J Clin Aesthet Dermatol. 2023;16(10):59–64.
J Clin Aesthet Dermatol. 2023;16(10):59–64.
by Alexis E. Carrington, MD; Jessica Maloh, ND; Yvonne Nong, MD, MS; Oma N. Agbai, MD, MS;
Apple A. Bodemer, MD; and Raja K. Sivamani, MD, MS, AP
Dr. Carrington is with the Department of Dermatology at George Washington School of Medicine and Health Sciences in Washington, DC. Dr. Nong and Drs. Maloh and Sivamani are with Integrative Skin Science and Research in Sacramento, California. Dr. Nong and Drs. Agbai and Sivamani are with the Department of Dermatology at the University of California-Davis in Sacramento, California. Additionally, Dr. Nong is with SUNY Downstate Medical Center in Brooklyn, New York. Dr. Bodemer is with the Department of Dermatology at University of Wisconsin’s School of Medicine and Public Health in Madison, Wisconsin. Additionally, Dr. Sivamani is with the College of Medicine at California Northstate University in Sacramento, California and Pacific Skin Institute in Sacramento, California.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Maloh serves as a consultant for Codex Labs Corp. Dr. Sivamani serves as a scientific advisor for LearnHealth, Arbonne, and Codex Labs and has served as a consultant or speaker for Burt’s Bees, Novozymes, Biogena, Novartis, Sanofi, Bristol Myers Squibb, Pfizer, Nutrafol, Galderma, Novartis, Abbvie, Leo, UCB, Sun and Regeneron Pharmaceuticals.
ABSTRACT: Objective. This review examines the current literature on the gut-skin connection in alopecia and summarizes interventions that impact hair growth by modulation of the gut or skin microbiome.
Methods. PubMed searches were done to assess studies of the gut and skin microbiome and forms of alopecia including, alopecia areata (AA), androgenic alopecia (AGA), alopecia universalis (AU), central centrifugal cicatricial alopecia (CCCA) and lichen planopilaris (LPP). Filters were applied for human and animal studies. Articles not translated to English and studies assessing supplemental therapies on alopecia were excluded.
Results. There is evidence that scalp, hair follicle, and gut microbiome alterations are associated with various types of alopecia. There is potential in the use of interventions targeting microbiome dysbiosis, including fecal transplants and probiotics.
Limitations. This field of study still requires more high-quality research and studies with larger participant .opulations.
Conclusion. Dysbiosis on the scalp, within the hair follicle and the gut seem to have a role in the pathophysiology of various forms of alopecia. There is evidence that interventions targeting dysbiosis may have potential in the treatment and management of hair loss. Further studies are needed to establish a direct connection and to clarify specific effects of these interventions.
Keywords. Alopecia, hair loss, microbiome, dysbiosis; probiotic
The microbiome is a diverse community of microorganisms including bacteria, fungi, and viruses, which interact with the physiology of the host and, in turn, contribute to health and disease. In the gut, these microorganisms can play active roles in digestion, nutrient synthesis, barrier function, and immunity.1 A disruption or imbalance in the composition of the gut microbiome, also referred to as dysbiosis, has been associated with conditions such as alopecia, or hair loss.2–5 This review summarizes the existing literature on the human gut and skin microbiome in patients with alopecia to provide a better understanding of its pathogenesis and potential therapeutic interventions.
The Gut-Skin Connection in Alopecia
Recent literature suggests that there is an interplay between the gut and skin microbiome, and that this interplay is relevant to hair biology. Two case reports demonstrate this link with fecal microbiota transplants. In both cases, the transplants were used to treat gastrointestinal (GI) concerns, but unexpectedly improved coexisting cases of alopecia areata.6
In animal studies, supplementing mice with the probiotic, Lactobacillus reuteri, led to an increased number of hairs in the anagen (growth) phase, as well as an increase of subcuticular hair follicles. Additionally, there was an increased sebocyte count, a higher sebocyte proliferation index, and a more acidic tissue pH, compared to mice in the control group.7 These changes were associated with improved hair growth and luster. The authors suggest an anti-inflammatory effect, as L. reuteri was found to upregulate the anti-inflammatory cytokine, IL-10, and downregulate the inflammatory cytokine, IL-17.7 In addition, another study found supplementation with Lactococcus lactis subsp. cremoris H61 to be associated with reduced hair loss in mice relative to control.8 Here, the authors suggest a potential immunomodulatory effect of the lactococcal strain since the probiotic group was found to produce more Th1-associated cytokines (IL-12 and interferon gamma) than the control group.
The Microbiome of the Scalp Surface vs. the Hair Follicle
To better understand scalp and hair biology with regards to the microbiome, it is important to identify the microbial community of both the scalp surface, as well as within the hair follicle. These two regions offer unique environmental conditions which can impact the colonization of microorganisms. Relative to the epidermis, the hair follicle has increased moisture, decreased acidity, and lower levels of UV exposure.9 Additionally, it is relatively anoxic, which may facilitate the growth of anaerobic microbes.10 From an immunology perspective, the hair follicle is unique in that the superficial component of the hair follicle is densely infiltrated with immune cells, such as dendritic cells and CD4+ cells, whereas the deeper levels from the bulge and downward are considered to be “immune privileged”.11 At this depth, keratinocytes produce immuno-inhibitors,9 and it is hypothesized that microbial changes in these deeper levels of the hair follicle may disrupt the follicular immune system, ultimately contributing to pathologic changes in hair growth.11 Unlike skin swab collections which can be used to analyze the microbiome of the scalp surface, accessing the microbiome within the hair follicle requires a follicular collection or a biopsy-based collection.11
Alopecia Areata and the Microbiome
Scalp microbiome. The most abundant bacteria on the scalp swabs of healthy individuals are Cutibacterium spp., and Staphylococcus spp., predominantly C. acnes and S. epidermidis, respectively. 12–14 Studies have shown that patients with alopecia areata (AA) have an increased abundance of C. acnes and decreased abundance of S. epidermidis and S. aureus in the scalp, relative to controls.9, 15–17 One pilot study employed a skin swab technique and internal transcribed spacer (ITS) 16S rRNA sequencing and found a significant decrease in Clostridia and Malasseziomycetes on the scalp of people with AA.18
Hair follicle microbiome. One group that used follicular biopsy samples found a decreased abundance of the Acinetobacter genus, Candidatus Aquiluna rubra, Staphylococcus epidermidis, and 2 genera of Microthrix Acea and ACK-M1 families in the hair follicles of people with AA relative to healthy controls.16 In the epidermal layer in particular, the genus SMB53 (family Clostridiaceae) was absent, and Anaerococcus and Neisseria and Neisseriaceae family were found to be increased in people with AA.16
The preliminary findings of Rinaldi et. al. found a higher incidence of C. acnes in AA, AGA and LPP compared to a control of 15 participants.17 Further, the ratio between S. epidermidis and S. aureus was diminished in AGA subjects compared to controls. AA participants found a more diminished S. epidermidis to S. aureus ratio, where S. aureus was more predominant than S. epidermidis. C. acnes appeared to be less prominent in LPP compared to AGA and AA, causing them to hypothesize that the loss of sebaceous glands commonly found in LPP may play a role in the reduction of C. acnes.17
Gut microbiome. It is unknown how the gut microbiota might influence AA pathogenesis, however the association of AA and gut dysbiosis diseases is prominent, as seen in inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD).2–5 The gut dysbiosis in IBD is demonstrated by findings of decreased Bifidobacteria abundance, and increased Bacteroides and Enterobacteriaceae.19 Interestingly, in cases of UC, there is a significantly higher occurrence of AA than in the healthy population. Furthermore, in patients with AA, there is a significantly higher occurrence of UC than in healthy patients.4 Additionally, there is a three-fold increased occurrence of AA in CD than in controls.4 Sobolewska-Wlodarczyk et al reviewed the evidence and hypothesized that this link may be related to the common molecular and inflammatory pathways involved in the pathogenesis of AA and IBD. For example, increased levels of Th-1 cytokines, such as TNF-alpha, have been demonstrated and implicated in both AA and IBD.4 However, whether these common pathways are related to common microbial changes is not yet established.
A recent study by Moreno-Arrones et al on 30 patients with alopecia universalis (AU) suggested AU may not affect the overall gut microbiome, finding no significant differences in α-diversity or ß-diversity of the AU patients’ gut microbiome compared to healthy controls. However, they found an increased abundance of Holdemania filiformis, Erysipelotrichacea, Lachnospiraceae, Parabacteroides johnsonii, Clostridiales vadin BB60 group, Bacteroides eggerthii and Parabacteroides distasonis in stool samples, describing these species as potential bacterial biomarkers associated with AU.20 These bacteria have associations to inflammatory processes and diseases. For example, Erysipelotrichaceae bacteria is coated with IgA and associated with gastrointestinal inflammatory process, hinting at a possible role of provoking an immune response which may be relevant in AU.21,22
In another study comparing stool samples from patients with alopecia areata and controls, a significant increase in the class Bacilli and order Lactobacillales has been found in the gut of people with AA.18 Future research is needed to better understand the gastrointestinal microbial community in people with alopecia, and its interplay with the immune pathways on a systemic level.
Therapies Targeting the Microbiome in Alopecia Areata
Although the exact pathogenesis of the microbiome involvement of AA is not fully known, successful therapies targeting the gut-skin axis serve as evidence of its possible involvement.
Borde and Astrand conducted multiple studies assessing the influence of the short chain fatty acid (SCFA), propionate, as treatment for AA in mice, yielding conflicting results.23 In a pilot study (n=5), hair growth was observed after 11 weeks of 200mM propionate treatment compared to controls. However, no significant difference was found in the levels of regulatory T-cells (Treg) count in those mice (p=0.16) despite an increased Treg/CD4+ ratio (p=0.08). In a larger subsequent study (n=6 per group), the same group did not observe increased hair growth.23
Recent cases of fecal transplants have shown improvement of hair growth in patients suffering from AA.6,24 Rebello et al reported on two patients with alopecia universalis who both received fecal microbiota transplantation as an intervention for coexisting and recurrent cases of C. difficile.6 In each of these patients, the C. difficile infections resolved, and interestingly, an improvement in hair growth was also noted. A study by Xie et al reported another patient with untreated AA who received a fecal transplant for gut dysbiosis-related diarrhea. Following the transplantation, there was an improvement in his gastrointestinal concerns as well as notable hair regrowth and restored hair pigmentation.24 These case reports suggest a clinically relevant link between the gut microbiome and hair health and call for further investigation to better establish this connection.
There is evidence that AA can be influenced by nutrients and nutrient activity. In particular, vitamin D deficiency25–27 and low levels of vitamin D receptors (VDR)28–30 have been associated with alopecia areata. Theories on how vitamin D deficiency contributes to gut dysbiosis includes an associated reduction in SCFAs as well as impaired activity of the gut microbiota regulating VDR.31 Interestingly, several studies have shown that the expression of VDR and Cyp27B1 is regulated by gut microbiota.32 The gut microbiome can influence nutrient absorption and it is hypothesized that reversing gut dysbiosis may lead to improved absorption of nutrients that could impact hair regrowth, such as small chain fatty acids and vitamin D.24
A recent study assessed the efficacy of platelet rich plasma (PRP)-like cosmetic gel containing postbiotics on AA.33 The gel has been found to have antimicrobial, antioxidant and immunomodulatory effects. The gel included ingredients like postbiotics (plantaricin A), and Lactobacillus kunkeei-fermented bee bread, produced from beneficial bacteria. The treatment group receiving the gel had a significant change from their baseline SALT (Severity of Alopecia Tool) at two and three months of treatment compared to the placebo group in which no significant changes from baseline were seen.33 Additionally, the daily use of a systemic probiotic, Synbiotic 2000, over 16 weeks did not reverse chronic AA but did increase the Treg/CD4+ ratio in skin draining lymph nodes from 12% at baseline to 15%, (p<0.01, n=6).23
Androgenic Alopecia and the Microbiome
Microbiome of the scalp and hair follicle. As with AA, studies have shown an increase in abundance of C. acnes and a decrease of S. epidermidis in AGA, suggesting a role of dysbiosis in AGA as well.12,13,15 The presence of porphyrins, which C. acnes is known to synthesize, and of C3 in the pilosebaceous unit in patients with AGA may contribute to oxidation and follicular inflammation known to occur in alopecia.13
Ho et al were the first to characterize the microbiota of the middle and lower portions of the hair follicle from both the vertex and occiput in patients with AGA. They found abundant Burkholderia genera in the middle portion of the follicle and higher bacterial diversity in the lower hair follicle.34–36 Burkholderia cepacia and Burkholderia contaminans have been isolated from patients with cystic fibrosis and pneumonia but had not previously been associated with skin related disorders.37,38 Furthermore, Ho et al provide more insight into the follicular miniaturization implicated in AGA pathophysiology. A difference between the microbial community in miniaturized follicles relative to non-miniaturized follicles was demonstrated. More specifically, in the middle portion of miniaturized follicles from the AGA vertex, there was an increase in C. acnes and a decrease in Burkholderia spp. relative to the middle portion of non-miniaturized follicles, while in the lower portions of miniaturized follicles from the AGA vertex, there was a decrease in M. komagatae, Sphingomonadaceae, and Brevibacterium when compared to non-miniaturized follicles. Next, when patients with AGA were compared to controls, more variation between the two groups was noted in the lower portions of follicles relative to the middle. For example, there was an increase in C. acnes and a decrease in Brevibacterium in the lower portions of AGA occipital follicles relative to control. In this study, the authors hypothesize that the increased abundance of C. acnes observed in AGA follicles may correspond with immune responses and inflammatory pathways involved in pathology.34
Limited evidence suggests that the prevalence of fungi in addition to C. acnes in the hair follicles of people with AGA may lead to increased hair shedding. Malassezia has been the predominant fungi present in the scalp microbiome in patients with AGA.39,40 Malassezia globosa and Malassezia restricta was found more abundantly on the crown and occipital areas in AGA relative to control.40 Contradictory to this finding, a recent study analyzing the scalp microbiome of 12 male AGA (stage II or IV on the Norwood Hamilton Classification) versus healthy subjects, found a lower proportion of Malassezia genus in the scalps of people with AGA and an increase in other bacterial genera. Furthermore, Malassezia restricta and Malassezia globosa were significantly lower (P<0.05) in abundance in the AGA group.12 This same study showed a significant increase in Stenotrophomonas geniculata (P< 0.01) in patients with AGA, leading to the hypothesis that S. geniculata may induce inner root sheath disintegration due to the promotion of a keratinolytic environment.
Due to the limited number of studies and conflicting results, multiple pathogenesis of Malassezia spp are suspected, yet the true involvement is not yet known. One proposed theory involves the proteolytic and lipase activity of Malassezia, of which seven species have been identified.41 Specifically, it is proposed that Malassezia could decompose keratin and collagen, providing nitrogen sources for bacterial growth and allowing its own adherence to host cells in the skin, which has been observed on microscopic imaging.40 In addition, previous studies found that Malassezia can increase the levels of IL-8, IL-10, and TGF-b1 in keratinocytes15 influencing apoptosis of keratinocytes, chemotactic activity on inflammatory cells and erosive damage to hair roots.42 These pathways could contribute to a cycle of furthering the abundance of Malassezia and general scalp microbiome dysbiosis.
Given these results, the presence of Malassezia spp. in addition to the scalp environment of the host seems to be one potential catalyst of AGA disease progression. In the healthy scalp, it appears that Malassezia maintains a symbiotic relationship with the microbiome, but alteration of the scalp environment may play a role in the pathogenesis of AGA.43 An increase in scalp oil content could be one of those environmental triggers leading to the overgrowth and reproduction of Malassezia.40
Some species of the scalp microbiota are thought to contribute to micro-inflammation which would promote the pathogenesis of AGA. There has been evidence that perifollicular inflammatory infiltration, prostaglandin and cytokine elevation in AGA possibly contributes to hair miniaturization.44–46 UV radiation, allergen exposure and porphyrin production are thought to contribute to inflammation in patients with AGA as well.44,47,48
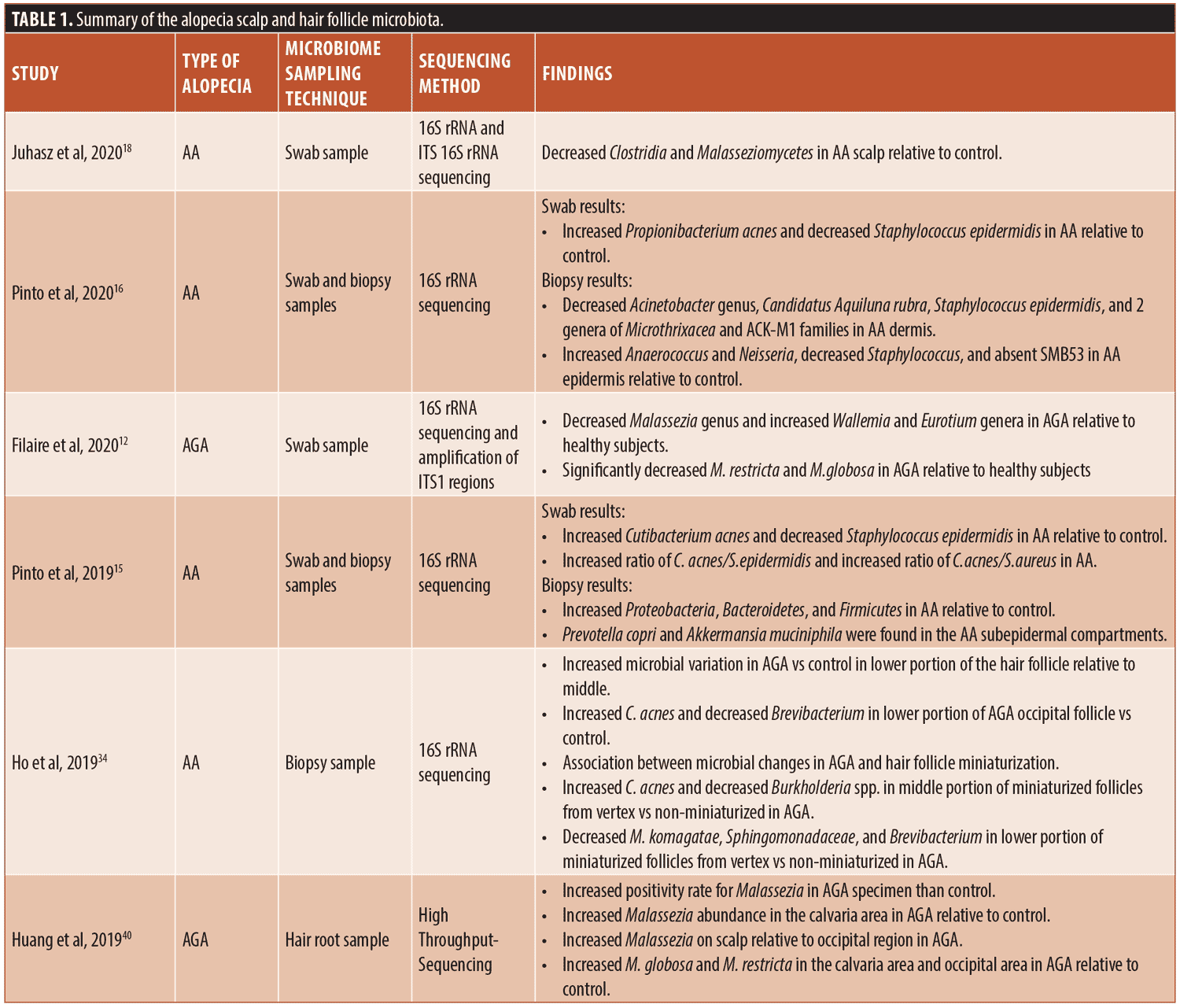
Knowledge of the scalp microbiome involvement in hair loss is limited to only a few studies focusing on the relationship of alopecia and scalp microbiota. The conflicting results could be due to the small sample sizes in the studies. In addition, as stated by Clavaud et al, the differences in results could be due to inaccurate dermatological diagnosis and/or species identification and sample populations in different geographic locations, including China and France.49 The literature on the scalp and hair follicle microbiome in conditions of alopecia has been summarized in Table 1.
Therapies Targeting the Microbiome in Androgenic Alopecia
Previous studies have shown improvement of AGA with antimicrobial therapies. Pierard-Franchimont et al showed that improvement in hair density was equivalent with the treatment of topical 2% minoxidil solution compared to 2% ketoconazole shampoo in patients with AGA.39 Antimicrobial lotion containing piroctone, olamine and triclosan applied for 18 months to the scalp of people with AGA resulted in signs of hair regrowth with moderate increase in density of hairs transitioning to growth phase. Components of inflammation were also reduced, including the density of activated T-cells in the follicular infra-infundibulum and isthmus and IgG deposits within the epithelial sheaths.50
Recently, a study showed a significant decrease in the abundance of C. acnes (P<0.05) and S. geniculata (P< 0.01), an increase of S. epidermidis (P< 0.05) and the proportion of M. restricta and M. globosa (P < 0.05) with an 83-day topical treatment of a Lindera strychnifolia root extract (LsR) in male patients with AGA,12 showing a potential therapy of this compound. LsR contains terpenes such as linderane, which is known to inhibit the cAMP/PKA/CREB pathway, and cAMP in particular is a known follicular growth inhibitor.24
Another study by Park et al examined the effect of an oral kimchi and cheonggukjang probiotic product on 23 male and 23 female subjects with androgenic alopecia.51 This drinkable product was consumed twice daily by the subjects for four months. Hair count and hair thickness were measured at baseline, after one month of treatment, and after four months of treatment. Hair count and thickness were found to significantly increase at one month and at four months relative to baseline (P<0.001). In 93 percent of the subjects, improvements in the outcome measures were observed. In this study, the authors hypothesize that the probiotic was able to exert these benefits through increasing blood flow to the scalp. Further research will be needed to more comprehensively understand the role of oral dietary probiotic supplementation on hair growth, along with larger sample sizes and the use of a control group.
Alterations in the gut microbiome have been demonstrated for the first time in patients suffering from post-finasteride syndrome (PFS), classified as persistent sexual, neurological, physical and mental adverse effects after taking finasteride to treat androgenic alopecia. Analysis of stool samples on 21 male patients with PFS were compared to ten male control patients. There was a significant reduction of richness, diversity, and composition in the PFS group using the α- and ß-diversity metrics.52 Through ß-diversity, a clustering effect in the gut microbiota was found in the patients with PFS, specifically showing a reduction in Faecalibacterium spp. and Ruminococcaceae UCG-005, and an increase in Alloprevotella and Odoribacter spp. compared to healthy controls. Of note, this small cohort study did not assess the scalp or follicular microbiome.
Scarring Alopecias and the Microbiome
The involvement of the microbiome in the pathogenesis of cicatricial alopecias has yet to be established. However, the associations of microbiological dysbiosis with scarring alopecia necessitate further research on this possible relationship. For example, we do know that S. aureus is implicated in the pathogenesis of folliculitis decalvans (FD),53, 54 however it has yet to be elucidated whether this superinfection is the primary cause of FD, or merely an exacerbating factor.
A recent case series demonstrated concomitant FD and LPP, or FD preceding LPP, resulting in speculation of dysbiosis inducing inflammation of the hair follicle in LPP.54 Further speculations about the link between FD and LPP include the koebnerization of LPP into chronically inflamed FD areas or possible undiagnosed LPP predisposing to secondary bacterial infection. Other viewpoints include the primary infectious agent causing the destruction of the hair follicles due to bacterial biofilms giving rise to the autoimmune reaction seen in LPP.55
Although studies assessing microbiome involvement with hair loss are limited, further studies are underway to evaluate the potential use of natural antimicrobial therapies for cases of alopecia, including garlic and tea tree oil.56, 57
The role the microbiome of the scalp and hair follicle play in various types of alopecia is an area of growing interest. Although the current state of research on microbiome involvement in alopecia is limited and requires more investigation, remarkable strides are being made. Further studies will be needed to expand knowledge about the microbiome’s influence on hair biology and pathology, as well as to identify potential therapies targeting the disease processes involved in hair loss.
References
- Kosiewicz MM, et al. Relationship between gut microbiota and development of T cell associated disease. FEBS Lett, 2014. 588(22):4195–4206.
- Safina DD, Abdulkhakov SR, Odintsova AK, et al. Clinical case of a combination of ulcerative colitis and alopecia areata. Experimental & Clinical Gastroenterology. 2013: p. 92–96.
- Patel KV, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013. 19(8):1753–1763.
- Sobolewska-Włodarczyk A, et al. Alopecia areata in patients with inflammatory bowel disease: an overview. Folia Med Cracov. 2016. 56(1):5-12.
- Muller SA and Winkelmann RK. ALOPECIA AREATA. AN EVALUATION OF 736 PATIENTS. Arch Dermatol. 1963. 88:290–297.
- Rebello D, et al. Hair Growth in Two Alopecia Patients after Fecal Microbiota Transplant. ACG Case Rep J. 2017. 4:e107.
- Levkovich T, et al. Probiotic bacteria induce a ‘glow of health’. PLoS One. 2013. 8(1):e53867.
- Kimoto-Nira H, et al. Anti-ageing effect of a lactococcal strain: analysis using senescence-accelerated mice. Br J Nutr. 2007. 98(6): 1178–1186.
- Lousada MB, et al. Exploring the human hair follicle microbiome. Br J Dermatol. 2021. 184(5):802–815.
- Grice EA and Segre JA. The skin microbiome. Nat Rev Microbiol, 2011. 9(4):244–253.
- Polak-Witka K, et al. The role of the microbiome in scalp hair follicle biology and disease. Exp Dermatol. 2020. 29(3):286–294.
- Filaire E, et al. Characteristics of healthy and androgenetic alopecia scalp microbiome: Effect of Lindera strychnifolia roots extract as a natural solution for its modulation. Int J Cosmet Sci. 2020. 42(6):615–621.
- Wang E, LJ-S, Hee TH. s Propionibacterium acnes associated with hair casts and alopecia? Int J Trichology. 2012. 4(93).
- Hosking AM, Juhasz M, Atanaskova N Mesinkovska, Complementary and Alternative Treatments for Alopecia: A Comprehensive Review. Skin Appendage Disord. 2019. 5(2):72–89.
- Pinto D, SE, and Marzani B. Scalp bacterial shift in Alopecia areata. PLoS One. 2019.14.
- Pinto D, et al. Predictive Metagenomic Profiling, Urine Metabolomics, and Human Marker Gene Expression as an Integrated Approach to Study Alopecia Areata. Front Cell Infect Microbiol. 2020. 10:146.
- Rinaldi F, PD, Marzani B, et al. Human microbiome: What’s new in scalp diseases. J Transl Sci. 2018. 4:1–4.
- Juhasz M, CS, Khosrovi-Eghbal A, et al. Characterizing the Skin and Gut Microbiome of Alopecia Areata Patients. J of Skin. 2020. 4:23–30.
- Tamboli CP, et al. Dysbiosis in inflammatory bowel disease. Gut. 2004. 53(1):1–4.
- Moreno-Arrones OM, et al. Analysis of the gut microbiota in alopecia areata: identification of bacterial biomarkers. J Eur Acad Dermatol Venereol. 2020. 34(2):400–405.
- Palm NW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014. 158(5):1000–1010.
- Kaakoush NO. Insights into the Role of Erysipelotrichaceae in the Human Host. Front Cell Infect Microbiol. 2015. 5:84.
- Borde A and Åstrand A. Alopecia areata and the gut-the link opens up for novel therapeutic interventions. Expert Opin Ther Targets. 2018. 22(6):503–511.
- Xie WR, et al. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: A case report and review of the literature. World J Clin Cases. 2019. 7(19):3074–3081.
- Thompson JM, et al. The Role of Micronutrients in Alopecia Areata: A Review. Am J Clin Dermatol. 2017. 18(5):663–679.
- Tsai TY and Huang YC. Vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J Am Acad Dermatol. 2018. 78(1):207–209.
- Lee S, et al. Increased prevalence of vitamin D deficiency in patients with alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018. 32(7): 1214–1221.
- Chen CH, Sakai Y, and Demay MB. Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology. 2001. 142(12):5386–5389.
- Xie Z, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002. 118(1):11–16.
- Fawzi MM, et al. Assessment of vitamin D receptors in alopecia areata and androgenetic alopecia. J Cosmet Dermatol. 2016. 15(4):318–323.
- Zhu W, et al. 1,25(OH)(2)D(3) deficiency-induced gut microbial dysbiosis degrades the colonic mucus barrier in Cyp27b1 knockout mouse model. Gut Pathog. 2019 Feb 20;11:8.
- Waterhouse JC, Perez TH, and Albert PJ. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann N Y Acad Sci. 2009.1173:757–765.
- Rinaldi F, Trink A, and Pinto D. Efficacy of Postbiotics in a PRP-Like Cosmetic Product for the Treatment of Alopecia Area Celsi: A Randomized Double-Blinded Parallel-Group Study. Dermatol Ther (Heidelb). 2020. 10(3):483–493.
- Ho BS, et al. Microbiome in the hair follicle of androgenetic alopecia patients. PLoS One. 2019. 14(5):e0216330.
- Tridico SR, et al. Metagenomic analyses of bacteria on human hairs: a qualitative assessment for applications in forensic science. Investig Genet. 2014. 5(1):16.
- Nakatsuji T, et al. The microbiome extends to subepidermal compartments of normal skin. Nat Commun. 2013. 4:1431.
- Martina P, et al. Genetic diversity of Burkholderia contaminans isolates from cystic fibrosis patients in Argentina. J Clin Microbiol. 2013. 51(1):339–344.
- Govan, JR and Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996. 60(3):539–574.
- Piérard-Franchimont C, et al. Ketoconazole shampoo: effect of long-term use in androgenic alopecia. Dermatology. 1998. 196(4): 474–477.
- Huang J et al. Investigation on Microecology of Hair Root Fungi in Androgenetic Alopecia Patients. Mycopathologia. 2019. 184(4): 505–515.
- Juntachai W and Kajiwara S. Differential Expression of Extracellular Lipase and Protease Activities of Mycelial and Yeast Forms in Malassezia furfur. Mycopathologia. 2015. 180(3-4):143–151.
- Pradhan S. et al. Cover Image: Naevus sebaceus affected by overgrowth of Malassezia globosa. Br J Dermatol. 2018. 179(6):1432–1433.
- DeAngelis YM, et al. Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J Investig Dermatol Symp Proc. 2005. 10(3):295–297.
- Mahé YF, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000. 39(8):576–584.
- Kwack MH, et al. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012. 132(1):43–49.
- Garza LA, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012. 4(126):126ra34.
- Hruza LL and Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993. 100(1): p. 35s–41s.
- Young JW, et al. Cutaneous immunopathology of androgenetic alopecia. J Am Osteopath Assoc. 1991. 91(8):765–771.
- Clavaud C, et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS One. 2013. 8(3):e58203.
- Piérard G, et al. Improvement in the inflammatory aspect of androgenetic alopecia. A pilot study with an antimicrobial lotion. J Dermatolog Treat. 1996. 7:153–157.
- Park DW, et al. Do Kimchi and Cheonggukjang Probiotics as a Functional Food Improve Androgenetic Alopecia? A Clinical Pilot Study. World J Mens Health. 2020. 38(1):95–102.
- Borgo F, et al. Alterations of gut microbiota composition in post-finasteride patients: a pilot study. J Endocrinol Invest. 2021. 44(6):1263–1273.
- Sillani C, et al. Effective treatment of folliculitis decalvans using selected antimicrobial agents. Int J Trichology. 2010. 2(1):20–23.
- Yip L, Barrett TH, and Harries MJ. Folliculitis decalvans and lichen planopilaris phenotypic spectrum: a case series of biphasic clinical presentation and theories on pathogenesis. Clin Exp Dermatol. 2020. 45(1):63–72.
- Trüeb RM, Rezende HD, and Diaz M. Dynamic Trichoscopy. JAMA Dermatol. 2018. 154(8): 877–878.
- Sakr FM, et al. Preparation and evaluation of a multimodal minoxidil microemulsion versus minoxidil alone in the treatment of androgenic alopecia of mixed etiology: a pilot study. Drug Des Devel Ther. 2013. 7:413–423.
- Pazyar N and Feily A. Garlic in dermatology. Dermatol Reports, 2011. 3(1):e4.