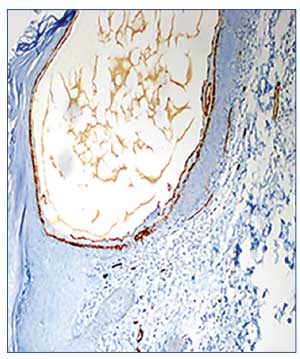
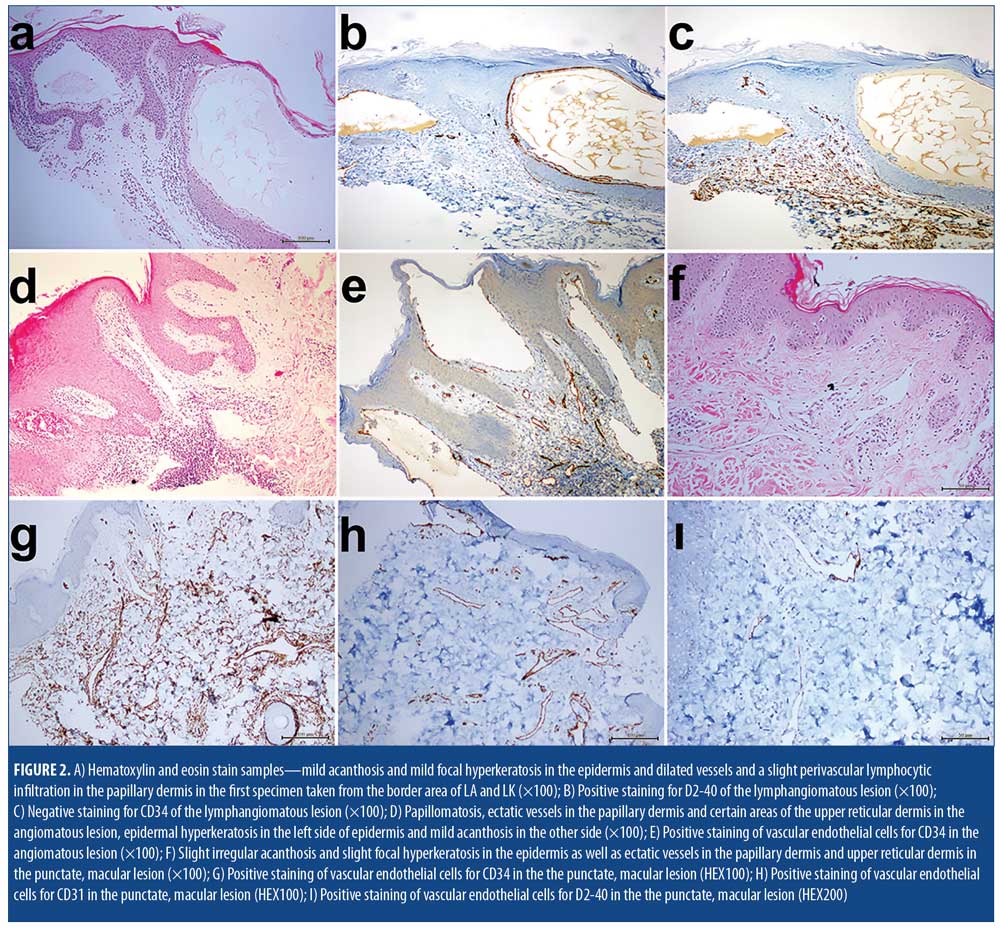
Histopathological examinations of the biopsy specimens showed three distinct histological features. The first biopsy specimen was taken from the area around the border of what clinically appeared to be LA and LK, and showed dilated vessels and a slight perivascular lymphocytic infiltration in the papillary dermis in both sites. Additionally, mild acanthosis and mild focal hyperkeratosis were detected in the epidermis in the LK site (Figure 2A). Immunohistochemial staining of this area revealed prevalence of D2-40 (Figure 2B), but not CD34 (Figure 2C).
An examination of the second specimen, which was taken from the area around the border of what clinically appeared to be HA and keratotic-HA, indicated papillomatosis and ectatic vessels in the papillary dermis and certain areas of the upper reticular dermis. These vessels had perivascular lymphocytic infiltrations and were full of erythrocytes. On the epidermis of the HA-appearing side of the lesion, there was a slight irregular acanthosis, while the other side resembling keratotic-HA had a slight hyperkeratosis (Figure 2D). Immunohistochemical staining of this area revealed presence of CD34 (Figure 2E).
The third specimen, which examined the punctate macules that clinically resembled AS, showed a slight irregular acanthosis and slight focal hyperkeratosis in the epidermis, as well as ectatic vessels in the papillary dermis and upper reticular dermis (Figure 2F). Immunohistochemical staining of the endothelial cells revealed a diffuse reticular pattern of CD34 (Figure 2G) and limited presence of CD31 (Figure 2H) and D2-40
(Figure 2I).
With the histopathological findings, the diagnoses of the lesions were made as LC, LK, and VH. Ablation of lesions using an erbium-doped yttrium aluminum garnet laser (Er:YAG) laser was planned. The patient, however, opted not to undergo invasive treatment, and thus a topical treatment regimen using imiquimod 5% was prescribed (application 3x/day). Unfortunately, due to lack of tolerance to the treatment (the patient experienced severe irritation) and with no improvement in the lesions, imiquimod therapy was discontinued after three months and the patient was lost to follow-up.
Discussion
Diagnosis of intertwined lesions, such as those presented in our case, can be challenging to the clinician. Current diagnostic criteria might not be helpful when dealing with a legion of unfamiliar etiology or unusual appearance, location, or histopathological results.
The first type of lesions found on our patient clinically appeared to be LA and LK. Lymphangioma is a rare and benign proliferation of the lymphatic system.4,10 There are three known types of LA: circumscriptum (or capillary), cavernous, and cystic.10 LC is characterized by clustered (localized) or diffused (classical) translucent vesicles that measure 1 to 5mm in diameter and are filled with clear lymphatic fluid. Congenital, or primary, forms are usually present at birth and represent the malformations of deep dermal and subcutaneous lymphatics with a secondary dilatation of superficial lymphatics.11 The localized forms occur less frequently and appear as small, discrete lesions that can appear anywhere on the body at any age.4 The lymphatic lesions present on our patient were congenital and localized and did not show any subcutaneous involvement. Some of the lesions were hyperkeratotic or verrucous in appearance.4,10 We hypothesized that the appearance of the lesions could have been due to the tissue organization, epithelial changes, and/or hyperkeratosis.4,12 The main histopathological finding was ectatic lymphatics in the papillary dermis. Scattered lymphocytes in the stroma were also seen. The lymphatic endothelial cells were positive for D2-40.11 D2-40 is a sensitive and relatively specific marker for the lymphatic endothelium but is not expressed in endothelial cells of the blood vessels. Though CD34 are occasionally present in lymphatics, according to Pusztaszeri et al,13 CD34 is not usually present in lymphatic vessels. However, the staining intensity for CD34 in the lymphatics is usually lower than in the endothelium of angiomatous vessels and is very weak when compared to D2-40. Histopathologically, the lymphangiomatous lesions on our patient were localized only in the papillary dermis, with focal hyperkeratosis present on some of them. These lesions were positive for D2-40, but not for CD34. Therefore, we concluded that these lesions were related to lymphatic system.
The second type of lesion clinically appeared to be HA, AC, or VH. Angiokeratomas are asymptomatic, 2- to 5mm, dark-red to blue-black hyperkeratotic papules that can be localized or generalized. Angiokeratoma circumscriptum naeviforme is the least common localized form of angiokeratoma. These types of lesions are typically multiple, hyperkeratotic, papules or plaque-like lesions usually found unilaterally on the lower leg, foot, or buttock.6,14 They are typically seen at birth. Sometimes, the lesions are initially present as multiple reddish macules, clinically resembling nevus flammeus, and then develop into plaques with a hyperkeratotic verrucous surface a few years later.14 Histologically, thin-walled ectatic vessels in the superficial dermis with overlying epidermal hyperplasia are found.6 Initially, the angiomatous lesions of our patient were similar to HA and AC clinically, yet the ectatic vessels were extended from the papillary dermis into the reticular dermis. Because of the deep angiomatous component, the diagnosis of AC was ruled out.
In the review article by Popadic,7 the author reports that VH, another rare, congenital keratotic hemangiomatous lesion, was first described by Imperial and Helwing in 1967. This lesion might appear at birth as a reddish macular area similar to the “port-wine” stain. Over the course of time, it transforms into bluish-black, verrucous, and hyperkeratotic plaques and nodules. This type of lesion can be single or grouped and can have a linear or serpiginous arrangement. The dimensions of the lesions are typically 4- to 8cm. These lesions occur unilaterally on the lower extremities 95 percent of the time. Reactive epidermal acanthosis, papillomatosis, hyperkeratosis, ectatic vessels that begin in the papillary dermis and extend into the reticular dermis or subcutaneous adipous tissue are seen histopathologically.7 The ectatic vessels in the lesions of our patient extended into the reticular dermis, and a prominent papillomatosis was seen. A moderate hyperkeratosis appeared only in the hyperkeratotic-looking area. Immunohistochemically, the dermal blood vessels were positive for both CD31 and CD34.13 Considering the clinical features, the way the lesions changed over time, and histopathological features of the keratotic angiomatous lesions of the patient, we believe the lesions were more consistent with VH than with AC.
The third type of the lesions clinically appeared to be AS. AS is a rare malformation of the blood vessels. The lesions are acquired, multiple, red to violaceous, punctate macules usually located in the lower extremities of girls under the age of 15 years. They enlarge peripherally, while those at the center fade, giving rise to a serpiginous pattern. They can occur in clusters and rarely follow Blaschko’s lines.3 Histologically, AS is characterized by clusters of ectatic capillaries in the papillary dermis.2,3 Sometimes, the lesions are hyperkeratotic.2 Considering that the lesions were present since birth, that the papules arose from punctate macules, and that the vessels extended into the reticular dermis, we ruled out AS. We believe the punctate patchy macules were more likely an early stage of VA.
In the histopathology, intertwined angiomatous and lymphamatous vessels were found in many areas. The third specimen was positive for CD34, D2-40, and CD31. Moreover, in one of the areas with a lymphangiomatous lesion, a big lymphatic vessel was negative for CD34 while the small vessels just under it were positive (Figure 2C). Debelenko et al15 stated that none of the nine verrucous angiomas were positive for D2-40 in their study. On the other hand, selective D2-40 immunoreactivity of lymphatics has been observed in normal tissues. It is unknown whether D2-40 reacts with the same or different antigens in normal lymphatics and vascular tumors. Although some markers are specific for lymphatics, they exhibit different expression patterns during prenatal development. Because some markers are expressed in both lymphatics and blood vessels in the early embryonal period, the respective immunopositivity might not indicate the lymphatic origin of a vascular tumor. In fact, because of these different staining features of vascular structures in different stages of their development, correctly interpreting the histopathology of congenital vascular tumors is a challenging and complex process. Some congenital vascular lesions might be misdiagnosed if the diagnosis is based on solely on clinical appearance without histopathological and immunohistochemical analysis, while others cannot be specifically diagnosed despite histopathological and immunohistochemical analysis. For example, Tennant et al16 clinically diagnosed 14 similar-looking hyperkeratotic vascular lesions (most of them were raised, red-to-purple in color, variably keratotic with irregular borders, and located on an extremity) as VH, AK, AC, or capillary-venous or capillary-lymphatic malformations before a detailed histopathological examination was made. After histopathological and immunohistochemical analysis, none of the lesions were diagnosed as AK, 11 of the 14 lesions were diagnosed as VH, and three of the lesions were diagnosed as combined vascular malformations composed of capillaries, lymphatics, and veins. Tennant et al16 also reported that, with the exception of the clinical verrucous appearances, the VHs in their patient sample had some common clinical and histopathological features, such as being present since birth, having proportionate growth, exhibiting hyperkeratotic epidermis, and containing thick-walled blood vessels that involved the entire dermis and subcutis. Our patient’s verrucous-hemangiomatous lesions had similar characteristics. Additionally, Popadic et al7 reported that there were changes in the appearance of their VH during the six-month follow-up period, according to both clinical and dermoscopic digital images. According to their report, the lesion was initially a solitary, purplish-brown plaque with a moderately rough surface. But three months later, the lesion showed significant regression and color change (turned bluish-purple). After an additional three months, the lesion had enlarged and developed into a dark, bluish-black, raised nodule with irregular borders and a hyperkeratotic surface. Based on our patient’s history, her angiomatous-verrucous lesions first emerged as erythematous, punctate, smooth patches that later developed hemangiomatous vesicles, which became hyperkeratotic. The manner in which the lesions evolved was compatible with a VH diagnosis. Yet, Kim et al17 described a 15-year-old boy who developed a solitary AK in an area of LC following repeated local trauma. They reported that, even though it was possible that the AK and LC arose independently from each other, they believed the AK was formed in the underlying LC through repeated injuries.
Chen et al2 reported a 48-year-old female patient with a late onset of AS whom developed atypical distributions (i.e., the lateral side, sole, and toes of her left foot) and had atypical clinical and histopathological (e.g., epidermal hyperkeratosis) findings. The authors reported that the lesions progressed quickly after the patient started hormone replacement therapy, and then slowed in progression after the therapy was stopped. They hypothesized that the increase in hormones induced receptors located on the surface of the endothelial cells in the skin lesions and contributed to the progression of the lesion. Similarly, regarding the genesis of AS, Neumann et al18 suggested that dermal vessel damage due to exposure to cold might lead to an abnormal vascular response, and the formation of new capillaries might subsequently aggregate to form larger ectatic vessels. However, the lesions on our patient had been present since birth, and she did not report any exposure to repeated trauma, severe cold, or hormonal therapy.
Unlike these similar combined and unusual congenital vascular lesions reported in the literature, the different lesions on our patient showed many transitions from one zone to an another, clinically and histologically. Even though, in whole, the lesion had clinical characteristics of five different types of lesions, after considering how the combined lesions changed over time, along with their intertwined histopathological features and unusual histopathological results, we concluded that the lesion was a combination of LA, LK, and VH—a hybrid entity not yet reported in the literature (to the best of our knowledge). We propose the name “Blaschko-linear congenital mixed hemato-lymphangio-keratoma serpiginosum” to represent the distinctive combination of lesions observed in our patient. Additionally, when considering the segmental location of the lesions, it seems likely that they formed due to some somatic mutations in the different developmental stages of pluripotent mesenchimal cells, which have both angiomatous (blood vessel) and lymphangiomatous (lymphatic vessel) potentials. In our opinion, the mutations were likely responsible for dysfunctional differentiation, migration, and placement of the vascular endothelial cells in the skin.
In 1976, Whimster et al19 proposed a model of pathogenesis for LC development and reported that, during embriogenesis, abnormal lymphatic cisterns grow independently from normal lymphatics in the deep subcutaneous tissue. Contraction of smooth muscle cells that line these cisterns cause dilatation and protrusion of lymphatic vesicles from the skin and result in the appearance of lesions.20 In 2004, Martinez-Menchon et al21 supported this hypothesis via their own imaging studies, which showed that large multi-lobular cisterns were located deep in the dermis and did not show any communication with adjacent normal lymphatics. In the light of this information, we also believe that external factors, such as trauma, friction, or cold temperatures, or internal factors, such as hormone imbalances, could be physical or biochemical triggers for an acquired transformation, proliferation, and migration to the upper layers of the pluripotent vascular rudimentary endothelial cells. Moreover, the transformed vascular endothelial cell type and degree of the transformation can be decisive in the clinical expression of the hybrid vascular malformations.
Regarding the treatment of LC, various modalities have been suggested. These include surgical excision; cryosurgery; electrocautery; surface ablation with laser (e.g., CO2, Er:YAG, pulsed-dye); sclerotherapy with sclerosing agents like OK-432 (picibanil); and superficial radiotherapy.22–24 However, for treatment of VH, superficial ablative procedures like electrocautery, cryosurgery, and laser ablation can lead to recurrence of the lesion due to its deeper components.25 The importance of complete excision and grafting is indicated in the successful therapy of the lesions.26 Recently, a topical application of imiquimod cream in the treatment of both LC27 and hemangioma28 has been recommended. In our case, the patient refused to undergo Er:YAG laser ablation; thus, we attempted to treat this unique combination of lesions with topical imiquimod. Unfortunately, due to lack of tolerance to the treatment by the patient and lack of improvement in the lesions, imiquimod therapy was discontinued after three months, and the patient was lost to follow-up. We hope that further studies in the fields of molecular genetics and immunobiology will elucidate the pathogenesis of such lesions.
References
- Requena L, Sangueza OP. Cutaneous vascular anomalies. Part I: Hamartomas, malformations and dilation of preexisting vessels. J Am Acad Dermatol. 1997;37(4):523–549.
- Chen JH, Wang KH, Hu CH, Chiu JS. Atypical angioma serpiginosum. Yonsei Med J. 2008;49(3):509–513.
- Bayramgurler D, Filinte D, Kiran R. Angioma serpiginosum with sole involvement. Eur J Dermatol. 2008;18:708–709.
- Aggarwal K, Gupta S, Jain VK, Marwah N. Congenital lymphangioma circumscriptum of the vulva. Indian Pediatr. 2009;46(5):428–429.
- Mülliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–422.
- Debbarman P, Roy S, Kumar P. Angiokeratoma circumscriptum neviforme. Indian Pediatr. 2012;49(1):80.
- Popadic M. Evolution of verrucous hemangioma. Indian J Dermatol Venereol Leprol. 2012;78(4):520.
- Wendt W, Kietzman H, Schubert C, Kaiserling E. Progressive lymphangiokeratoma and angiosarcoma (Stewart-Treves syndrome) in congenital lymphedema. Hautarzt. 1988;39(3):155–160.
- Terushkin V, Marmon S, Fischer M, et al. Verrucous lymphangioma circumscriptum. Dermatol Online J. 2012;18(12):9.
- Amouri M, Masmoudi A, Boudaya S, et al. Acquired lymphangioma circumscriptum of the vulva. Dermatol Online J. 2007;13(4):10.
- Gnanaraj P, Revathy V, Venugopal V, et al. Secondary lymphangioma of vulva: a report of two cases. Indian J Dermatol. 2012;57(2):149–151.
- Harwood CA, Mortimer PS. Acquired vulval lymphangiomata mimicking genital warts. Br J Dermatol. 1993;129(3):334–336.
- Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers of CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54(4):385–395.
- Ghosh SK, Bandyopadhyay D, Gloshal L, Haldar S. Angiokeratoma circumscriptum naeviforme: a case report of a rare disease. Dermatol Online J. 2011;17(9):11.
- Debelenko LV, Perez-Atayde AR, Mulliken JB, et al. D2-40 immunohistochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioentothelioma. Mod Pathol. 2005;18(11):1454–1460.
- Tennant LB, Mulliken JB, Perez-Atayde AR, Kozakewich HP. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23(3):208–215.
- Kim JH, Nam TS, Kim SH. Solitary angiokeratoma developed in one area lymphangioma circumscriptum. J Korean Med Sci. 1988;3(4):169–170.
- Neumann E. Some new observations on the genesis of angioma serpiginosum. Acta Derm Venereol. 1971;51(3):194–198.
- Whimster IW. The pathology of lymphangioma circumscriptum. Br J Dermatol. 1976;94(5):473–486.
- Terushkin V, Marmon S, Fischer M, et al. Verrucous lymphangioma circumscriptum. Dermatol Online J. 2012;18(12):9.
- Martinez-Menchon T, Mahiques-Santos L, Febrer-Bosch I, et al. Lymphangioma circumscriptum: an example of Whimster’s hypothesis. Pediatr Dermatol. 2004;21(6):652–554.
- Aggarwal K, Gupta S, Jain VK, Marwah N. Congenital lymphangioma circumscriptum of the vulva. Indian Pediatr. 2009;46(5):428–429.
- Mehta V, Nayak S, Balachandran C, et al. Extensive congenital vulvar lymphangioma mimicking genital warts. Indian J Dermatol. 2010;55(1):121–122.
- Gnanaraj P, Revathy V, Venugopal V, et al. Secondary lymphangioma of vulva: a report of two cases. Indian J Dermatol. 2012;57(2):149–151.
- Yasar A, Ermertcan AT, Bilal C, et al. Verrucous hemangioma. Indian J Dermatol Venereol Leprol. 2009;75(5):528–530.
- Pavithra S, Mallya H, Kini H, Pai GS. Verrucous hemangioma or angiokeratoma? A missed diagnosis. Indian J Dermatol. 2011;56(5):599–600.
- Wang JY, Liu LF, Mao XH. Treatment of lymphangioma circumscriptum with topical imiquimod %5 cream. Dermatol Surg. 2012;38(9):1566–1569.
- Sun ZJ, Zhao YF, Zhang WF. Immune response: a possible role in the pathophysiology of hemangioma. Med Hypotheses. 2007;68(2):353–355.

