 J Clin Aesthet Dermatol. 2020;13(12):22–26.
J Clin Aesthet Dermatol. 2020;13(12):22–26.
by Enayat Mohamed Atwa, MD; Mohamed Mahmoud Nasr, MD; and Howyda M. Ebrahim, MD
Dr. Atwa is a Professor of Dermatology, Venereology, and Andrology and member of the Faculty of Medicine at Zagazig University in Ash Sharqia Governorate, Egypt. Dr. Nasr is an Associate Professor of Dermatology, Venereology, and Andrology and member of the Faculty of Medicine at Zagazig University in Ash Sharqia Governorate, Egypt. Dr. Ebrahim is an Associate Professor of Dermatology, Venereology, and Andrology and member of the Faculty of Medicine at Zagazig University in Ash Sharqia Governorate, Egypt.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Intradermal botolinum toxin A (BTXA) is an advanced technique that emerged in response to the increased demand for noninvasive facial lifting and skin rejuvenation.
Objective: We sought to evaluate the safety and efficacy of intradermal injections of BTXA for facial lifting.
Methods: Twenty-five female patients with mild symmetrical facial skin laxity were enrolled in this study. All patients were treated with BTXA in one side of the face while the other side was injected with normal saline. The response to treatment was assessed by two dermatologists who evaluated global photographs using a quartile grading scale (QGS). The patient self-assessment and satisfaction questionnaires were recorded.
Results: A highly significant difference was found between the side injected with BTXA and the saline injected side (control) (p<0.001). Facial lifting was achieved in 58.66 percent of the sides injected with BTXA. Forty-four percent of patients were very satisfied. Older patients showed better improvement than younger patients (p=<0.001). The results persisted for 16 weeks. No adverse effects were observed.
Conclusion: Our results suggest that intradermal injection of BTXA could be a safe and effective therapeutic option for face lifting.
KEYWORDS: Nonsurgical face lifting, intradermal botulinum toxin A injection, microbotox, mesobotox
Face lifting surgery has been used to treat aging-related facial laxity and create a more youthful appearance, but is accompanied by a risk of scarring and infection.1 Botulinum toxin A (BTXA) is one of the most popular and less invasive aesthetic procedures available for the improvement of wrinkles.1–3 Treatment with intradermal BTXA is still a relatively new concept compared with conventional BTXA, which has been used for many years.4 Intradermal BTXA comprises multiple, smaller injections of diluted BTXA into the skin to achieve facial lifting and skin rejuvenation.5,6 Intradermal BTXA mainly targets the skin, not the muscle. Muscles responsible for facial expressions originate from the bone, and most of these muscles have an insertion in the skin.7 Therefore, using intradermal injection of BTXA, the function of the muscle is retained, resulting in preservation of a natural look compared to conventional BTXA, which targets mainly the muscles. Conventional BTXA treatment can lead to undesirable outcomes, such as a less natural, frozen expression.8 It has been reported that intradermal BTXA injection also can reduce pore size and decrease sebum secretion.9–11 Moreover, its injection into the jaw line results in lifting of the cheeks and jowls through its effect on the platysma muscle.12–14 It creates a more youthful appearance and can be used to rejuvenate the skin of the whole face and improve fine wrinkles under and around the eyes.15
Methods
Study design. This study was a double-blind, placebo-controlled, split-face design. All of the patients were injected with BTXA in the left side and saline in the right side of the face. It was carried out at the Dermatology, Venereology, and Andrology Department, Faculty of Medicine, at Zagazig University Hospitals in Egypt from March 2017 to October 2017 and was approved by the local ethics committee.
Patients. Twenty-five women, aged 30 to 50 years, with symmetrical facial skin laxity and a mild-to-moderate deepening in the nasolabial folds with mild sagging in the jowls (Grade 1, 2 score of facial sagging)16 participated in this study. Patients with a history of keloids formation, hypertrophic scars, human albumin allergy, hepatitis infection, facial asymmetry or eyebrow ptosis, active herpes simplex infection, autoimmune disorders, or neuromuscular diseases were excluded. Patients were also excluded if they were taking anticoagulants, aspirin, amino-glycosides, antibiotics, or if they were pregnant or lactating. Patients who had previously received other aesthetic treatments in the face or BTXA therapy within eight months prior to the study were also excluded. Informed consent was obtained from all patients. Full clinical and dermatological examinations were performed.
Reconstitution of BTXA vials. Each vial contained 50 units of botulinum toxin A (Refinex Botox Ltd, KC Pharmaceuticals, Pomona, California). The vial was diluted with 3.5cm of unpreserved saline. This gave the vial a final concentration of about 15U/mL. The vial was stored at 2 to 8°C and was used within 2 to 4 hours after reconstitution.
Technique of BTXA injection. The injection was performed with the patient in a semi-sitting position. Topical anesthetic EMLA cream (Eutectic Mixture of 2.5% Lidocaine HCl and 2.5% prilocaine; AstraZeneca AB, Sweden) was applied one hour before injection to diminish the discomfort during injection. The sites of injection were marked with a pen marker, and about 15 points were injected on each side. All subjects were injected on one side of the face with BTXA and with 0.9% normal saline (NS) on the other side. The intradermal injection was performed using 1mL insulin syringe of 32-gauge needle. At each point, 0.02mL per point was injected into the dermis to produce a wheal, and resistance should be felt, which indicated the injection was at the intradermal level. The injection points at one side of the face were as follows: four points arranged in two rows over the frontalis muscles below the hairline, two points at the temporal area along the hair line, one centimeter apart, points injected at one centimeter intervals in the crow’s feet area, and another two points in the front of the ear at the level of the tragus and auricle. Another four points along the mandibular line were injected at one-centimeter intervals. The same points on the other side of the face were injected with saline. All patients were instructed to avoid facial massage in the first 4 to 8 hours after injection.
Assessment. Photographs were taken using identical camera (Nikon Corporation, Tokyo, Japan) settings at baseline and two weeks after the injection. The clinical response was based on evaluation of the pretreatment and two weeks’ posttreatment photographs. Two independent dermatologists compared the photographs using quartile grading scale (QGS) as follows: less than (1–25%)=no improvement, 1=mild improvement (1–25%), 2=moderate improvement (26–50%), 3=good improvement (51–75%) and 4=excellent improvement (>75%). The participants rated their satisfaction with the treatment as very satisfied, satisfied, slightly satisfied, and unsatisfied. Regarding the self-assessment, the patients scored their assessment two weeks after the treatment based on four levels: no improvement, mild improvement, good improvement, and excellent improvement. Patients were followed up with for six months following treatment.
Statistical analysis. All analyses were performed with the Statistical Package for the Social Sciences (SPSS Inc., Chicago, Illinois) using program version 18.0. Spearman correlation coefficient was used. Logistic regression analysis was done to calculate the odds ratio of age. P-value of less than 0.05 was considered significant for all previous tests.
Results
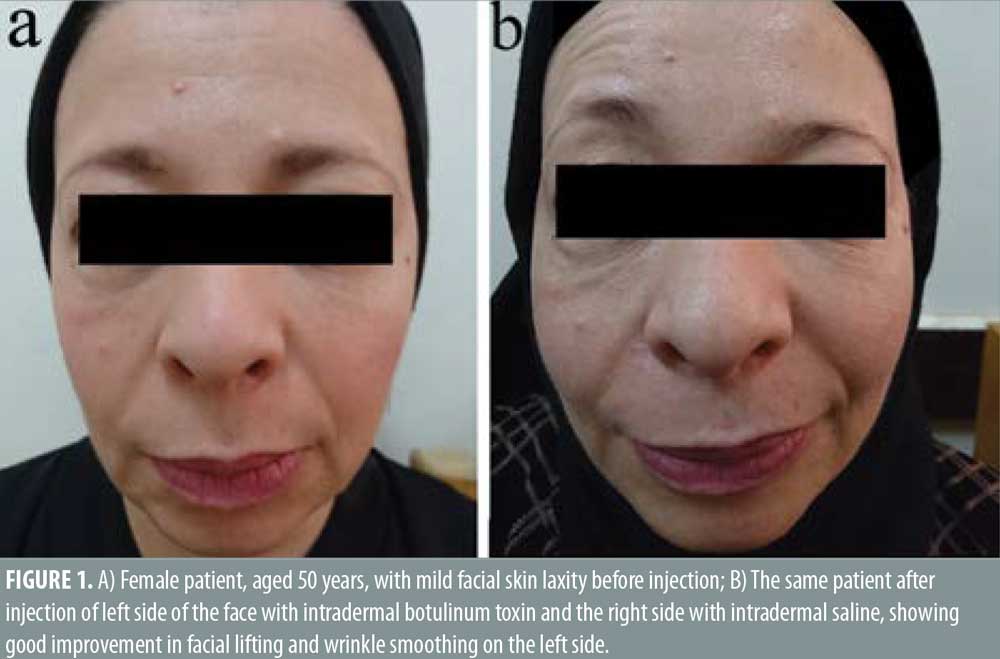
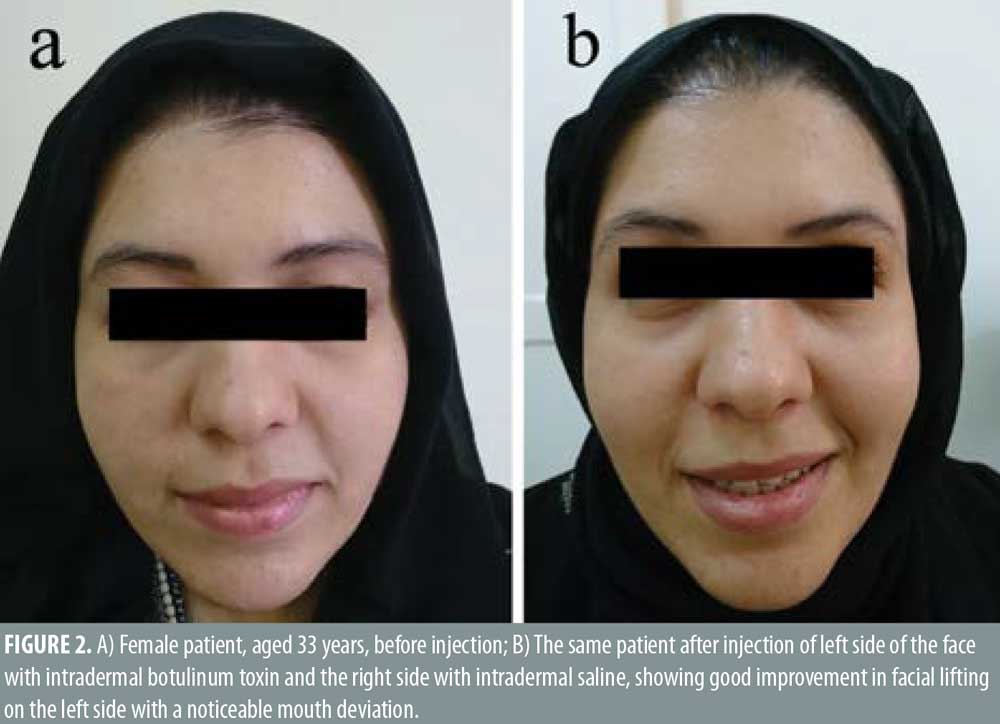
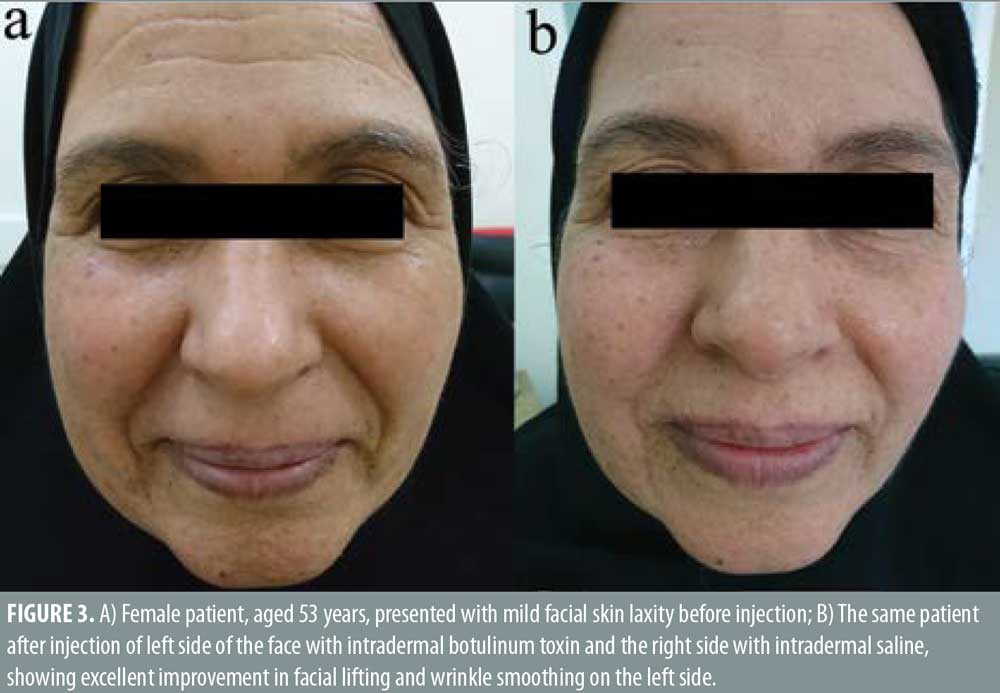
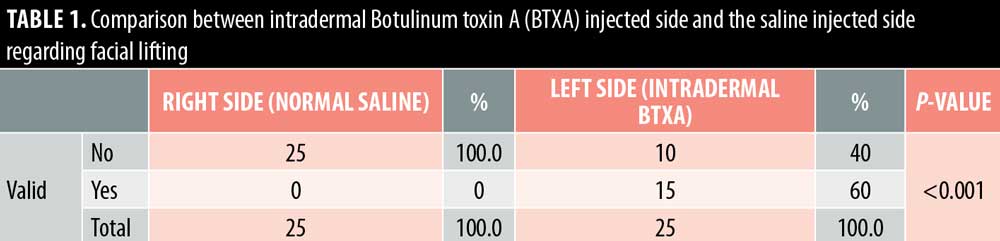
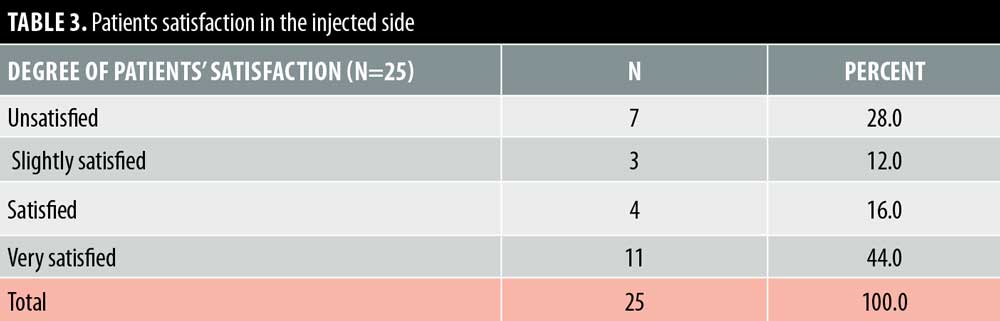
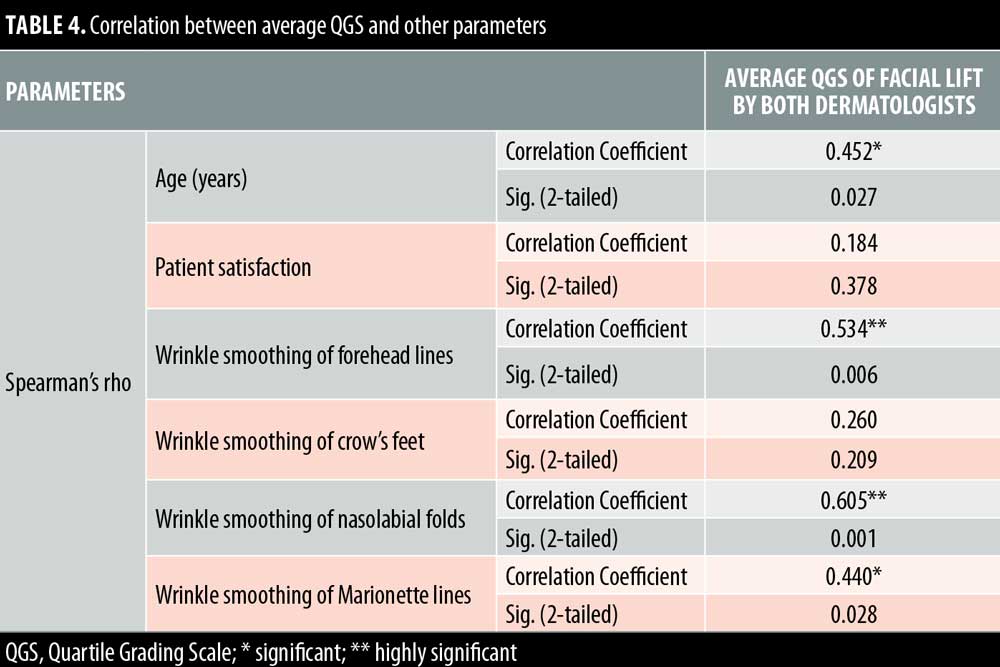
Twenty- five female patients with a mean age of 39.80±9.27 years were enrolled in this study. All patients completed the study. Facial lifting was achieved in 15 out of 25 patients (60%) on the side injected with BTXA intradermal (Figures 1–3). There was a statistically highly significant difference between the BTXA injected side compared to the normal saline injected side (p<0.001) (Table 1). Twenty-three patients out of 25 (92%) were able to identify the side injected with BTXA. Patients’ self-assessment revealed that 28 percent perceived excellent improvement, 16 percent perceived good improvement, 12 percent perceived moderate improvement, another 12 percent perceived mild, and 32 percent perceived no improvement (Table 2). Regarding wrinkle improvement, the forehead lines improved in 52 percent of patients compared to 16 percent in the glabellar lines (Table 3). Forty-four percent of patients reported a high degree of satisfaction, 16 percent of patients were satisfied, 12 percent were slightly satisfied, while 28 percent were unsatisfied (Table 4). Patients younger than 35 years achieved more than 50-percent improvement in the QGS (Table 4). There was a significant positive correlation between the average QGS and age (P=0.027). There was an improvement of the forehead lines (P=0.006), nasolabial folds (P=0.001), crow’s feet (P=209), and of the marionette lines (P=0.028) (Table 4).
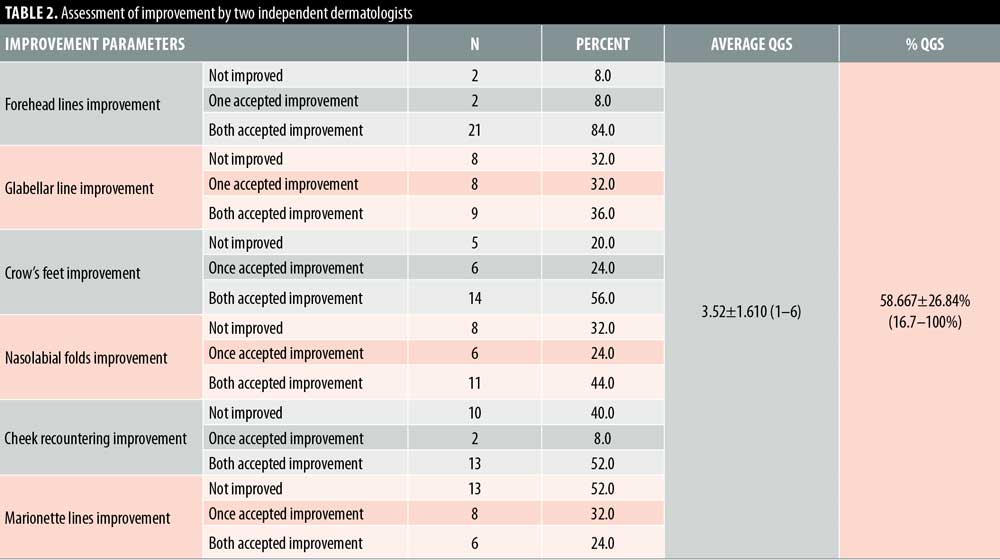
Side effects. One patient had moderate discomfort at the injection sites, which improved immediately after the injection.
Follow-up results. The effect of intradermal BTXA treatment lasted for 16 weeks.
Discussion
Facial aging is a challenging cosmetic problem. Facial aging is characterized by an imbalance between the levator and depressor muscles of facial expression together with gravity, which has a more pronounced effect on the depressor muscles, leading to dropping and sagging of the face.17–19 Intradermal BTXA is a technique used to correct mild sagging in the face.20 It can help to relax the depressor muscles and the lateral fibers of the orbicularis oculi. Moreover, it elevates the eyebrows, smooths forehead lines, realigns the jaw line.21,22 In this study, facial lifting was achieved in 58.667±26.84 of patients injected with intradermal BTXA, with a highly significant difference between the BTXA-injected side and the saline-injected side (P<0.001). In regard to improvement, 28 percent of the patients reported excellent improvement, 16 percent had good improvement, 12 percent had moderate improvement, 12 percent showed mild improvement, and 32 percent revealed no improvement. Twenty-three patients (92%) were able to identify the side injected with BTXA, which reflects an overall improvement of the wrinkle’s appearance. In regard to the degree of patient’s satisfaction, 11 patients (44%) were very satisfied, four patients (16%) were satisfied, and three patients (12%) were slightly satisfied, while seven patients (28%) were unsatisfied. Forehead lines showed a higher degree of improvement in (52%) of patients compared to improvement in the glabellar lines (32%). Intradermal BTXA seems to be more suitable for patients with flat and flaccid muscles, which are often associated with loose skin. It can soften wrinkles; however, it was unable to improve glabellar lines, as the muscles in this area are strong and might respond better to the conventional intramuscular injection. Our findings were consistent with another study that used intradermal abobotulinumtoxin A (ABO).20 They demonstrated that facial lifting occurred in 40.9 percent of the side injected with ABO compared to 4.5 percent on the side injected with saline (P=0.021). Nine cases had mild improvement and two cases showed moderate improvement. Smoothing of the wrinkles occurred in two cases.
Our results revealed that the facial lifting of older patients was higher than in the younger patients, while Wanitphakdeedecha et al20 reported that the facial lifting was higher in the patients younger than 32 years. In our study, the older patients with mild laxity responded better as a result of their weaker facial muscles and lax skin; therefore, any improvement would be noticeable. Also, Petchngaovilai21 injected 261 patients with intradermal BTXA for mid-face lifting. A greater improvement of 24.90 percent of cases was achieved in cheek lifting with smoothing of the nasolabial folds and recontouring of the face while 65.52 percent showed moderate improvement, and 9.58 percent reported minimal improvement of the facial recontouring. Petchngaovilai concluded that intradermal BTXA is effective in mid-face lifting.21 On the other hand, Chang et al22 used intradermal BTXA in nine patients to achieve facial lifting and wrinkle smoothing on the lower face. The authors noted an improvement of 66.6 percent in both skin texture and the wrinkles in the injected side; however, no facial lifting occurred, which might be attributed to a decrease in the number of participants. They concluded that the intradermal injection of BTXA can decrease the wrinkles.22 Wolffles et al23 used intradermal BTXA to treat the lower face and neck area. Here, patients showed an improvement in skin texture, neck creases, and contour of cervicomental angle. In contrast, Kapoor et al24 studied intradermal BTXA injection for face lifting in 10 participants. Half of their faces were injected with intradermal BTXA and the other half with saline. The results demonstrated that no difference between both treated sides. They reported that the negative results might be due to limited number of patients.24
In the current study, no severe adverse effects were reported except slight discomfort observed in most of the patients during the injection, which resolved immediately after the injection. Chang et al22 also reported that pain was tolerable among all of the patients in their study. Intradermal BTXA can be combined with the conventional type, particularly in the areas of deep muscles, such as corrugators, and with other cosmetic procedures, such as fillers or threads, to achieve additional favorable outcomes.
Conclusion
Intradermal BTXA injection appears to be safe and an effective alternative therapeutic modality for mild facial lifting especially in patients who do not prefer surgical methods. Our study was limited based on the subjective assessment methods and an absence of an objective scale of measurement. A larger sample is recommended to verify our results. Further studies are advised to compare intradermal and intramuscular BTXA injection for facial lifting.
References
- Ascher B, Zakine B, Kestemont P, et al. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol. 2004;51:223–233.
- Austin HW. The lip lift. Plast Reconstr Surg. 1968;77:990–994.
- Berry MG, Stanek JJ. Botulinum neurotoxin A: a review. J Plast Reconstr Aesthet Surg. 2012;65:1283–1291.
- Bonaparte JP, Ellis D, Quinn JG, et al. A comparative assessment of three formulations of botulinum toxin A for facial rhytides: a systematic review and meta-analyses. Syst Rev. 2013;2:40.
- Seo KK. Multiple Intradermal Botulinum Toxin Injections. In: Seo KK. Botulinum Toxin for Asians. Springer Singapore, 2017.
- Carruthers A, Bruce S, de Coninck A, et al. Efficacy and safety of onabotulinumtoxinA for the treatment of crow’s feet lines: a multicenter, randomized, controlled trial. Dermatol Surg. 2014; 40:1181–1190.
- Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008;7:847–850.
- Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg. 2013;39(3 Pt 1):443–448.
- Jerdan K, Fabi S. A noninvasive approach to off-face skin laxity and tightening: a review of literature. Semin Cutan Med Surg. 2013;34:118–128.
- Cavallini M, Cirillo P, Fundarò SP, et al. Safety of botulinum toxin A in aesthetic treatments: a systematic review of clinical studies. Dermatol Surg. 2014;40:525–536.
- Cerrene N, Giordano, Seth L, et al. Injectable and topical neurotoxin in dermatology, basic science, anatomy, and therapeutic agents. J Am Acad Dermatol. 2017;76:1013–1024.
- Chang SP, Tsai HH, Chen WY, et al. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Inter J Dermatol. 2008;47:1287–1294.
- Campanati A, Martina E, Giuliodori K, Consales V et al. Botulinum toxin off-label use in dermatology: a review. Skin Appendage Disord. 2017;3:39–56.
- Jia Z, Lu H, Zang X, et al. Adverse events of botulinum toxin Type A in facial rejuvenation: a systematic review and meta-analysis. Aesth Plast Surg. 2016; 40:769–777.
- Zhu J, Ji X, Xu Y, et al. The efficacy of intradermal injection of type A botulinum toxin for facial rejuvenation. Dermatol Ther. 2017;30(1).
- Alexiades AM. A quantitative and comprehensive grading scale for rhytides, laxity and photoaging. J Drug Dermatol. 2006;5:808–809.
- Noland ME, Lalonde DH, Yee GL, et al. Current uses of botulinum neurotoxins in plastic surgery. Plast Reconstr Surg. 2016;138: 519–530.
- Mahmoud BH, Burnett Cet, Ozog D. Prospective randomized split-face comparative study between topical botulinum toxin A surface application and local injection for crow’s feet. Dermatol Surg. 2016;42:554–556.
- Lee SK. Multiple intradermal small bolus injection of botulinum toxin: the limit and the potentiality. J Cosmet Laser Ther. 2012;14(6):304–306.
- Wanitphakdeedecha R, Ungaksornpairote C, Kaewkes A, et al. The comparison between intradermal injection of abobotulinumtoxin A and normal saline for face-lifting: a split-face randomized controlled trial. J Cosmet Dermatol. 2016;0:1–6.
- Petchngaovilai C. Mid face lifting with botulinum toxin: intradermal technique. J Cosmet Dermatol. 2009;8:312–316.
- Chang SP, Tsai HH, Chen WY, et al. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47:1287–1294.
- Wolffles TL. Microbotox of the lower face and neck: evolution of a personal technique and its clinical effects. Plast Reconstr Surg. 2015;136:92S–100S.
- Kapoor R, Shome D, Jain V, Dikshit R. Facial rejuvenation after intradermal botulinum toxin: is it really the botulinum toxin or is it the pricks? Dermatol Surg. 2010;Suppl 4:2098–2105.

