 J Clin Aesthet Dermatol. 2020;13(10):32–37
J Clin Aesthet Dermatol. 2020;13(10):32–37
by Suparuj Lueangarun, MD, MSc; and Ratchathorn Panchaprateep, MD, PhD
Dr. Lueangarun is with the Division of Dermatology at Chulabhorn International College of Medicine at Thammasat University in Pathumthani, Thailand. Dr. Panchaprateep is with the Division of Dermatology, Faculty of Medicine at Chulalongkorn University, in Bangkok, Thailand.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest related to the content of this article.
ABSTRACT: Objective: We sought to evaluate the efficacy and safety profile of an herbal extract combination comprising biochanin A, acetyl tetrapeptide-3, and ginseng extracts, and compare this to 3% minoxidil solution for the treatment of andogenetic alopecia (AGA).
Methods: A 24-week, triple-blinded, randomized controlled study was conducted in male and female subjects (N=32) with mild to moderate AGA. All were randomized to receive twice-daily, 1mL applications of the herbal extract combination or 3% minoxidil solution. Clinical efficacy from photographic assessment and adverse reactions were evaluated.
Results: There were thirty-two subjects (16 male, mean age 41.3±13.8 years), with AGA onset and duration of 35.5±13.6 and 6.5±5.1 years, respectively. The herbal extract combination demonstrated a comparable efficacy to 3% minoxidil solution. Expert panel photographic assessment observed a response to both treatments in most patients at 24 weeks, with no statistically significant difference in an increase of terminal hair counts (8.3% [P=0.009] and 8.7% [P=0.002] at 24 weeks in the herbal extract combinations and the 3% minoxidil solution groups, respectively). No local adverse reactions from the herbal extract combination were observed, but one subject developed scalp eczema after using the 3% minoxidil solution.
Conclusion: The non-significant difference in clinical efficacy and safety to 3% minoxidil solution suggests that the herbal extract combination evaluated here could potentially be an alternative treatment with for AGA. Further studies with larger groups and longer follow-up periods are recommended to verify our results.
Keywords: Androgenetic alopecia, drug therapy, herbal medicine, biochanin A, acetyl tetrapeptide-3, panax ginseng, minoxidil
Androgenic alopecia (AGA) is a common non-scarring hair loss disorder that affects both men and women that can become more severe over time.1,2 AGA can be caused by androgens, genetics, a loss of extracellular matrix (ECM) proteins in the follicular bed, and micro-inflammation.3 This results in a shortened anagen phase of the hair cycle that leads to the production of shorter, thinner hair shafts, or a process called follicular miniaturization.1 The clinical manifestations of AGA include miniaturization of terminal hair, increased ratio of telogen hair, decrease of hair life, and thinning hair.1
Currently, oral finasteride and topical minoxidil are approved as standard treatments for AGA.4 However, there are some limitations to the efficacy and side effects of those treatments, such as sexual dysfunction and contact dermatitis.5,6 A recent systemic review showed that among men taking finasteride for AGA treatment, the evidence for sexual dysfunction is less conclusive, with 10 studies demonstrating sexual adverse effects, including ED and decreased libido.7 Thus, several novel modalities for AGA have been evaluated to enhance treatment efficacy and lower systemic side effects.8–12
Phytopharmaceutical extracts have been investigated for AGA treatment in an attempt to increase patient adherence, decrease side effects, and target more than one mode of action.4 The combination of biochanin A, acetyl tetrapeptide-3, and ginseng extracts has been shown to stimulate dermal papilla ECM proteins by increasing hydroxyproline, Collagen Type 3, and laminin, yielding a significant improvement in hair follicle size and hair anchoring.13 Increased hair growth has been observed after the seven-day culture by biochanin A and acetyl tetrapeptide-3 compared to minoxidil in part of the activation of hair growth.13–15 Observed in in-vitro studies, biochanin A and acetyl tetrapeptide-3 appear to exert an anti-inflammatory effect by decreasing pro-inflammatory cytokines.13,16 A four-month, randomized, placebo-controlled study evaluated the efficacy of biochanin A and acetyl tetrapeptide-3 compared to placebo for the treatment of AGA using microscopic evaluation with a phototrichogram revealed that the herbal lotion increased the anagen hair density (13%) with greater ratio from the anagen to telogen (46%) when compared to a reduction of –33 percent for the placebo.13 Panax ginseng root extract appears to exert an anti-apoptotic effect with dermal papilla cell proliferation16 in the pathogenesis of both male and female pattern hair loss.9
This study aimed to evaluate the clinical efficacy and safety profile of an herbal extract combination compared to 3% minoxidil solution for the potential treatment of AGA in both male and female subjects.
Methods
This was a 24-week, prospective, randomized, triple-blind, controlled study conducted at King Chulalongkorn Memorial Hospital between June 2013 and May 2014 in male and female subjects, aged 18 to 55 years, with male pattern hair loss (MPHL) Hamilton-Norwood Stage III to IV vertex and female pattern hair loss (FPHL) Ludwig Type I to II.17,18
Exclusion criteria included use of topical medical therapies within six months prior to the study, treatment with systemic therapies (finasteride within one year and dutasteride within 1.5 years), previous hair restoration surgery, use of shampoo for hair growth or hair supplements within three months prior, and other alternative treatments for hair growth within one month prior to the study. In addition, patients with FPHL and a hyperandrogen condition and those with diabetes mellitus, nutritional deficiency, autoimmune disease, immunocompromised conditions, HIV-infection, cancer, as well as pregnant and lactating women were excluded.
Study design. This study aimed to compare the efficacy of an herbal extract combination to 3% minoxidil solution without a placebo group. The number of subjects was calculated using the t-test analysis with alpha error = 0.05, power of the test = 80 percent, and intercept = 2.5, which yielded a sample size of 32 subjects. They were then randomized using predetermined computer-generated blocked randomization lists to apply the assigned 1mL of herbal extracts (n=17) or 3% minoxidil solution (n=15) twice daily at approximately 12-hour intervals (total daily dose of 2mL) to the vertex area of the scalp for 24 weeks. The two treatment medications were prepared in identically-appearing, pre-packaged, and pre-labeled bottles. Efficacy, safety, and patient adherence were evaluated at 12 weeks and the end of 24 weeks. Adherence was assessed from the daily use of the study drugs. All subjects were also asked to return the bottles to measure the residual volumes of the two medications.
The herbal extract combination (Hirsuit™ hair tonic, Dermcor Pharmaceutical, Bangkok, Thailand) evaluated here comprises acetyl tetrapeptide-3, biochanin A (red clover extracts), Panax Ginseng extract, and Salvia officinalis oil. Other ingredients include water, alcohol, panthenol, PEG-7 glyceryl cocoate, inositol, fragrance, PEG-40, hydrogenated castor oil, menthol and DMDM hydantoin. The 3% minoxidil lotion was manufactured by the Pharmaceutical Division of King Chulalongkorn Memorial Hospital in Bangkok, Thailand.
The study protocol was approved by the Ethics Committee of Faculty of Medicine, Chulalongkorn University, Thailand (IRB 515/56) and conducted in accordance with the Declaration of Helsinki of 1975, as revised in 1983, and Good Clinical Practice Guidelines. All subjects were informed about the study and provided written informed consent before participation.
Efficacy evaluation procedure. The global photography taken from digital camera with stereotactic device at Day 0, Week 12, and Week 24 compared to baseline photographs was clinically evaluated by three blinded dermatologists, using the standardized seven-point rating scale (-3=greatly decreased, -2=moderately decreased, -1=slightly decreased, 0=no change, +1=slightly increased, +2=moderately increased, and +3=greatly increased).19,20
Target area hair count was also taken at baseline, Week 12 and Week 24. Terminal hair counts were measured on one-cm2 areas of the clipped hairs, selected from the balding area on the mid scalp. A small cosmetic ink tattoo in the center of the square was used as a marker to identify the area of hair counting. Dermoscopic photographs of the target area were taken by dermoscopy with one-cm2 square grid (DermLite II pro HR; 3 Gen LLC, California) of the ink tattoo at the center. Hair count per cm2 square was performed by two blinded dermatologists, using photo-editing software.
Hair Mass Index (HMI) was evaluated using cross-section trichometry (HairCheck®, Divi Int’l Co., Miami, Florida), measured on area 2x2cm2 to reflect hair density and hair diameter at baseline and week 24.21–23
Patient satisfaction was assessed at Week 12 and Week 24 using questionnaires with a 7-point rating scale (-3=significantly worse, -2=moderately worse, -1=minimally worse, 0=no change, +1=minimally improved, +2 =moderately improved, and +3= significantly improved).
Safety assessment. A safety assessment was performed using the results of history taking and physical examination of adverse events, such as itchiness, dermatitis, dryness, and dandruff, with 4 rating scale (0=none, 1=mild, 2=moderate and 3=severe) at baseline, Week 12, and Week 24.
Statistical analysis. Patient demographic data and other descriptive statistics were analyzed using percentage and mean±SD. Also, t-test and Mann-Whitney U test were applied for normal and non-normal distribution data respectively. Fisher exact and chi-square were employed for category data. The statistician was blinded from the study information. P-value <0.05 was considered as statistically significant.
Results
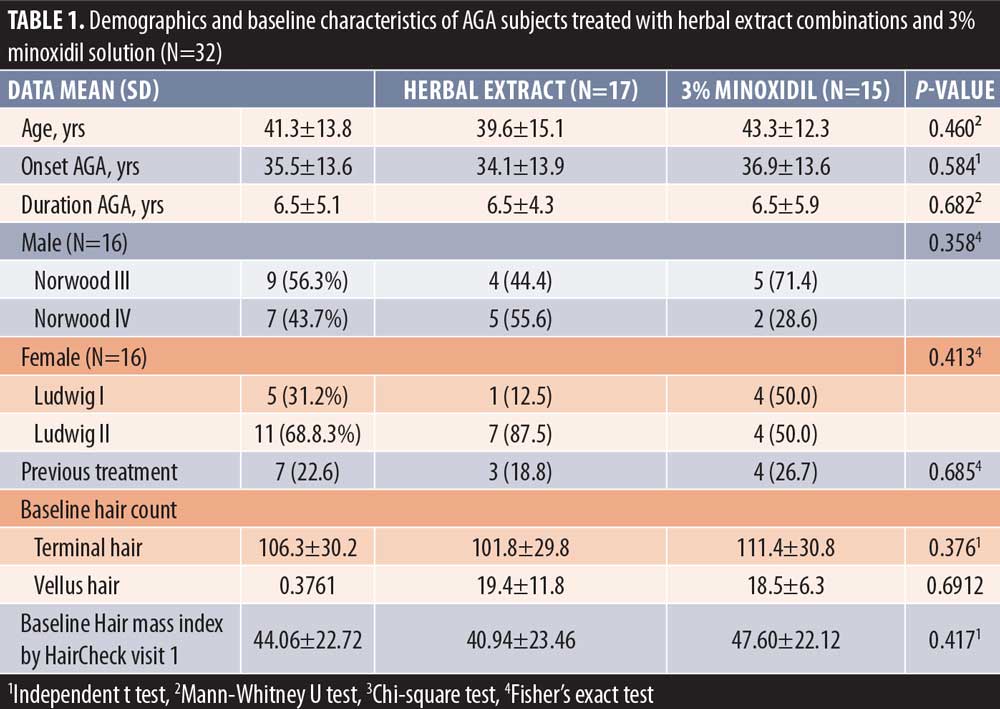
Thirty-two subjects (16 males and 16 females, mean + age = 41.3 ± 13.8 years) with mild-to-moderate AGA were included and completed the study. The age of AGA onset and duration of AGA were 35.5 ± 13.6 and 6.5 ± 5.1 years, respectively. The distributions of AGA severity were Norwood class IIIv and IVv for 28 percent and 22 percent in the male participants, and Ludwig class I and II for 27 percent and 34 percent in female participants, respectively. Eighteen percent of the subjects had previous treatment with minoxidil solution or oral finasteride. There was no history of allergy to either medications in all subjects. There were no statistically significant baseline characteristics between the herbal extract combinations and the 3% minoxidil solution treated groups. The mean amount of daily use of treatment medications was 2.1mL and was similar in both groups. (Table 1)
Efficacy evaluation. The expert panel photographic assessment (with the intraclass correlation coefficient=0.805, substantial agreement) on the vertex scalp at Week 24 revealed 100-percent response (score at least 1) in both the herbal extract combination and the 3% minoxidil solution treated groups. Furthermore, 12.5 percent and 21 percent of the herbal extract combination and the 3% minoxidil solution treated groups showed excellent improvement (score = +3), respectively, without statistical difference between the two groups.
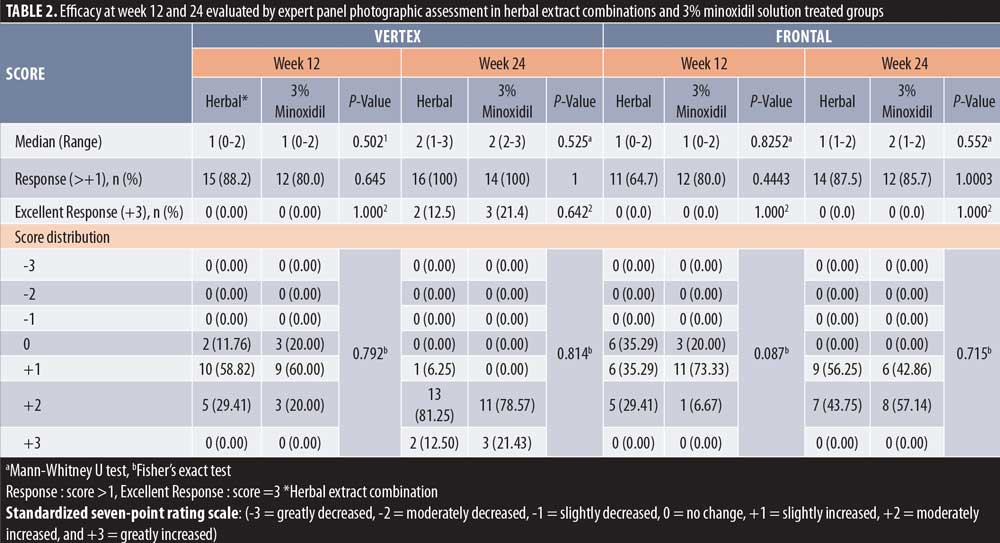
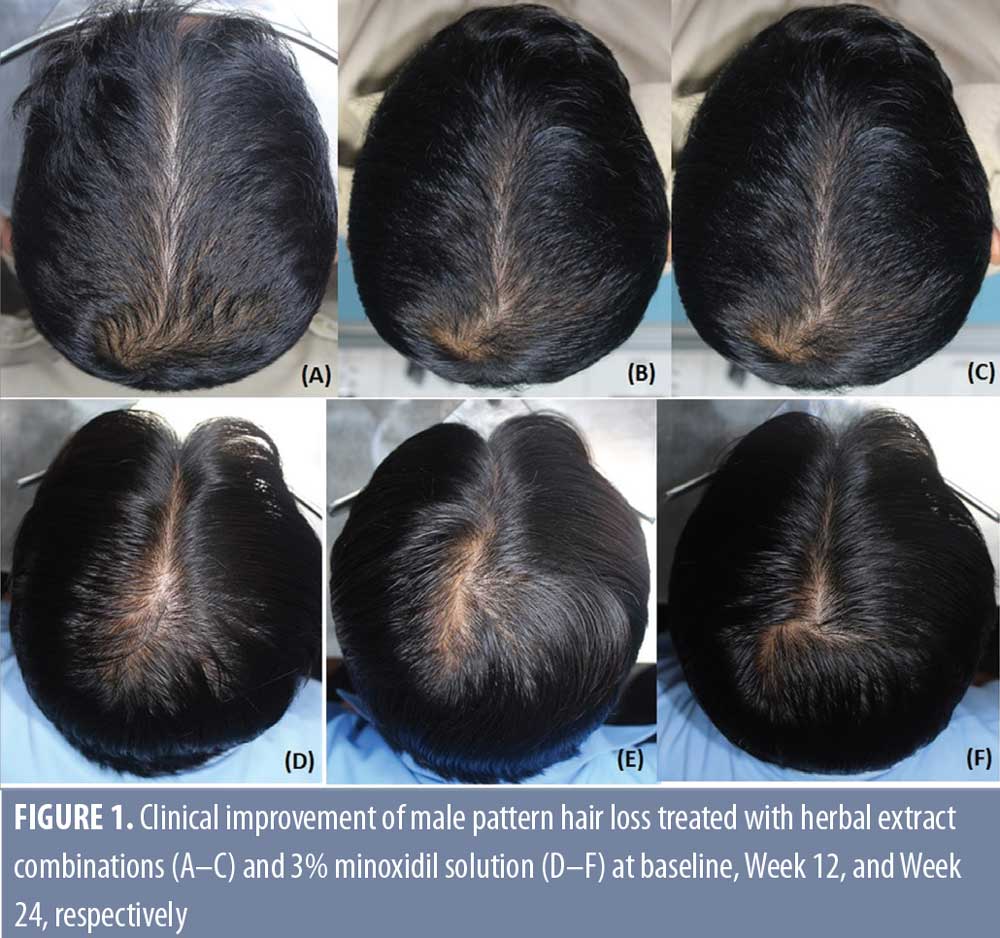
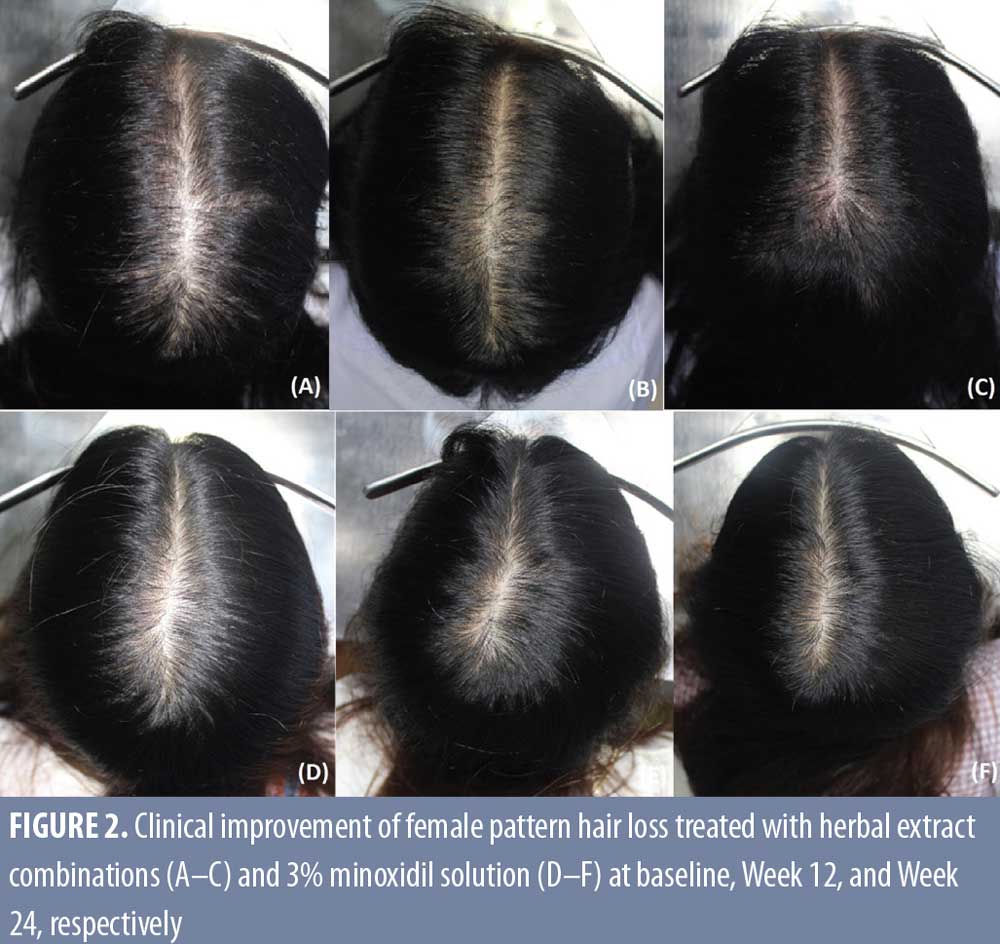 However, the response at the frontal area was less than that of the vertex area in both the herbal extract combination (87.5%) and the 3% minoxidil solution (85%) treated groups, without statistical difference. (Table 2, Figures 1 and 2)
However, the response at the frontal area was less than that of the vertex area in both the herbal extract combination (87.5%) and the 3% minoxidil solution (85%) treated groups, without statistical difference. (Table 2, Figures 1 and 2)
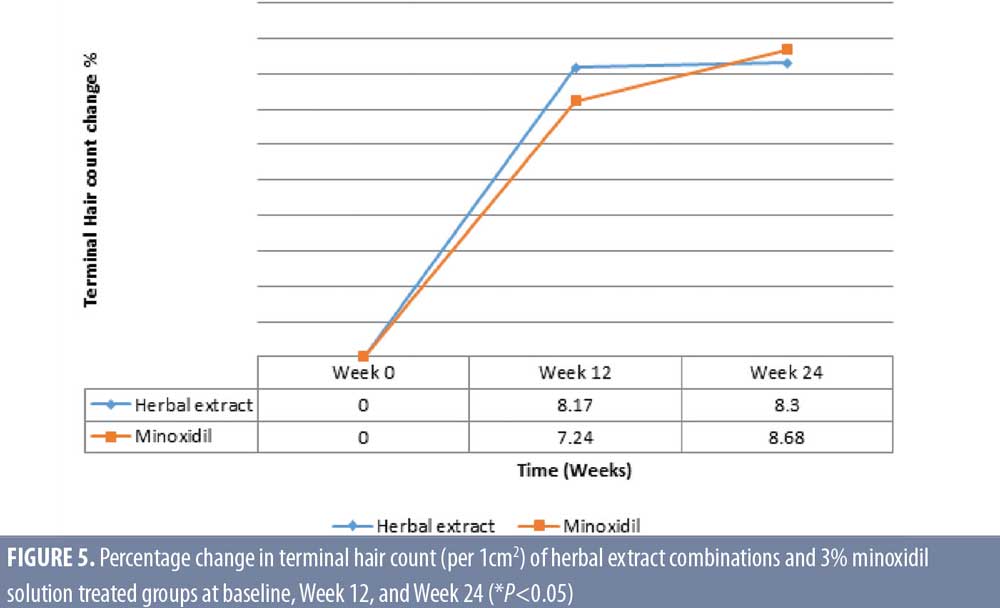
Target area hair count. The herbal extract combination treated group showed a significant increase of mean ± SD terminal hair count per cm2 for 101.8 ± 29.8, 110.07 ± 29.3, and 110.2 ± 28.7, (P=0.009) at baseline, Week 12, and Week 24, respectively. The 3% minoxidil solution treated group also demonstrated a significant increase of terminal hair count per cm2 for 111.4 ± 30.8, 119.5 ± 24.5, and 122.1 ± 29.07, (P=0.002) at baseline, Week 12, and Week 24, respectively.
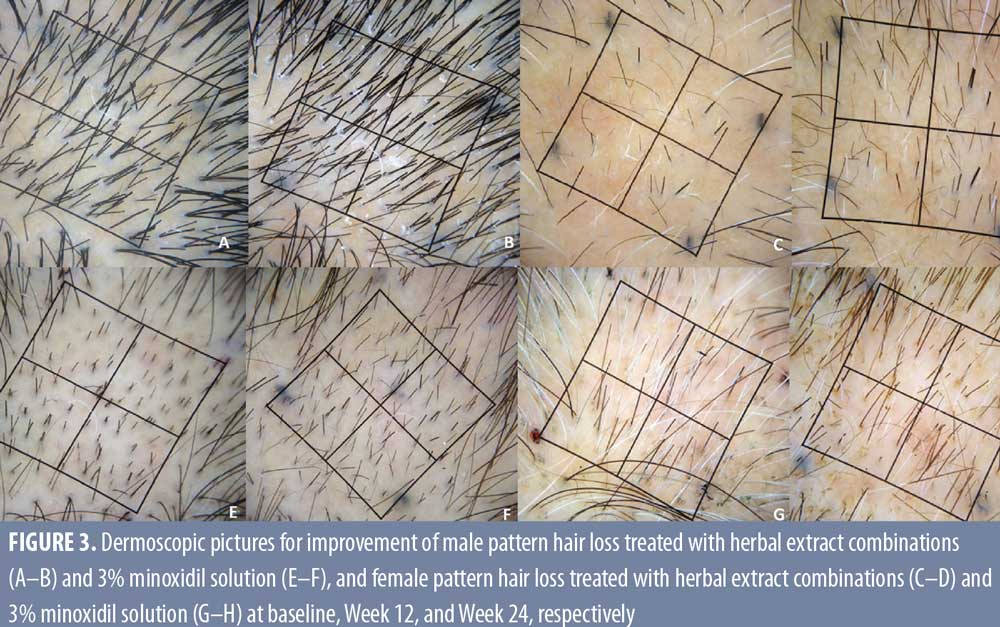
There was a significant clinical improvement at Week 24, with an 8.3 percent (P=0.009) and 8.68 percent (P=0.002) increase for terminal hair count in the herbal extract combination and the 3% minoxidil solution treated groups, respectively, with no statistical difference between the two groups (P= 0.370 at Week 12 and P=0.306 at Week 24) (Figure 3).
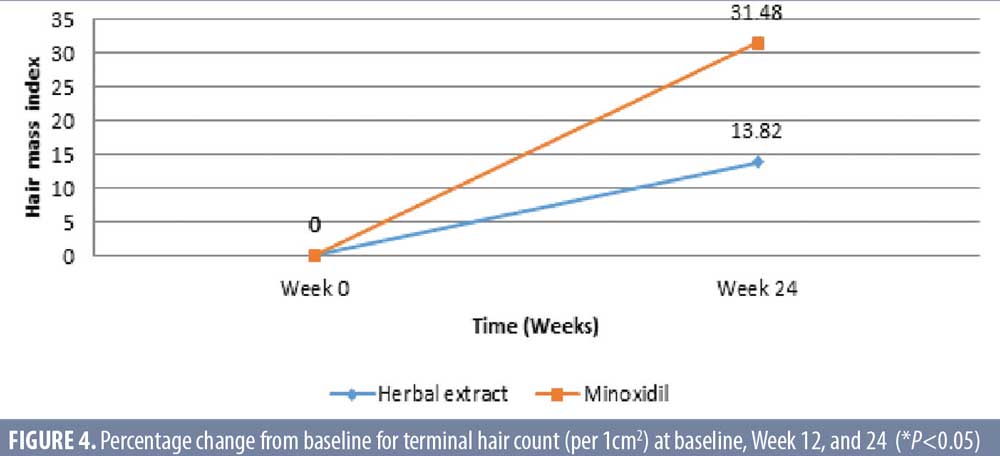
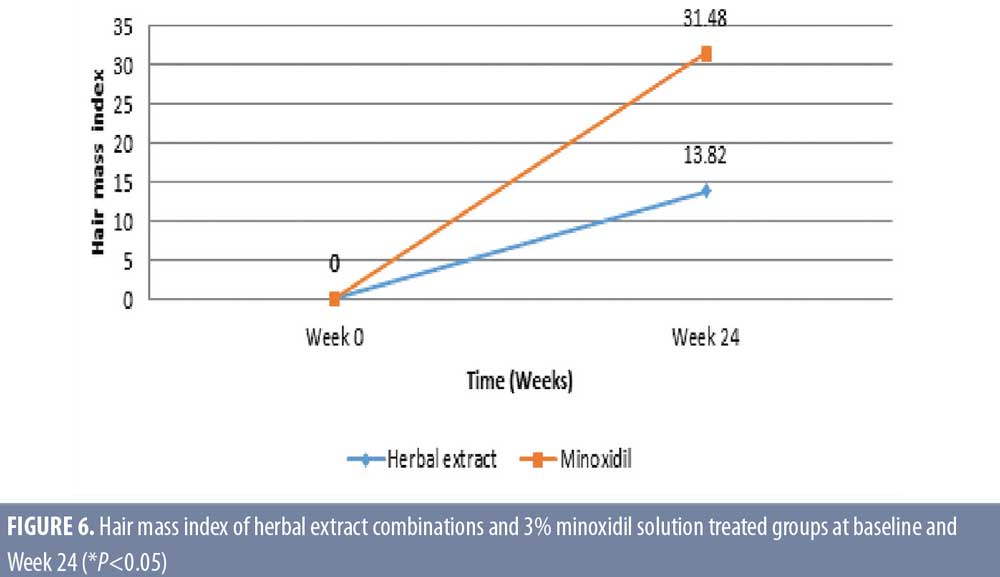
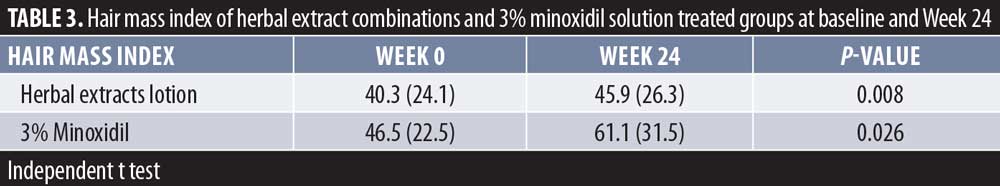 Hair mass index (HMI). The herbal extract combinations treated group showed a significant increase of mean ± SD HMI for 40.3 ± 24.1 and 45.9 ± 26.3 (P=0.008) at baseline and week 24, respectively. The 3% minoxidil solution treated group also demonstrated a significant increase of HMI for 46.5 ± 22.5 and 61.1 ± 31.5 (P=0.026) at baseline and week 24, respectively (Table 3). There was a significant increase of HMI in both groups, with 13.8-percent response in the herbal extract combination (P=0.008) and a 31.48-percent response in the 3% minoxidil solution treated groups (P=0.026) at Week 24 after treatment, without statistical difference between the two groups (P=0.158) (Figures 4 and 5).
Hair mass index (HMI). The herbal extract combinations treated group showed a significant increase of mean ± SD HMI for 40.3 ± 24.1 and 45.9 ± 26.3 (P=0.008) at baseline and week 24, respectively. The 3% minoxidil solution treated group also demonstrated a significant increase of HMI for 46.5 ± 22.5 and 61.1 ± 31.5 (P=0.026) at baseline and week 24, respectively (Table 3). There was a significant increase of HMI in both groups, with 13.8-percent response in the herbal extract combination (P=0.008) and a 31.48-percent response in the 3% minoxidil solution treated groups (P=0.026) at Week 24 after treatment, without statistical difference between the two groups (P=0.158) (Figures 4 and 5).
Patient satisfaction. Patient satisfaction with hair density and hair thickness in the herbal extract combinations and the 3% minoxidil solution treated groups were 68.75 percent versus 76.92 (P=0.85) and 78.92 percent versus 69.23 percent (P=0.92), respectively. There was no statistically significant difference in patient satisfaction at Week 24.
Safety assessment. No local adverse reactions were observed in the herbal extract combination treated group. One female patient developed scalp eczema after using the 3% minoxidil solution for six weeks. Nonetheless, there was a satisfactory response after switching to the herbal extract combinations without adverse reactions at the end of study.
Discussion
The herbal extract combinations showed the efficacy in AGA treatment with comparable efficacy to the 3% minoxidil solution for treatment of mild-to-moderate AGA in both male and female participants. This was evaluated by a macrographic expert panel of global assessment (almost all response), microscopic evaluation including target area hair count (increase of terminal hair 8.3% vs. 8.68%, P=0.306), HMI (increase of 13.8% vs. 31.5%, P=0.158), and patient self assessment. Also, the satisfactory safety profile of the herbal extract combination was observed with no eczematous reaction and only one participant in the minoxidil group.
A wide range of molecules, products, and interventions claim to promote hair growth in patients with AGA, including as phytopharmaceutical agents and natural products.24,25 The hair growth properties in most of these products are from the mechanism of DHT-inhibitory and anti-inflammatory activities, with improved perifollicular vascularization and hair follicle nutrition, or not be precisely reported or likely caused by unknown mechanism of action.25 There have been several randomized controlled trials that are usually studied in the limited sample sizes and durations (ranged from 18 to 40 weeks) with diverse results. The studies have mostly been evaluated using only microscopic evaluation, such as hair count or only macroscopic assessment.26–30
The improvements in AGA in the patients who received the herbal extract combination in our study could be the result of multiple mechanisms based on previously published study data, including the inhibition of 5?-reductase, increase of hair anchoring, and hair diameter via the stimulation of extracellular matrix (ECM) production of dermal papilla, and decrease of micro-inflammation in the pathogenesis of both male and female pattern hair loss.9,13,16,32,33,34
Limitations
Despite thoroughly designed to cover all aspects of hair loss testing conducted in this triple-blinded, randomized trial, evaluated by both global and microscopic assessments, our study had limitations, including a small sample size and a short period of treatment. Hair Mass Index is one of the hair-growth evaluation methods, but it is not a well-accepted measurement in hair research due to a lack of studies that validate its accuracy.21–23
Hence, a larger sample size and a longer duration of randomized trial, with subgroup analysis according to gender and combination treatments of herbal extracts and minoxidil solution is recommended to verify the efficacy and safety profile of the herbal extract combination as an alternative treatment for AGA.
Conclusion
Due to the non-significant difference in clinical efficacy and safety to the 3% minoxidil solution, the herbal extract combination evaluated here could be a viable phytopharmaceutical for the treatment of mild to moderate AGA in both male and female patients. However, a study with a larger sample size and a longer follow-up is recommended to verify our results.
Acknowledgments
The authors would like to extend our special thanks to Dr. Patcharin Prechanond and Dr. Kangsadan Roongrodwongsiri for their assistance in recruiting subjects and managing the database, Ms. Sunattee Kessung for her assistance in editing and revising this manuscript, and Ms. Kamonwan Soonklang for her statistical analysis.
References
- Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4(22):1-11.
- Paik JH, Yoon JB, Sim WY, et al. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001;145(1):95-99.
- Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993;28(5 Pt 1):755-763.
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136-141.
- Blumeyer A, Tosti A, Messenger A, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9 Suppl 6:S1-57.
- Lueangarun S, Subpayasarn U, Chakavittumrong P, et al. Lupus panniculitis of the scalp presenting with linear alopecia along the lines of Blaschko. Clin Exp Dermatol. 2017;42(6):705-707.
- Zakhem GA, Goldberg JE, Motosko CC, et al. Sexual dysfunction in men taking systemic dermatologic medication: A systematic review. J Am Acad Dermatol. 2019;81(1):163-172.
- Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008;59(4):547-566; quiz 567-548
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341(13):964-973.
- Price VH. Androgenetic alopecia in women. J Investig Dermatol Symp Proc. 2003;8(1):24-27.
- Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377-385.
- Finasteride Male Pattern Hair Loss Study G. Long-term (5-year) multinational experience with finasteride 1 mg in the treatment of men with androgenetic alopecia. Eur J Dermatol. 2002;12(1):38-49.
- Loing E, Lachance R, Ollier V, et al. A new strategy to modulate alopecia using a combination of two specific and unique ingredients. J Cosmet Sci. 2013;64(1):45-58.
- Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97 ( Pt 3):463-471.
- Lucas Meyer Cosmetics. Technical File CapixylTM. 2013; http://www.redenhair.com/img/cms/CAPIXYL-Techfile.pdf. Accessed 5 Dec, 2019.
- Park S, Shin WS, Ho J. Fructus panax ginseng extract promotes hair regeneration in C57BL/6 mice. J Ethnopharmacol. 2011;138(2):340-344.
- Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68(11):1359-1365.
- Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97(3):247-254.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 Pt 1):578-589.
- Suchonwanit P, Iamsumang W, Rojhirunsakool S. Efficacy of Topical Combination of 0.25% Finasteride and 3% Minoxidil Versus 3% Minoxidil Solution in Female Pattern Hair Loss: A Randomized, Double-Blind, Controlled Study. Am J Clin Dermatol. 2019;20(1):147-153.
- Nichols AJ, Hughes OB, Canazza A, et al. An Open-Label Evaluator Blinded Study of the Efficacy and Safety of a New Nutritional Supplement in Androgenetic Alopecia: A Pilot Study. J Clin Aesthet Dermatol. 2017;10(2):52-56.
- Del Campo R, Zhang Y, Wakeford C. Effect of Miracle Fruit (Synsepalum dulcificum) Seed Oil (MFSO((R))) on the Measurable Improvement of Hair Breakage in Women with Damaged Hair: A Randomized, Double-blind, Placebo-controlled, Eight-month Trial. J Clin Aesthet Dermatol. 2017;10(11):39-48.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section Trichometry: A Clinical Tool for Assessing the Progression and Treatment Response of Alopecia. Int J Trichology. 2012;4(4):259-264.
- Lueangarun S, Subpayasarn U, Tempark T. Distinctive lupus panniculitis of scalp with linear alopecia along Blaschko’s lines: a review of the literature. Int J Dermatol. 2019;58(2):144-150.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men – short version. J Eur Acad Dermatol Venereol. 2018;32(1):11-22.
- Draelos ZD, Jacobson EL, Kim H, et al. A pilot study evaluating the efficacy of topically applied niacin derivatives for treatment of female pattern alopecia. J Cosmet Dermatol. 2005;4(4):258-261.
- Kessels AG, Cardynaals RL, Borger RL, et al. The effectiveness of the hair-restorer “Dabao” in males with alopecia androgenetica. A clinical experiment. J Clin Epidemiol. 1991;44(4-5):439-447.
- Kamimura A, Takahashi T, Watanabe Y. Investigation of topical application of procyanidin B-2 from apple to identify its potential use as a hair growing agent. Phytomedicine. 2000;7(6):529-536.
- Nagasawa A, Wakisaka E, Kidena H, et al. t-Flavanone Improves the Male Pattern of Hair Loss by Enhancing Hair-Anchoring Strength: A Randomized, Double-Blind, Placebo-Controlled Study. Dermatol Ther (Heidelb). 2016;6(1):59-68.
- Takahashi T, Kamimura A, Kagoura M, et al. Investigation of the topical application of procyanidin oligomers from apples to identify their potential use as a hair-growing agent. J Cosmet Dermatol. 2005;4(4):245-249.
- Greenberg J, Katz M. Treatment of androgenetic alopecia with a 7.5% herbal preparation. J Dermatolog Treat. 1996;7(3):159-162.
- Hiipakka RA, Zhang HZ, Dai W, et al. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 2002;63(6):1165-1176.
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341(7):491-497.
- Panchaprateep R, Lueangarun S. Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. Dermatol Ther (Heidelb). 2020. https://doi.org/10.1007/s13555-020-00448-x

