 by Saakshi Khattri, MD; Orin Goldblum, MD; Kathleen Solotkin, MSN; Yasmin Amir, MD; Michelle S. Min, MD, MSci; Terri Ridenour, BSN, MBA; Fan Emily Yang, PhD; and Mark Lebwohl, MD, FAAD
by Saakshi Khattri, MD; Orin Goldblum, MD; Kathleen Solotkin, MSN; Yasmin Amir, MD; Michelle S. Min, MD, MSci; Terri Ridenour, BSN, MBA; Fan Emily Yang, PhD; and Mark Lebwohl, MD, FAAD
Dr. Khattri, Dr. Amir, Dr. Min, and Dr. Lebwohl are with the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Khattri is also with the Laboratory for Investigative Dermatology at the Rockefeller University in New York, New York. Dr. Min is also with Boston University School of Medicine in Boston, Massachusetts. Dr. Goldblum, Ms. Solotkin, Ms. Ridenour, and Dr. Yang are with Eli Lilly and Company in Indianapolis, Indiana.
J Clin Aesthet Dermatol. 2018;11(5):33–37
Funding: Funding provided by Eli Lilly and Company.
Disclosures: Dr. Khattri has received grant/research support from and is an investigator for Eli Lilly and Company. Dr. Lebwohl is an employee of Mount Sinai, which receives research funds from AbGenomics, Amgen, Anacor, Boehringer Ingelheim, Celgene, Ferndale, Janssen Biotech, Kadmon, LEO Pharma, Eli Lilly and Company, Medimmune, Novartis, Pfizer, Sun Pharma, and Valeant. Dr. Goldblum, Ms. Solotkin, Ms. Ridenour, and Dr. Yang own stock and are employees of Eli Lilly and Company. Dr. Amir and Dr. Min have no conflicts of interest relevant to the content of this article.
Abstract: Objective. The purpose of this study was to evaluate the speed of onset of clinical response to ixekizumab (IXE) and assess the progression of visible improvement in patients with moderate-to-severe plaque psoriasis.
Design. This was an interventional, randomized, open-label, Phase IIIb clinical trial.
Setting. This was a single center study at the Mount Sinai School of Medicine.
Participants. Twelve patients were randomized at a ratio of 1:1 to receive 80mg of ixekizumab every two (IXE Q2W) or four (IXE Q4W) weeks following a starting dose of 160mg of ixekizumab. After Week 12, all patients received 80mg IXE Q4W through Week 44.
Measurements. Clinical response was measured using the Patient’s Global Assessment (PatGA), the Psoriasis Area and Severity Index (PASI), the static Physician’s Global Assessment (sPGA), and the Itch Numeric Rating Scale (Itch NRS). Sequential patient photographs were taken at regular intervals during the study to evaluate visible improvement in plaque psoriasis.
Results. The median time to an improvement of at least 1 point or 2 points from baseline in PatGA score was 5.0 and 10.0 days for patients randomized to IXE Q2W and 6.0 and 13.5 days for patients randomized to IXE Q4W. All patients achieved at least a 50- or 75-percent improvement in PASI from baseline by Weeks 2 and 4, respectively. At least half of the patients achieved at least a 4-point improvement from baseline in Itch NRS by Day 14. Improvement in disease was visibly evident within one week of treatment in patient photographs.
Conclusion. Ixekizumab results in a rapid and visible improvement in plaque psoriasis in as early as one week of treatment.
Keywords: Plaque psoriasis, ixekizumab, early onset, randomized, Phase IIIb, photographs
Introduction
Plaque psoriasis is a common immune-mediated skin condition propagated by interleukin (IL)-17A and other pro-inflammatory cytokines.1 Ixekizumab, a high-affinity monoclonal antibody that selectively targets IL-17A, has been shown to result in a significant improvement in psoriasis following 12 and 60 weeks of treatment.2,3,4 Although the long-term efficacy of ixekizumab has been evaluated, the time to meaningful clinical improvement has not been fully assessed. In addition, the relationship between ixekizumab treatment and a visual improvement in skin over time has not been reported.
The primary objective of this study was to determine the time to improvement in disease as measured by a 1-point or greater improvement from baseline in the 6-point Patient’s Global Assessment (PatGA) scale. Secondary objectives included evaluation of the time course of clinical response to ixekizumab, which was measured by the percent improvement from baseline in the Psoriasis Area and Severity Index (PASI), the change from baseline in the Itch Numeric Rating Scale (NRS), and the time to a 2-point or greater improvement from baseline in the PatGA. Efficacy was also measured by the percentage of patients achieving a static Physician’s Global Assessment (sPGA) score of 0 or 1 or an sPGA score of 0. Standardized, sequential photographs were taken of each patient as visual evidence of improvement.
Methods
Patients were at least 18 years old with a history of moderate-to-severe plaque psoriasis spanning six months or longer. Patients were candidates for phototherapy and/or systemic therapy. Inclusion criteria also required that patients had at least a 10-percent body surface area (BSA) involvement, a PASI score of at least 12, a sPGA score of at least 3 (6-point scale), and a PatGA score of at least 3 at baseline. Patients were excluded if they were unwilling or unable to commit to the photography schedule for the study duration, if they had a form of psoriasis other than plaque psoriasis, if they had a serious disorder or illness other than psoriasis, if they had a serious infection within three months prior to baseline, if there was evidence or suspicion of active or latent tuberculosis, or if they were women who were pregnant or lactating. Patients were also excluded if they had previously received IL-17 or IL-23 antagonists, as both agents affect similar cytokines and signaling pathways and prior exposure could potentially confound interpretation of study results. All enrolled patients provided written informed consent prior to undergoing study related procedures.
For this 48-week, randomized, single-center, open-label study, patients were randomized at a ratio of 1:1 to receive 80mg of ixekizumab either every two (Q2W) or four (Q4W) weeks during the induction dosing period (0–12 weeks) following an initial 160mg dose of ixekizumab. All patients received 80mg ixekizumab Q4W during the maintenance dosing period (12–48 weeks), with the final dose of ixekizumab administered at Week 44. PatGA and Itch NRS were reported daily by patients using an electronic diary between Weeks 0 and 2, then twice a week from Weeks 2 to 4, once a week from Weeks 4 to 12, once per month from Weeks 12 to 24, and once every three months from Weeks 24 to 48. Clinicians assessed sPGA and PASI during each visit at Weeks 0, 1, 2, 3, 4, 8, 12, 24, 36, and 48. The study protocol was approved by the site’s institutional review board and the study was performed in accordance with the ethical standards of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study prior to undergoing study related procedures. Clinicaltrials.gov identifier: NCT02387801.
Anterior and posterior photographs were taken of the torso, upper extremities, groin, buttocks, and lower extremities of each patient under similar lighting and magnification conditions by a professional photographer. Photographs were taken twice-weekly (Weeks 0–3), once-weekly (Weeks 4–8), every two weeks (Weeks 8–12), then every 12 weeks (Weeks 12–48) with a two-day minimum between photos.
Time-to-event analysis was used to assess the time to a 1-point or greater improvement or a 2-point or greater improvement from baseline in the PatGA using the Kaplan-Meier product limit method. Response rates were summarized using nonresponder imputation to account for missing data. Safety data were summarized by frequency of adverse events occurring during the induction dosing period and the maintenance dosing period.
Results
Twelve patients were randomized to receive either IXE Q2W (6 patients) or IXE Q4W (6 patients) through Week 12. The baseline demographics and disease characteristics for each treatment regimen are provided in
Table 1.
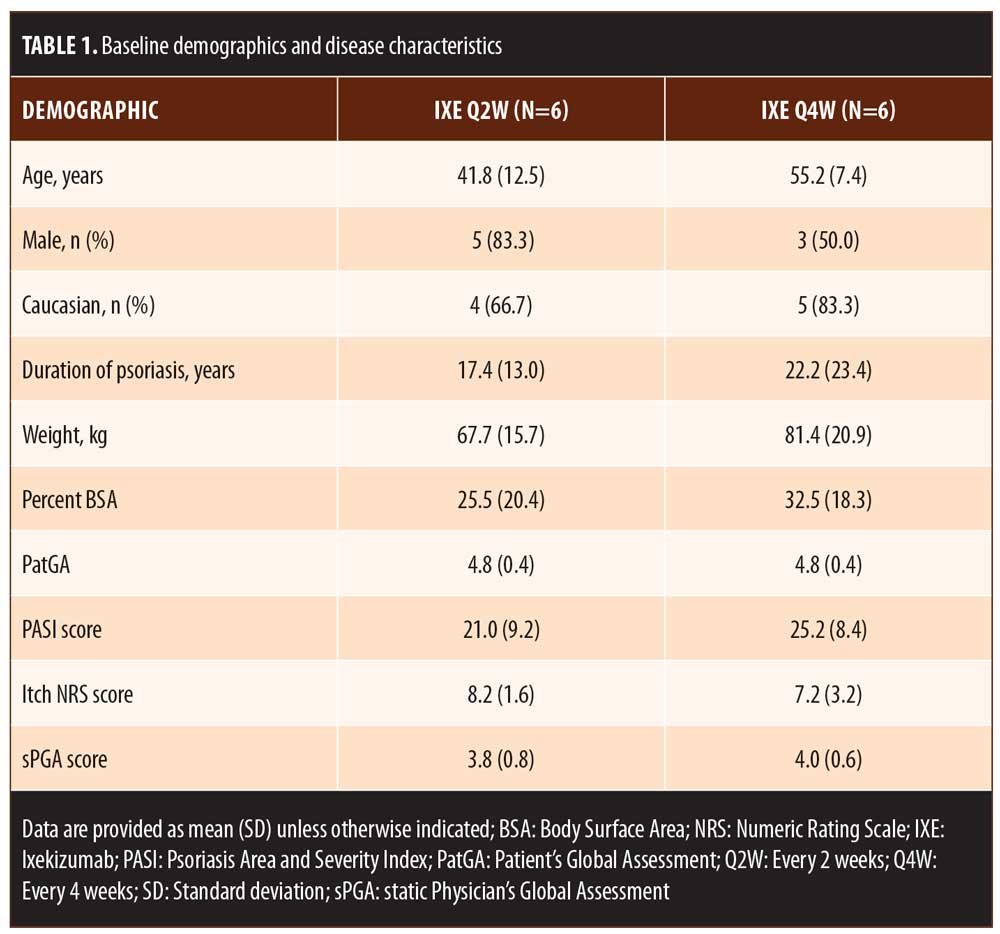
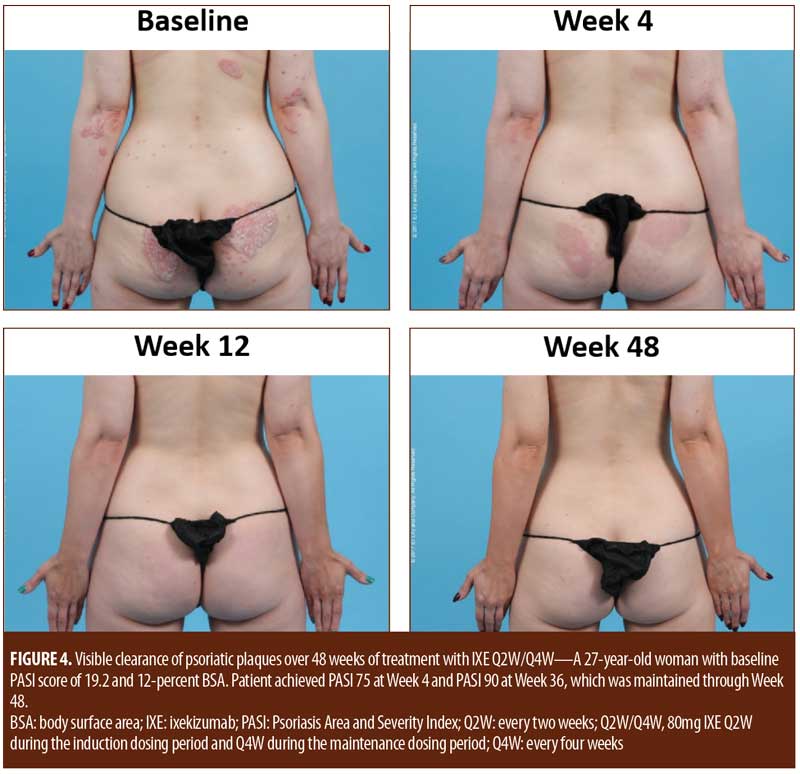
For patients randomized to IXE Q2W, the median time to a 1-point or greater improvement and a 2-point or greater improvement in the PatGA from baseline was 5.0 and 10.0 days, respectively. The median time to a 1-point or greater improvement and a 2-point or greater improvement from baseline in PatGA was 6.0 and 13.5 days for patients randomized to IXE Q4W, respectively. By Weeks 2 and 4, all patients achieved at least 50- or 75-percent PASI improvement from baseline, respectively (Figure 1). Of 11 patients with a baseline Itch NRS score of at least 4 (1 patient had a baseline Itch NRS score of 1), at least half (Q2W: 3 patients, 50.0%; Q4W: 3 patients, 60.0%) achieved at least a 4-point Itch NRS improvement from baseline by Day 14 (Figure 1C). Patient photographs demonstrated visible improvement in disease within one week of treatment (Figures 2–4 and Supplemental Video [click HERE to access video].
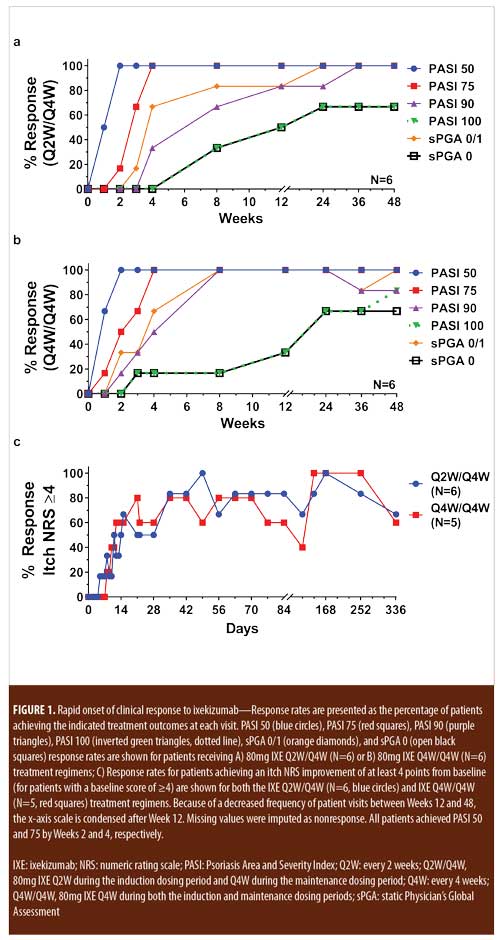
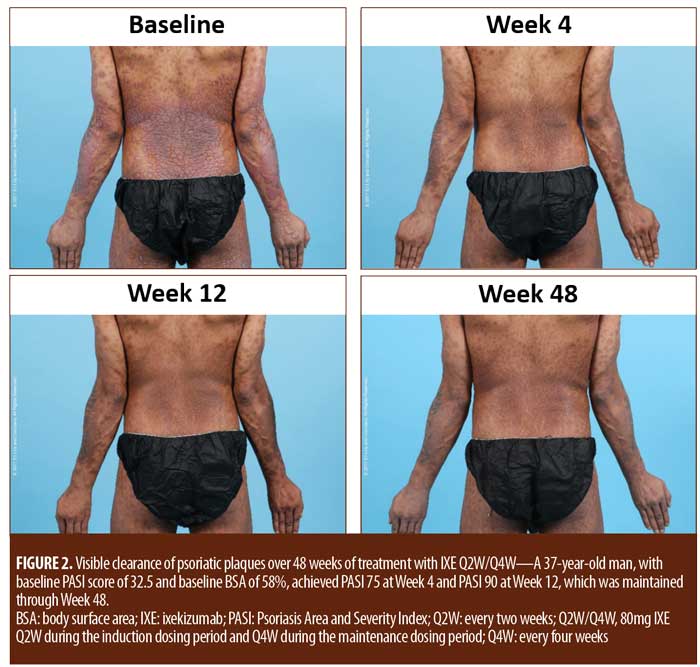
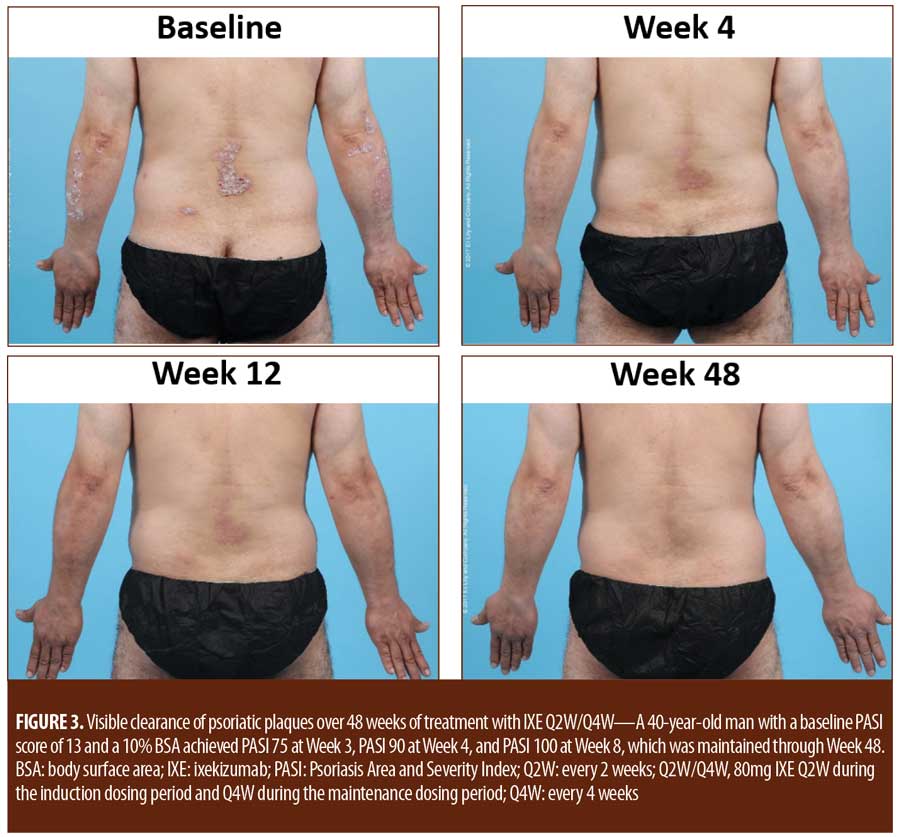
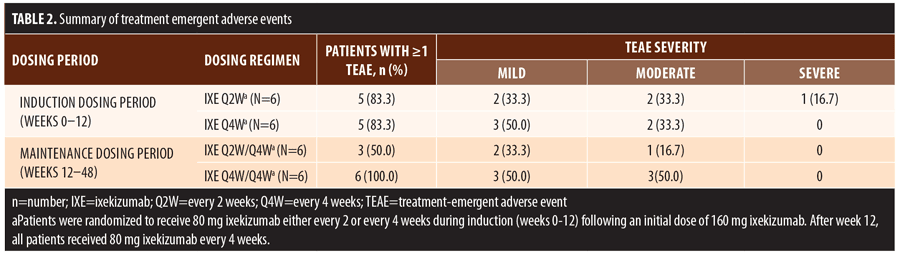
Treatment emergent adverse events (TEAEs) were mild to moderate in severity except for one severe TEAE of arthralgia
(Table 2). This single severe TEAE was adjudicated as unrelated to ixekizumab and resolved without changing treatment. There were no serious adverse events, deaths, or discontinuations during the trial.
Discussion
Previous studies of the efficacy of ixekizumab in patients with moderate-to-severe plaque psoriasis established the efficacy and safety of ixekizumab over long-term treatment in patients with moderate-to-severe plaque psoriasis.3,4 The current study was designed specifically to evaluate the early improvement in disease in response to ixekizumab and to determine the speed of onset of efficacy. In parallel to these quantitative assessments of efficacy, this study also assessed the qualitative visual improvement in psoriasis symptoms in response to ixekizumab treatment by obtaining sequential photographs of participants over time.
Although limited by a small patient population and lack of a placebo comparator, this study demonstrated that ixekizumab treatment resulted in a rapid and early onset of efficacy in as early as one week of treatment. In addition, the early clinical responses were maintained throughout the 48-week study, consistent with previously published results showing long-term maintenance of efficacy for patients receiving ixekizumab.3,4 Sequential patient photographs also demonstrated a rapid improvement in plaque psoriasis. Visible improvement was evident within one week of treatment and continued to improve over time. At 48 weeks of treatment, photographs demonstrated the maintenance of visible improvement in psoriasis symptoms, consistent with a persistent clinical response.
Conclusion
Patients with moderate-to-severe plaque psoriasis exhibited a rapid clinical response and visible improvement in psoriasis in response to treatment with 80mg of ixekizumab every two or four weeks. Both clinical response and visible improvement in disease were evident within one week and persisted through 48 weeks of treatment. There were no unexpected safety concerns observed during the study.
Acknowledgments
The authors thank Clinton Bertram, PhD, an employee of Eli Lilly and Company, for writing and editorial support.
References
- Campa M, Mansouri B, Warren R, Menter A. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelb). 2016;6(1): 1–12. doi:10.1007/s13555-015-0092-3.
- Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39–50. doi:10.2147/JIR.S100940.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–56. doi:10.1056/NEJMoa1512711.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541-51. doi:10.1016/S0140–6736(15)60125-8.

