 J Clin Aesthet Dermatol. 2020;13(10):28–31
J Clin Aesthet Dermatol. 2020;13(10):28–31
by Theodore Rosen, MD, and Kami Lowery, BS
Dr. Rosen and Ms. Lowery are with the Department of Dermatology at the Baylor College of Medicine in Houston, Texas.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: A 64 year-old Caucasian male patient with a long history of ultraviolet light exposure and multiple actinic keratoses presented with a large, erythematous, and scaly plaque on his forehead. Biopsies revealed superficial basal cell carcinoma (sBCC). Because the patient wanted the shortest possible topical regimen, his sBCC was treated with two overnight ingenol mebutate (IM) 0.05% gel applications. He tolerated the local skin reaction (LSR) well, and at approximately six weeks post-treatment, biopsies showed no evidence of sBCC. The patient was happy with the cosmetic outcome and has remained free of clinical recurrence for 18 months. Although IM gel is only FDA approved for the treatment of actinic keratosis, it has also been used off-label to treat other epithelial lesions, including basal cell carcinoma (BCC), anogenital warts, and Bowen’s disease. One clinical trial, multiple case series and case reports, and now this report, have demonstrated IM’s utility in treating BCC. IM treatment is therefore a promising alternative to surgery for select BCC, with major advantages, including a short treatment duration and generally favorable cosmetic outcome.
Keywords: Superficial basal cell carcinoma, ingenol mebutate gel, topical treatment, nodular basal cell carcinoma, Bowen’s disease, squamous cell carcinoma in situ, anogenital warts, condylomata acuminata
Excisional and Mohs micrographic surgery are often considered to be the gold standard and the optimal treatment options for the majority of basal cell carcinomas (BCC). These modalities are most commonly employed because of historically demonstrated low recurrence rates and the ability to confirm residual tumor pathologically.1
Select BCCs can also be successfully managed with various destructive techniques (e.g. curettage and electrodesiccation, curettage and cryosurgery, temperature-monitored cryosurgery, or ablative laser therapy), several types of photodynamic therapy, topical application of various drugs (e.g. 5-fluorouracil, imiquimod, or ingenol mebutate), radiotherapy, or hedgehog pathway inhibitors (the latter being reserved for locally aggressive, recurrent or metastatic lesions).2 With the ever increasing number of patients with garden variety superficial and nodular BCC, escalating healthcare costs associated with surgery performed on such lesions, and the lack of timely access to dermatologists in some locales, nonsurgical options should always at least be considered.3 In fact, many low-risk tumors can be successfully managed using non-surgical methods.
When choosing between these many alternatives, healthcare providers must take into consideration the tumor’s size and location, history of response to prior interventions (if any), and histopathological subtype. In addition, provider familiarity and experience with each possible therapeutic modality is important in selecting and recommending specific treatments. Finally, the patient’s age and societal activity level, the patient’s preferences, as well as the potential for adverse events, and likely cosmetic outcome should also all be considered.2,3
A case is presented herein which posed a treatment challenge. A low risk, but fairly large superficial BCC of the face required therapy in a somewhat demanding and questionably adherent patient. After exhaustive discussion, the patient agreed to an unconventional treatment, notably the use of ingenol mebutate. The results of that treatment are presented and discussed.
Case Report
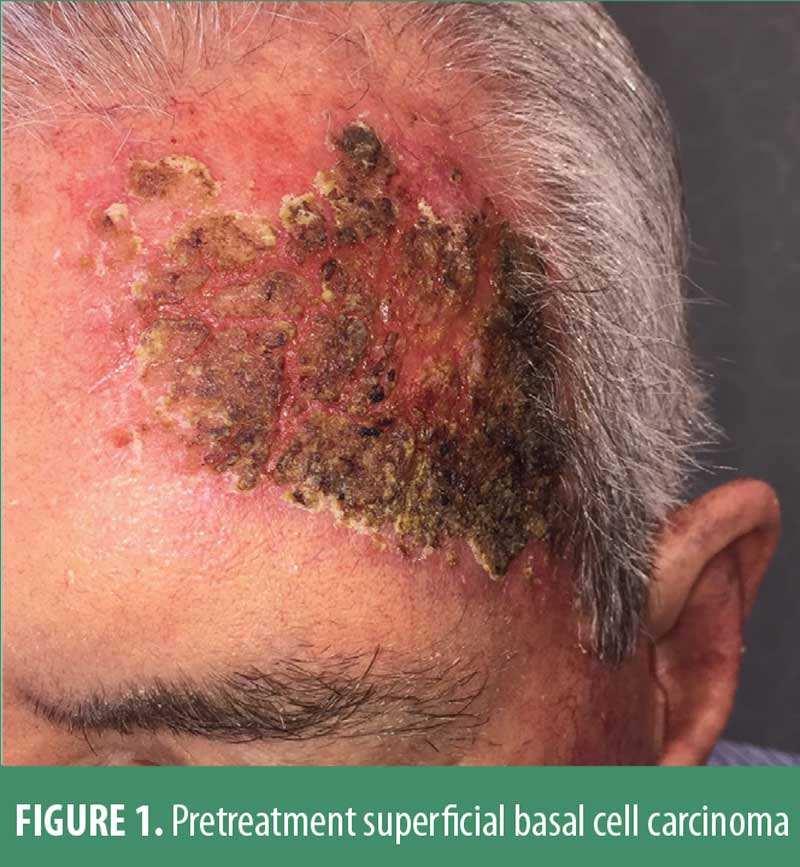
A 64-year-old Caucasian male patient had a 35-year history of extensive facial ultraviolet light exposure. Despite repeated warnings, this avid fisherman and motorboat enthusiast neither wore a hat nor reliably applied sunscreen. Moreover, he had been treated for a decade elsewhere for severe psoriasis with loosely supervised use of a home UVB unit. Not surprisingly, he developed and was treated for actinic keratoses with periodic liquid nitrogen cryosurgery, as he adamantly refused topical field therapy. He ultimately presented with a 5.3cm by 8.1cm, irregularly shaped, erythematous, scaly and somewhat atrophic-appearing plaque on the forehead. (Figure 1)
Multiple small (2mm) scout biopsies all disclosed superficial basal cell carcinoma without dermal invasion. When the various therapeutic options were discussed, he chose topical treatment rather than either surgical intervention or radiation therapy. Because he vigorously insisted upon the shortest possible topical regimen, and based on prior cases and case series in the literature, the patient was treated with overnight applications, for two consecutive days, of ingenol mebutate 0.05% gel. The patient was asked to apply a kidney bean-sized dab of medication, enough to cover the clinically visible lesion and a rim of 1 cm of normal skin beyond the obvious neoplasm. It was carefully explained that this modality was off label, and he was asked to sign an informed consent.
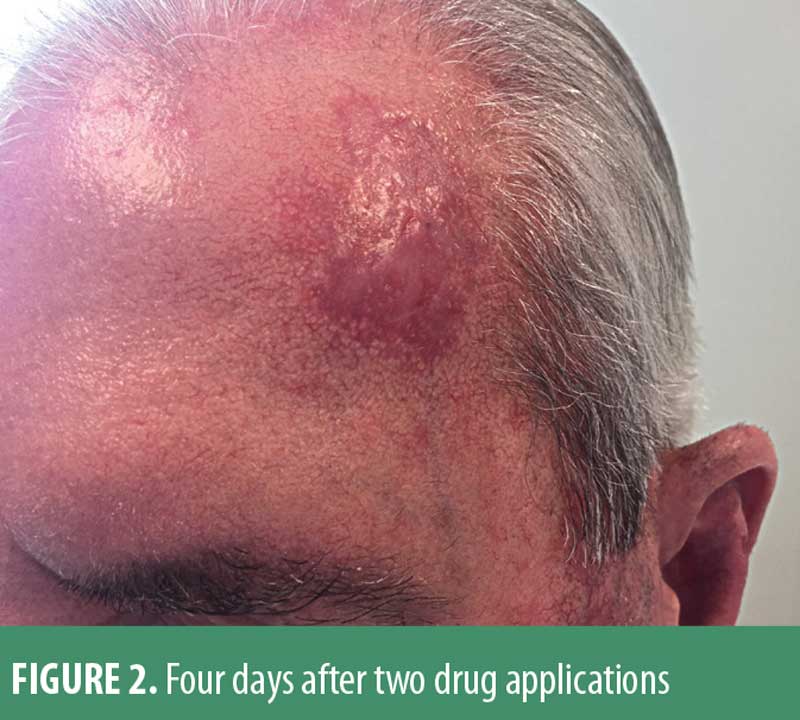
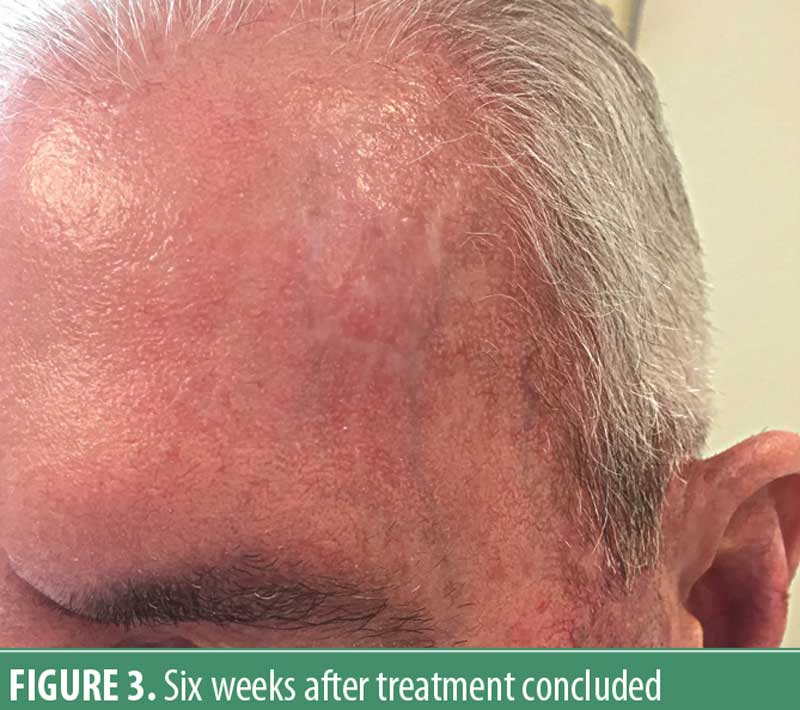
The patient was seen four days after the two applications, and demonstrated a rather striking crusted erosion. (Figure 2) He felt systemically well otherwise and did not complain of significant pain in the treatment area. The patient was then instructed to liberally apply a vaseline-based bland ointment 3 to 4 times daily. At approximately six weeks following treatment completion, he had totally re-epithelialized, was free of any discomfort, and was pleased with the cosmetic outcome. (Figure 3) Three small biopsies done in close proximity to prior scout samplings showed no evidence of basal cell carcinoma. The patient has remained free of clinical recurrence for 18 months, but continues to be monitored/examined every 3 to 4 months.
Discussion
Ingenol mebutate (IM) gel is a topical agent derived from the sap of the plant Euphorbia peplus.4 Although the plant has long been used as a home remedy for a variety of skin lesions, in 2012 the USFDA approved IM gel for the treatment of actinic keratosis.5,6 The 0.015% gel is applied once daily for three days on the face and scalp, and the 0.05% gel is used once daily for two days on the trunk and extremities.6 IM acts in a dose-dependent manner through two known mechanisms. It induces primary necrosis of tumor cells and causes a robust immune reaction (via neutrophil-mediated, antibody-dependent cellular cytotoxicity of residual malignant cells).7,8 Advantages of this treatment include the brief treatment duration and rapid wound healing.7
Investigators have also successfully used IM gel off-label for the treatment of other epithelial lesions. In one case series, 16/17 (94.1%) of patients with anogenital warts (condylomata acuminata) experienced complete wart clearance after treatment with IM gel and remained in remission at 240 days.9 IM may also have some utility in treating Bowen’s disease (squamous cell carcinoma in situ). One study found that 8/9 (88.9%) patients with this condition achieved complete lesion clearance after combined treatment with fractional CO2 laser before IM gel.10 The authors concluded that IM treatment alone had limited value in treating Bowen’s disease, although there are also reports of complete resolution with IM administered as monotherapy.10,12
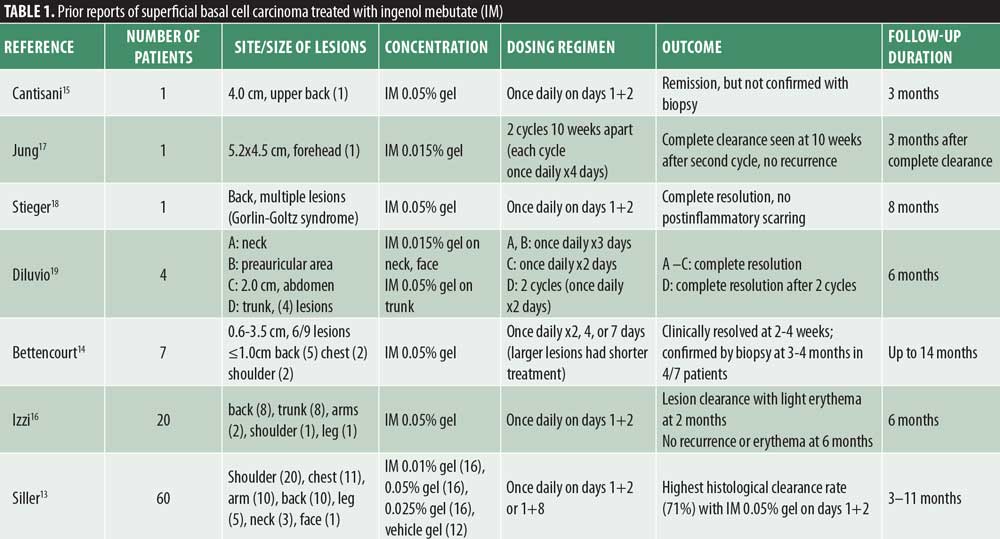
This novel drug also has favorable evidence for the treatment of superficial basal cell carcinoma (sBCC) (Table 1). A randomized, vehicle-controlled, Phase IIa study of 60 patients with sBCC observed histological clearance rates of up to 71 percent with IM 0.05% gel applied once daily on two consecutive days. This combination was superior to regimens that dosed IM on Days 1+8 and/or used IM gel 0.01% or 0.025%. No serious adverse events were observed in this trial, although six patients experienced severe local skin reactions (LSRs) such as vesicle formation. The most common LSRs were scaling, flaking, and dryness, and erythema.13
Among other cases of sBCC successfully treated with IM gel, researchers found that the LSR was generally well tolerated and lasted about two weeks.14–18 Most patients experienced complete resolution of sBCC after one cycle of IM treatment; however, two patients required two cycles for complete clearance.17,19 One retrospective case series reported that an additional patient (not included in the study) experienced recurrence of sBCC a few months after IM treatment and was sent for surgery.14
Although IM is known to produce pleasing cosmetic results, one correspondence reported a scarring reaction at a patient’s IM treatment site. Investigators pretreated a 1.0 cm superficial and micronodular BCC on the chest with a light curettage prior to starting IM 0.05% gel once daily for two days. The wound created by curettage likely increased the surface area for gel absorption and resulted in scar formation. Therefore, the authors recommend allowing wound sites to heal before starting IM treatment.20
The evidence is less clear for treating BCCs that are not the superficial subtype with IM. Nodular BCC (nBCC) is the most common variant; however, compared to over 90 cases of sBCC treated with IM, there is only one report of nBCC treated with IM.1,21 A patient’s large facial nBCC resolved after seven cycles of IM treatment over eleven months (each cycle required application of 0.015% gel once daily for three days).21 This protracted treatment duration suggests that IM might be less effective in the treatment of nBCC compared to sBCC. Also, a patient with multiple BCCs (subtype unspecified) within a verrucous epidermal nevus experienced a poor response to IM. Only partial clearance was achieved after three treatment cycles (once daily, 3 days each week) with IM 0.05% gel.22 However, a trial of IM treatment might still be considered for some patients with nodular BCC, particularly patients who cannot or will not undergo traditional surgical interventions.
This case is unique from previous reports of using IM to treat sBCC for two reasons. First, the patient discussed here has been monitored for clinical recurrence for 18 months posttreatment. In comparison, the follow-up period for other sBCC cases ranged from three to 14 months. In order to validate the long-term efficacy of IM gel for sBCC, future large-scale trials should follow larger groups of patients for extended periods of time after treatment. These data will better capture the sBCC recurrence rate after IM treatment and the proportion of patients who need multiple treatment cycles.
Second, this case presents the first reported use of IM 0.05% gel on the face for sBCC. There are only four other reports of using IM to treat BCC on the face (one nBCC and three sBCC), and the other investigators used the less potent IM 0.01% (not commercially available) and 0.015% gel formulations on this sensitive area.13,17,19,21 Our patient tolerated the LSR well and had no pain after his treatment. However, because only IM 0.015% gel is FDA approved for use on the face (in the treatment of actinic keratosis), dermatologists may want to treat facial sBCC more conservatively with this gel.6 Also, the previously mentioned clinical trial found that the incidence of some adverse events, such as vesicles, ulceration/erosion, scabbing/crusting, edema, and hypopigmentation was higher in patients treated with IM 0.05% gel.13
Conclusion
We successfully treated a patient’s large facial sBCC with IM 0.05% gel that was applied on two consecutive days. The patient tolerated the treatment well, was happy with the cosmetic outcome, and has remained in remission for 18 months. These results are consistent with previous reports of this novel treatment for sBCC. There is no current sign that this drug will ever be on label for other indications besides actinic keratosis. However, IM should at least be considered in difficult cases of sBCC—and other non-melanoma skin cancer—when first-line, traditional and other creative modalities are less attractive.
References
- Marzuka AG, Book SE. Basal Cell Carcinoma: Pathogenesis, Epidemiology, Clinical Features, Diagnosis, Histopathology, and Management. Yale J Bio Med. 2015; 88(2): 167–179.
- Paoli J, Gyllencretuz JD, Fougelberg J, et al. Nonsurgical Options for the Treatment of Basal Cell Carcinoma. Dermatol Pract Concept. 2019; 9(2): 75–81.
- Tanese K. Diagnosis and Management of Basal Cell Carcinoma. Curr Treat Options Oncol. 2019; 20(2):13.
- Ramsay JR, Suhrbier A, Aylward JH, et al. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br J Dermatol. 2011;164(3):633–636.
- Green AC, Beardmore GL. Home treatment of skin cancer and solar keratoses. Australas J Dermatol. 1988;29(3):127–130.
- PICATO® gel [package insert]. Parsippany, NJ: LEO Pharma Inc; 2012.
- Braun SA, Baran J, Schrumpf H, et al. Ingenol mebutate induces a tumor cell-directed inflammatory response and antimicrobial peptides thereby promoting rapid tumor destruction and wound healing. Eur J Med Res. 2018:23:45.
- Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol. 2012;66(3):486–493.
- Schopf RE. Ingenol mebutate gel is effective against anogenital warts – a case series in 17 patients. J Eur Acad Dermatol Venereol. 2016;30(6):1041–1043.
- Lee DW, Ahn HH, Kye YC, et al. Clinical experience of ingenol mebutate gel for the treatment of Bowen’s disease. J Dermatol. 2018;45(4):425–430
- Salleras Redonnet M, Quintana Codina M. Ingenol mebutate gel for the treatment of Bowen’s disease: a case report of three patients. Dermatol Ther. 2016;29(4):236–239.
- Alkhalaf A, Hofbauer GF. Ingenol Mebutate 150 mg as Physician-Directed Treatment of Bowen’s Disease Under Occlusion. Dermatology. 2016;232(Suppl 1):17–19.
- Siller G, Rosen R, Freeman M, et al. PEP005 (ingenol mebutate) gel for the topical treatment of superficial basal cell carcinoma: results of a randomized phase IIa trial. Australas J Dermatol. 2010;51(2):99–105.
- Bettencourt MS. Treatment of superficial basal cell carcinoma with ingenol mebutate gel, 0.05%. Clin Comset Investig Dermatol. 2016;9:205–209.
- Cantisani C, Paolino G, Cantoresi F, et al. Superficial basal cell carcinoma successfully treated with ingenol mebutate gel 0.05%. Dermatol Ther. 2014;27(6):352–354.
- Izzi S, Sorgi P, Piemonte P, et al. Successfully treated superficial basal cell carcinomas with ingenol mebutate 0.05% gel: report of twenty cases. Dermatol Ther. 2016;29(6):470–472.
- Jung YS, Lee JH, Bae JM, et al. Superficial basal cell carcinoma treated with two cycles of ingenol mebutate gel 0.015. Ann Dermatol. 2016;28(6): 796–797.
- Stieger M, Hunger RE. Ingenol mebutate treatment in a patient with Gorlin syndrome. Dermatology. 2016;232(Suppl 1):29–31.
- Diluvio L, Bavetta M, Di Prete M, et al. Dermoscopic monitoring of efficacy of ingenol mebutate in the treatment of pigmented and non-pigmented basal cell carcinomas. Dermatol Ther. 2017;30(1).
- Nguyen NM, Tremaine AM, Zachary CB. A scarring reaction to the treatment of basal cell carcinoma with ingenol mebutate. Skinmed. 2014;12(5): 317–318.
- Iannazzone SS, Ingordo V. Nodular basal cell carcinoma of the face successfully treated with ingenol mebutate 0.015% gel. Dermatol Pract Concept. 2018;8(2):129–131.
- Satolli F, Rovesti M, De Felici MB, et al. Multiple Basal Cell Carcinoma Arising in a Verrucous Epidermal Naevus: Clinical, Histological and Therapeutic Observations. Acta Derm Venereol. 2018;98(1): 132–133.

