J Clin Aesthet Dermatol. 2019;12(12):E53–E57
 by Yates Yen-Yu Chao, MD; Fang-wen Tseng, MD; Yu-li Yang, MD; Ya-hui Chen, MD; Nai-jen Hsu, MD; and Li-yen Chang, MD
by Yates Yen-Yu Chao, MD; Fang-wen Tseng, MD; Yu-li Yang, MD; Ya-hui Chen, MD; Nai-jen Hsu, MD; and Li-yen Chang, MD
Dr. Chao is with the CHAO Institute of Aesthetic Medicine in Taipei, Taiwan. Dr. Tseng is with the Milano Aesthetic Clinic in Taipei, Taiwan. Dr. Yang is with the MED Aesthetic Clinic in Kaohsiung, Taiwan. Dr. Chen is with the YaSkin Dermatology Clinic in Kaohsiung, Taiwan. Dr. Hsu is with the Nai Jen Hsu Dermatology Clinic in Tainan, Taiwan. Dr. Chang is with Liyen’s Clinic of Plastic Surgery in Taichung, Taiwan.
FUNDING: Funding was provided by Merz Asia-Pacific Pte. Ltd., Taiwan Branch.
DISCLOSURES: Dr. Chao is the speaker, key opinion leader, and advisor of and has received honoraria for speeches, studies and travels from Merz, the manufacturer of incobotulinumtoxinA. Drs. Tseng and Chen have received speaker honoraria from Merz. Dr. Chang is a speaker and member of advisory boards for Merz. The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective: We assessed clinical effectiveness, longevity of treatment effects, and patient satisfaction with incobotulinumtoxinA for glabellar frown lines (GFL) treatment in Asian patients.
Design, Setting, and Participants: This was a prospective, multicenter, single-arm, open-label study at six sites in Taiwan. Patients aged 20 to 65 years with mild to very severe GFLs (Merz scale: 1–4 points) were eligible; 45 patients [including 23 BoNT/A-naïve and 22 previously-treated (“switch”) patients were enrolled. Patients received intramuscular incobotulinumtoxinA injection at up to five injection points. Total doses ranged from 12 to 20U.
Measurements: Investigators assessed improvements in dynamic GFLs at Days 14 and 120 using the validated five-point Merz scale (0=no lines; 4=very severe lines). Treatment satisfaction was self-reported by patients via questionnaire.
Results: All patients showed excellent response to treatment in that Merz scores at Day 14 were 0 or 1 point(s). Both groups showed a mean improvement of 2.9 points; the response rate (1-point improvement or more from baseline) was 100 percent. GFL improvement was maintained over at least four months in both groups (mean improvements at Day 120: 1.5 points, naïve; 1.7 points, switch). Patient satisfaction ratings remained high (almost 100% in both groups) throughout the study. There were no statistically significant differences between groups regarding treatment satisfaction or GFL improvement (Merz score) at Days 14 and 120. No adverse events occurred.
Conclusion: In Asian patients, incobotulinumtoxinA treatment for dynamic GFLs is effective and long lasting, with no expected differences between BoNT/A-naïve patients and those switching from other BoNT/As. IncobotulinumtoxinA yields consistent and natural-looking results for first and subsequent treatments.
KEYWORDS: Botulinum neurotoxin type A, incobotulinumtoxinA, glabellar frown lines, patient satisfaction, Asian
Botulinum neurotoxin type A (BoNT/A) injection is the most widely used nonsurgical cosmetic procedure for correcting glabellar frown lines (GFLs) and other facial wrinkles caused mainly by hyperdynamic muscles. Once BoNT/A is injected into the target facial muscles, it rapidly inhibits acetylcholine release at the neuromuscular junction, relaxing the muscles and preventing wrinkle formation.1,2 To eliminate facial wrinkles while retaining natural expressiveness, the practitioner must carefully assess a subject’s facial features and musculature and use optimal BoNT/A toxin doses and appropriate injection sites for each case. Treatment effects might be visible as early as one week after BoNT/A injection and can last several months.3–8 Reported patient satisfaction with BoNT/A treatment is generally high.9 However, as patients will need repeated treatments with BoNT/A, it is important to select a preparation with lower potential immunogenicity to minimize the risk of primary and secondary nonresponse to treatment.10
IncobotulinumtoxinA (Xeomin®; Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) was used in this clinical study.IncobotulinumtoxinA is a highly purified BoNT/A preparation free from complexing proteins, which are thought to contribute to partial or complete treatment failure secondary to neutralizing antibody formation.11 The efficacy and safety of incobotulinumtoxinA for GFL treatment has been demonstrated previously in clinical trials.3,4,12 Among four BoNT/A preparations commercially available in Asia, incobotulinumtoxinA was found to have the highest specific potency and lowest protein content, based on the quantitation of BoNTA neurotoxin content using sandwich enzyme-linked immunosorbent assays.10
Few studies have addressed the use of incobotulinumtoxinA for treating GFLs in Asian subjects, either in controlled studies or in daily practice settings.13–15 The Asian Doctors Hands on Experience through Real-life Efficacy (ADHERE) program was designed to expand on the limited available data. We conducted the Taiwanese ADHERE study amongst a typical sample of East Asian patients with GFLs and prospectively assessed both incobotulinumtoxinA treatment response and associated levels of patient satisfaction over four months. To our knowledge, this is the first study to examine potential differences in treatment outcome and patient satisfaction with incobotulinumtoxinA injection in BoNT/A-naïve and non-naïve patients.
Methods
This single-arm, prospective study enrolled patients with GFLs from six participating sites in Taiwan from February through September 2018. The study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki. The respective appropriate institutional review boards approved the study protocol and the patient informed consent document prior to the start of the study.
Patients. Eligible patients were male or female, aged 20 to 65 years, and scheduled to undergo treatment for GFLs. Patients were excluded if they had (1) received treatment with resorbable fillers and botulinum toxins in the preceding six months or treatment with nonresorbable fillers or surgery in the treatment area; (2) allergies to the study product; (3) contraindications against botulinum toxin treatment (including pregnancy and breastfeeding); (4) blood disorders, severe infection, septicemia, oncologic conditions, or neuromuscular diseases; or (5) circumstances that would not allow regular participation in the study. Patients who were naïve to BoNT/A treatment and those who were switching to
incobotulinumtoxinA treatment (after a washout period of at least six months) were included to evaluate potential differences between these groups. All patients provided their informed consent prior to the start of the study.
Study design. This single-arm prospective study enrolled 45 patients (23 BoNT/A treatment-naïve and 22 previously treated with other BoNT/A preparations). The target sample size was estimated based on earlier dose-ranging and proof-of-concept studies that included approximately 20 patients per group or treatment tested.8,14 The study product, incobotulinumtoxinA, was supplied in a single-vial pack of 100U of lyophilized powder for reconstitution with preservative-free 0.9% sodium chloride solution. The standard GFL treatment administered in this study was based on consensus recommendations on the aesthetic usage of BoNT/A in Asians.16 In a single treatment session, patients received intramuscular incobotulinumtoxinA injections at up to five injection points in the corrugator supercilii and procerus muscles (Figure 1). The maximum recommended dose for treatment of GFL is 20U per treatment session, divided among the injection points. In this study, within the range of 12 to 20U for the total dose, the treating physicians determined the dose used for each patient based on their clinical judgment, taking into account anatomical variation between individuals and GFL severity.
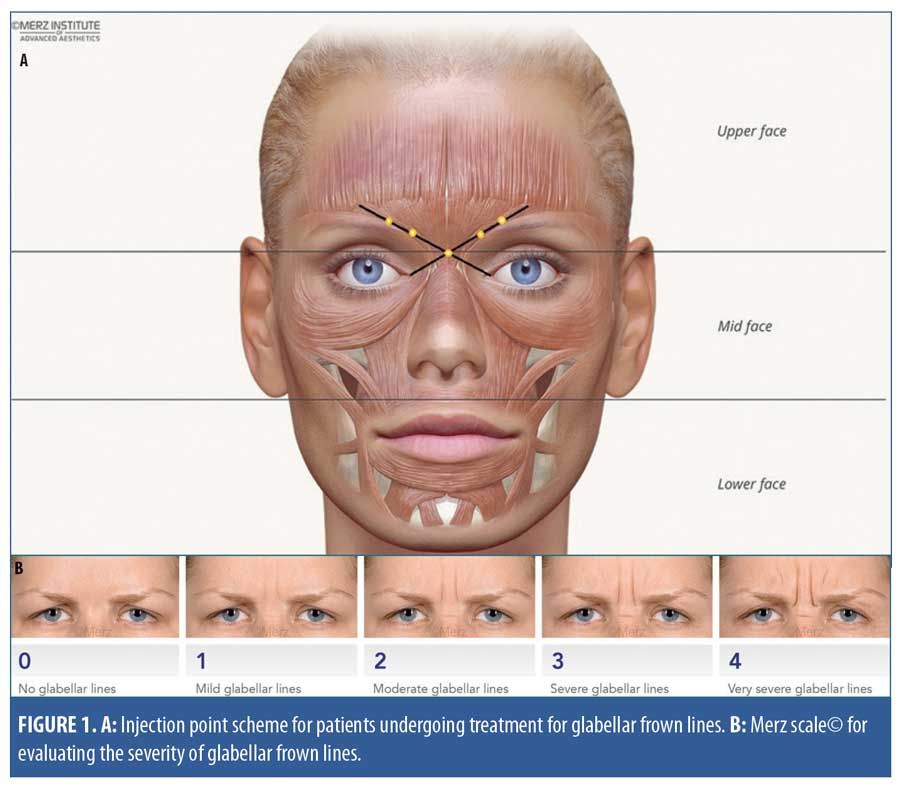
Study assessments and data analysis. All study assessments were performed at Day 0 (baseline) and on Day 14 and Day 120 after treatment. Photographs of the treatment site were taken before and after treatment. The physicians assessed posttreatment improvements in GFL (dynamic, at maximum frown) using the validated Merz scale (Appendix A).17,18 This is a five-point scale used to rate the severity of GFLs and other facial lines. Scores range from zero points (“no lines”) to four points (“very severe lines”) (Figure 1B), and a change of one point or more is considered clinically meaningful.
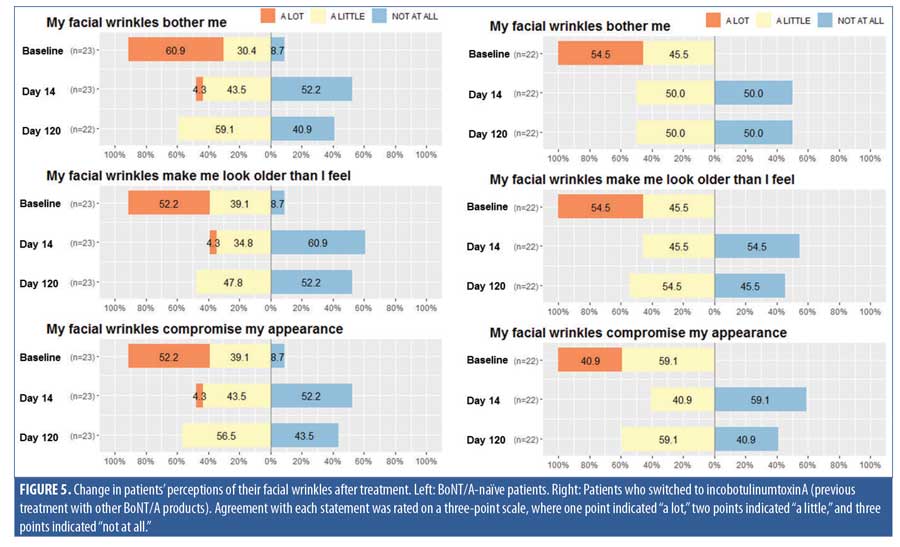
Patients completed a self-reported questionnaire (Appendix B) to rate their overall satisfaction with treatment on a four-point scale (4=very satisfied, 3=satisfied, 2=disappointed, and 1=very disappointed). Patients were also asked for their perceptions concerning their facial wrinkles (agreement/disagreement with statements on a three-point scale, with three points indicating “a lot,” two points indicating “a little,” and one point indicating “not at all”).
Patients’ baseline characteristics, Merz scale scores for GFLs, and scores for treatment satisfaction and the perception of facial wrinkles were summarized and presented using descriptive statistics and graphics. The Wilcoxon signed-rank test was used to evaluate whether the mean Merz scores at each visit (Day 4 and Day 120) were significantly different from the mean scores at baseline. The chi-squared test or Fisher’s exact test was used to evaluate the significance of differences between groups in terms of treatment response rate and patient satisfaction at each visit. All tests of significance were conducted at the five-percent level. Statistical analyses were performed using R (version 3.5.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics. Of 45 patients enrolled, 23 (51.1%) were treatment-naïve and 22 (48.9%) previously received GFL treatment with BoNT/A other than incobotulinumtoxinA. Most patients were female (n=40; 88.9%). The mean age was 41.4 (standard deviation: 9.8) years and the age range was 24 to 58 years.
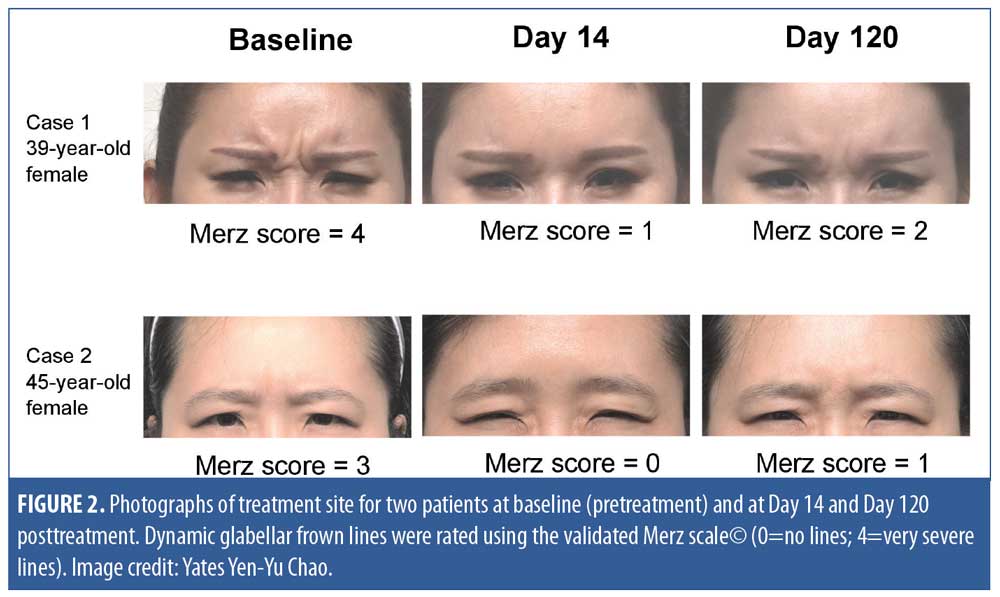
Treatment effectiveness. Figure 2 shows the changes in dynamic GFL severity (at maximum frown) and corresponding Merz scale scores in two patients, from baseline to Day 14 and Day 120 after treatment.
IncobotulinumtoxinA treatment was effective in both BoNT/A-naïve and switch patients, as shown by the changes in mean Merz scores from baseline to Day 14 and Day 120 in the two groups (Figure 3). At baseline, GFLs were assessed as “severe” in both groups, with mean Merz scores of 3.2 (standard deviation: 0.8) (naïve) and 3.0 (standard deviation: 0.7) (switch) points. Within 14 days after treatment, GFL severity was reduced to “minimal” in both groups, with mean scores of 0.3 (standard deviation: 0.4) (BoNT/A-naïve) and 0.1(standard deviation: 0.4) (switch) points. At Day 14, both BoNT/A-naïve and switch groups showed a mean improvement of 2.9 points from baseline (p<0.05 for both).
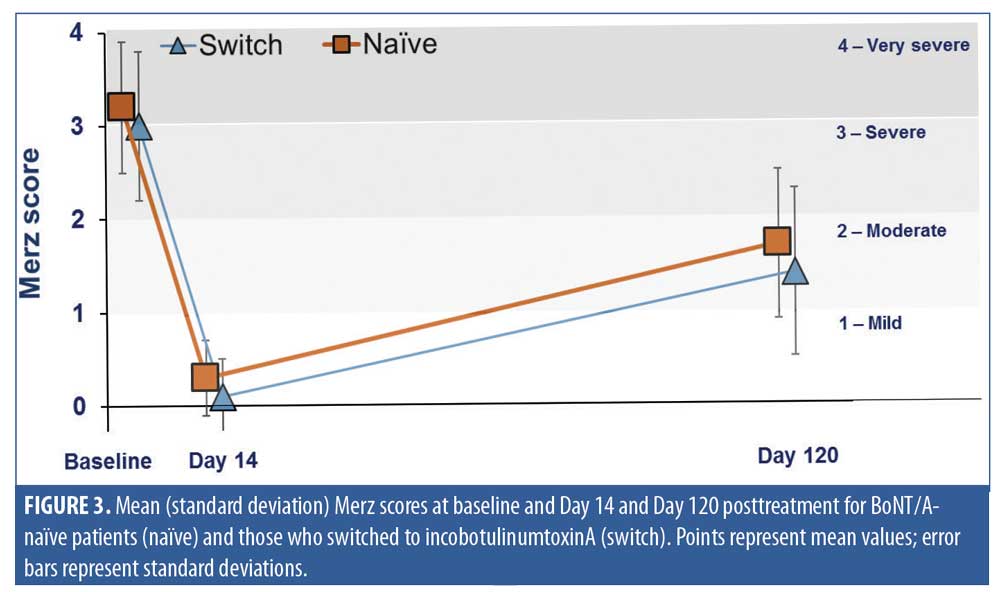
Improvement in GFL appearance was maintained for at least four months. At the end of the study (Day 120), the mean GFL severity was “mild” in both groups (Figure 3), with mean improvements of 1.5 points in the BoNT/A-naïve group and 1.7 points in the switch group (p<0.05 for both).
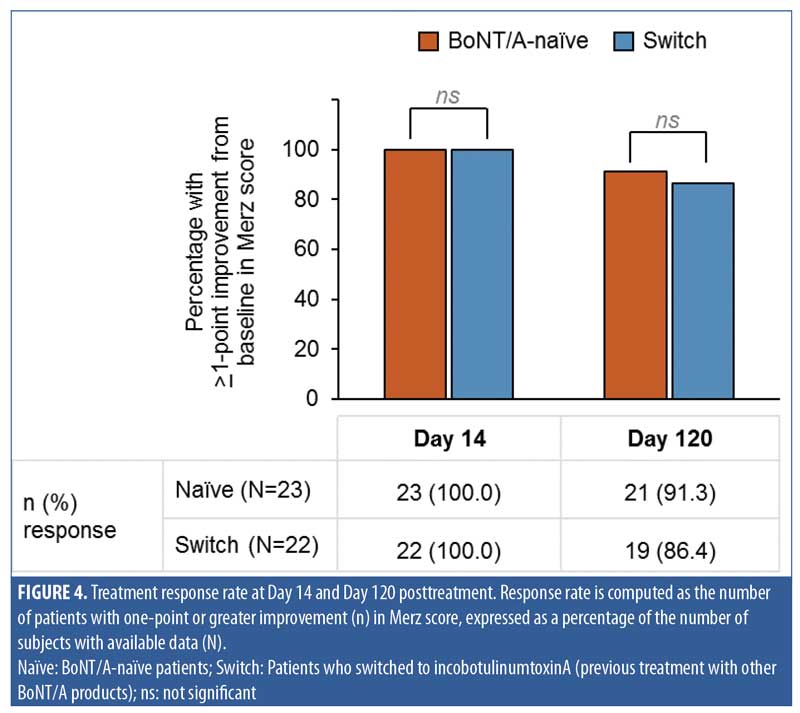
The treatment response rate (percentage of patients with a greater than 1-point improvement from baseline in Merz score) was 100 percent at Day 14 and 88.8 percent (40/45 patients) at Day 120, confirming the longevity of the treatment effects in the majority of patients. At each visit (Day 14 and Day 120), there was no significant difference between the naïve and switch groups observed in terms of response rate (Figure 4).
Treatment satisfaction. Treatment satisfaction (percentage of patients who were “satisfied” or “very satisfied”) was more than 95 percent in both the naïve and switch groups at Day 14. The level of treatment satisfaction was high among both BoNT/A-naïve and switch patients (Table 1). At Day 14, 95.7 percent and 100 percent of naïve and switch patients, respectively, were “satisfied” or “very satisfied” with their treatment. At Day 120, all patients in both groups reported that they were “satisfied” or “very satisfied” with their treatment. Patients in both groups agreed that the results of incobotulinumtoxinA treatment looked natural to them (95.6% of all patients at Day 14 and 100% at Day 120).
At baseline, the majority of patients reported that their facial wrinkles bothered them, made them look older, and/or compromised their appearance “a lot” (Figure 5). For both BoNT/A-naïve and switch patients, perceptions of their wrinkles were markedly improved after treatment, with most patients reporting at Day 14 and Day 120 that their wrinkles bothered them only “a little” or “not at all.” The pattern of improvement was not statistically different (p>0.05) for BoNT/A-naïve and switch patients (Figure 5).
Adverse events. No adverse events or study-related adverse events were reported during the study period.
Discussion
Treatment of GFLs is one of the most common cosmetic applications of BoNT/A injection. Our prospective study expands on currently limited data on incobotulinumtoxinA for GFL treatment in Asian patients in routine practice settings.14,15 From across six study sites in Taiwan, we recruited a typical cross-section of Asian patients seeking GFL treatment in terms of age and sex, as well as equal proportions of patients with and without previous BoNT/A treatment experience. Our results demonstrate the effectiveness and longevity of incobotulinumtoxinA treatment effects in Asians, accompanied by high levels of patient satisfaction. No adverse events were reported in this study, confirming the favorable safety profile of this BoNT/A preparation already demonstrated in clinical trials.3,19
With BoNT/A-based wrinkle treatment, the optimal outcome is a natural-looking smoothing of the facial lines while preserving the normal expressiveness. Our patients’ perceptions of their wrinkles were rapidly and dramatically improved, and they agreed that the results looked natural. For patients, the quality of treatment outcomes and longevity of effects are key considerations, whether they are receiving their first treatment or seeking retreatment for GFL reappearance. In all of our patients, excellent results were obtained within 14 days of incobotulinumtoxinA injection (Figures 2 and 3). This is comparable to results among Caucasians, where treatment response and a reduction of facial wrinkle scores to minimal or mild levels were seen within 14 to 30 days.3,8,12
Importantly, we observed that the treatment was just as effective and long-lasting in BoNT/A-naïve patients as in those who switched to incobotulinumtoxinA (Figures 3 and 4). Both groups also reported high levels of satisfaction over the four-month study period (Table 1). At four months, both naïve and switch patients retained at least a one and a half-point improvement of average from baseline, indicating that meaningful improvements in GFL appearance can last for more than four months. Moreover, the high satisfaction reported in both groups over the study period (Table 1) indicates that this improvement was both easily perceived and meaningful to patients.
These observations suggest that many patients could continue to experience treatment benefits and improved appearance for four months or more after incobotulinumtoxinA injection. Although a longer follow-up period might be desirable to confirm the maximum effect duration, four months is a practical and clinically relevant period over which to assess treatment effects. Our results are compatible with expected intervals of five months or more between repeat treatments.6
The evidence from comparative studies indicates that major commercially available BoNT/A products have similar aesthetic efficacy,20–22 taking into account their different unit potencies and dose response curves.23 This is important to note, although individual or brand preference can also influence the decision to switch or remain with a particular BoNT/A preparation for specific applications. Our clinical observations confirm that, in the hands of experienced practitioners and taking into account product-specific characteristics, incobotulinumtoxinA treatment has a similar effect onset and yields excellent and long-lasting results in Asian patients, coupled with high patient satisfaction, as seen among Caucasian patients.
In conclusion, our clinical experience in Taiwan suggests incobotulinumtoxinA is an effective tool for facial rejuvenation in Asian patients with GFLs.
Acknowledgments
Manuscript drafting and technical editorial support was provided by Tech Observer Asia Pacific Pte. Ltd., Singapore. Funding for this was provided by Merz Asia-Pacific Pte. Ltd., Taiwan Branch, Taipei, Taiwan.
References
- Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry. 2004;75(7):951–957.
- Carruthers JA, Lowe NJ, Menter MA et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol 2002;46(6):840–849.
- Carruthers A, Carruthers J, Coleman WP, 3rd, et al. Multicenter, randomized, phase III study of a single dose of incobotulinumtoxinA, free from complexing proteins, in the treatment of glabellar frown lines. Dermatol Surg. 2013;39(4):551–558.
- Hanke CW, Narins RS, Brandt F et al. A randomized, placebo-controlled, double-blind phase III trial investigating the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg 2013;39(6):891–899.
- Nestor M, Ablon G , Pickett A. Key parameters for the use of abobotulinumtoxinA in aesthetics: onset and duration. Aesthet Surg J. 2017;37(Suppl_1):S20–S31.
- Pavicic T, Prager W, Kloppel M et al. IncobotulinumtoxinA use in aesthetic indications in daily practice: a European multicenter, noninterventional, retrospective study. Clin Cosmet Investig Dermatol. 2015;8:135–142.
- Rappl T, Parvizi D, Friedl H et al. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double-blind study. Clin Cosmet Investig Dermatol. 2013;6:211–219.
- Prager W, Bee EK, Havermann I , Zschocke I. Onset, longevity, and patient satisfaction with incobotulinumtoxinA for the treatment of glabellar frown lines: a single-arm, prospective clinical study. Clin Interv Aging. 2013;8:449–456.
- Fagien S , Carruthers JD. A comprehensive review of patient-reported satisfaction with botulinum toxin type a for aesthetic procedures. Plast Reconstr Surg. 2008;122(6):1915–1925.
- Frevert J, Ahn KY, Park MY , Sunga O. Comparison of botulinum neurotoxin type A formulations in Asia. Clin Cosmet Investig Dermatol. 2018;11:327–331.
- Benecke R. Clinical relevance of botulinum toxin immunogenicity. BioDrugs. 2012;26(2):e1–e9.
- Imhof M , Kuhne U. A Phase III Ssudy of incobotulinumtoxinA in the treatment of glabellar frown lines. J Clin Aesthet Dermatol. 2011;4(10):28–34.
- Seo K, Tsai TF, Chao YY , Goodman GJ. Efficacy and safety of incobotulinumtoxinA in Asian subjects: a pooled analysis of clinical trials in the treatment of glabellar frown lines. J Drugs Dermatol. 2016;15(9):1084–1087.
- Lim JTE, Loh DK, Soh K , Sunga O. Efficacy and patient satisfaction with incobotulinumtoxinA for the treatment of glabellar frown lines. Singapore Med J. 2017;58(10):606–609.
- Park JY, Sung NK , Pitt JM. Efficacy and tolerability of incobotulinumtoxinA for treating glabellar frown lines in Korean adults: a postmarketing observational study. Dermatol Surg. 2017 Sep 28. [Epub ahead of print].
- Sundaram H, Huang PH, Hsu NJ et al. Aesthetic applications of botulinum toxin A in Asians: an international, multidisciplinary, Pan-Asian consensus. Plast Reconstr Surg Glob Open. 2016;4(12):e872.
- Flynn TC, Carruthers A, Carruthers J et al. Validated assessment scales for the upper face. Dermatol Surg. 2012;38(2 Spec No.):309–319.
- Carruthers A , Carruthers J. A validated facial grading scale: the future of facial ageing measurement tools?. J Cosmet Laser Ther. 2010;12(5):235–241.
- Sattler G, Callander MJ, Grablowitz D et al. Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatol Surg. 2010;36 Suppl 4:2146–2154.
- Prager W, Nogueira Teixeira D , Leventhal PS. IncobotulinumtoxinA for aesthetic indications: a systematic review of prospective comparative trials. Dermatol Surg. 2017;43(7):959–966.
- Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15(1):1–9.
- Walker TJ , Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7(2):31–39.
- Brin MF, James C , Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227–241.

