 Dear Editor:
Dear Editor:
Topical field-directed treatment is well-established for actinic keratosis (AK) lesions, which are considered premalignant sun-related skin lesions. The most commonly used local treatments for AK lesions are diclofenac, 5-fluroouracil, imiquimod, and ingenol mebutate.1
Ingenol mebutate, the latest introduction to the dermatologist’s therapeutic quiver for actinic keratosis, is an effective and well-tolerated option, with minimal local reactions and no reported systemic effects. Ingenol mebutate, a diterpene ester, is the active ingredient in the sap of the plant Euphorbia peplus. Here, we discuss the first reported case of severe pustular eruption of the face with systemic symptoms induced by ingenol mebutate.
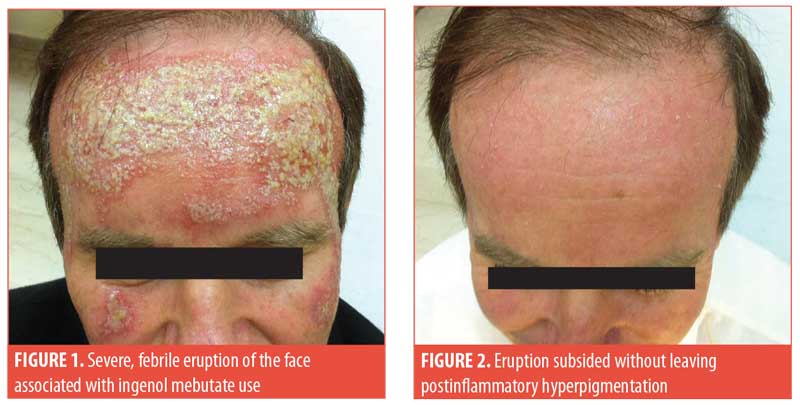
Case presentation. A 57-year-old man presented to our clinic with severe erythema, pustules, and crusts on his entire forehead and cheeks (Figure 1). He also complained of a burning sensation and headache. Upon examination, he was febrile up to 100.6°F. According to his history, he had previously used ingenol mebutate 0.015% gel but only for two (instead of three) consecutive nights as had been prescribed for his AK. On the second day of use, he developed a moderate skin reaction, as expected; however, this led him to discontinue use after the second day. In the three days that followed, the local reaction became more severe and the patient also experienced systemic symptoms. Cultures obtained from the pustules were negative for pathogenic organisms. Blood test findings were all within normal ranges, whereas chest X-ray and urine culture results were unremarkable. The fever subsided within a few days without any treatment. The rash was treated with normal saline compresses that were applied three times per day for 10 days, with excellent results (Figure 2).
Discussion. Local skin reactions are common after the topical application of ingenol mebutate and range from mild to moderate erythema, dryness, burning, pruritus and scaling. Swelling and vesicles/pustules have also been reported.2 The composite local skin response score on the face and scalp studies peaks around Day 4 and declines thereafter; whereas, on trunk and extremity studies, maximum composite scores occur on Days 3, 8 and 15.2 The majority of local skin responses resolve within two weeks.
Pharmacokinetic data from ingenol mebutate 0.05% applied on AK lesions on the forearms for two consecutive days did not reveal any quantifiable systemic absorption for areas up to 100cm2.3 Although there was no anticipated systemic exposure following the application of ingenol mebutate gel to the scalp or face due to the lower concentration used in these areas,3 a study from Bucko et al4 demonstrated quantifiable concentrations of ingenol mebutate and its metabolites via a validated bioanalytical assay in whole blood after treatment in patients who received 0.027% ingenol mebutate for AK lesions on the face. The United States Food and Drug Administration has indicated that the subnanomolar levels of ingenol mebutate often detected are not clinically relevant, taking into consideration in-vitro drug interactions, nonclinical pharmacology/toxicology studies, and electrocardiogram results. Nonetheless, the clinical data needed to assess the impact of such exposure are lacking.4 Contrary to treatment with ingenol mebutate, systemic absorption of other topical treatments, such as imiquimod, diclofenac, and fluorouracil, is well known; systemic side effects, including headache, malaise, and flu-like symptoms, have been reported following treament with these agents.5
In our case, the patient developed fever and malaise on the grounds of a severe topical reaction to ingenol mebutate application. Due to the minimal systemic bioavailability of ingenol mebutate that has only been described in higher concentrations prescribed to our patient, we speculate that the extensive inflammation induced by ingenol mebutate triggered a more generalized response. This individual event that has not been previously described in the literature highlights the potential for systemic adverse effects to arise from using this topical medication.
With regard,
Efstathios Rallis, MD, PhD and Angeliki Liakopoulou, MD
Affiliations. Dr. Rallis is with the Department of Biomedical Sciences at the University of West Attica in Athens, Greece. Dr. Liakopoulou is with the Department of Dermatology at Princess Alexandra Hospital in Harlow, United Kingdom.
Disclosures. The authors have no conflicts of interest relevant to the content of this article.
Correspondence. Efstathios Rallis, MD, PhD; Email: efrall@otenet.gr
References
- Goldenberg G, Prel M. Actinic keratosis: update on field therapy. J Clin Aesthet Dermatol. 2014;7(10): 28–31.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010–1019.
- Anderson L, Jarratt M, Schmieder G, et al. Tolerability and pharmacokinetics of ingenol mebutate 0.05% gel applied to treatment areas up to 100cm2 on the forearm(s) of patients with actinic keratosis. J Clin Aesthet Dermatol. 2014;7(12):19–29.
- Bucko AD, Jarratt M, Stough DB, et al. Pharmacokinetics of ingenol mebutate gel under maximum use conditions in large treatment areas. J Dermatolog Treat. 2018;29(1):74–79.
- Berman B. Safety and tolerability of ingenol mebutate in the treatment of actinic keratosis. Expert Opin Drug Saf. 2015;14(12):1969–1978.

