
J Clin Aesthet Dermatol. 2021;14(2):26–33.
by Brianna De Souza, MD; Andrea Tovar-Garza, MD; Laura N. Uwakwe, MD; and Amy McMichael, MD
All authors are with the Department of Dermatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Bi-temporal hair loss can be a diagnostic challenge because several entities may affect this region of the scalp, including both scarring and nonscarring conditions. Although traction alopecia is the most common cause of bi-temporal hair loss, no studies to date have outlined all of the potential causes.
Objective: We sought to review nonscarring and scarring conditions that have a clinical presentation of bi-temporal hair loss, including traction alopecia, telogen effluvium, female pattern hair loss, frontal fibrosing alopecia, central centrifugal cicatricial alopecia, and seborrheic dermatitis.
Methods: A Google Scholar and PubMed literature search were conducted for this review. The keywords used in the search included the following: “traction alopecia”, “telogen effluvium”, “androgenic alopecia”, “androgenetic alopecia”, “female pattern hair loss”, “alopecia areata”, “frontal fibrosing alopecia”, “central centrifugal cicatricial alopecia”, and “seborrheic dermatitis”. The scope of our search included all research articles published from 1957 to February 2019. In total, 94 articles regarding non-scarring and scarring hair loss were selected and included according to topic relevance. Exclusion criteria included articles that did not address the epidemiology and/or clinicopathologic or dermatoscopic findings of non-scarring and scarring forms of alopecia. Inclusion criteria included articles that addressed a clinical presentation of bitemporal hair loss; or addressed epidemiology, clinical presentation, dermatoscopic findings, and/or treatment.
Results: Bi-temporal hair loss is a common and often distressing condition with a broad differential.
Conclusion: Clinicians must be aware of the potential causes of bi-temporal hair loss. Prompt diagnosis is essential to prevent further hair loss, especially in scarring conditions.
KEYWORDS: Bi-temporal, alopecia, traction, telogen effluvium, female pattern hair loss, alopecia areata, frontal fibrosing alopecia, central centrifugal cicatricial alopecia
Hair loss, or alopecia, is a common condition that is seen in both men and women, affecting patients of all racial and ethnic backgrounds. It has a negative impact on the majority of affected individuals, with 75 percent of patients demonstrating low self-esteem and 50 percent experiencing social problems.1 Often, the clinical severity does not reliably predict the quality of life in patients with alopecia; therefore, prompt diagnosis is essential when managing any type of hair loss.2
Bi-temporal hair loss is a common finding in clinical practice and demands thorough evaluation. Accurate diagnosis requires detailed medical history, grooming practices, physical examination, dermatoscopic evaluation, and sometimes histopathologic evaluation. Clinicians should be aware that normal variants exist in a natural hairline, as such measurements of the frontotemporal scalp are a critical part of the diagnosis.3 The purpose of this review is to outline the clinical, dermatoscopic, and histopathological characteristics of different types of alopecia that may clinically present with hair loss in the bilateral temporal scalp.
Methods
A Google Scholar and PubMed literature search were conducted for this review. The keywords used in the search included the following: “traction alopecia”, “telogen effluvium”, “androgenic alopecia”, “androgenetic alopecia”, “female pattern hair loss”, “alopecia areata”, “frontal fibrosing alopecia”, “central centrifugal cicatricial alopecia”, and “seborrheic dermatitis”. The scope of our search included all research articles published from 1957 to February 2019. In total, 94 articles regarding non-scarring and scarring hair loss were selected and included according to topic relevance. Exclusion criteria included articles that did not address the epidemiology and/or clinicopathologic or dermatoscopic findings of non-scarring and scarring forms of alopecia. Inclusion criteria included articles that addressed a clinical presentation of bitemporal hair loss; or addressed epidemiology, clinical presentation, dermatoscopic findings, and/or treatment.
Results
Traction alopecia. Traction alopecia is a kind of hair loss caused by prolonged tension to the hair, originally described in Greenland girls in 1957.4 This type of alopecia is typically nonscarring; however, prolonged trauma may lead to a scarring hair loss.
TA is mainly characterized by symmetrical loss and thinning of hair along the frontotemporal margins, though any area of the scalp can be affected. This condition is commonly seen in African-American women and children in association with the use of tight braids, dread-locks, and ponytails.5 Yet, traction alopecia is not exclusive to African-Americans; it can occur in patients of varying racial and ethnic backgrounds and is dependent on individual hair styling. For example, Japanese patients can develop a form of traction alopecia known as alopecia atrophic symmetrica temporalis following repeated use of traditional Japanese hairstyles.6 Therefore, a detailed history of traction-related hair care practices is essential for the diagnosis.
The earliest clinical signs in traction alopecia are perifollicular erythema and pruritus, though papules and pustules may be present.7 The fringe sign was described by Samrao et al9 and is characterized by the presence of retained hairs along the frontal and/or temporal hairline, and has a high sensitivity and specificity for both early- and late-stage traction alopecia8 (Figure 1). Traction alopecia in the occipital scalp is commonly seen in patients who wear tight chignon or buns.9 Hair pull test can be positive in active disease.
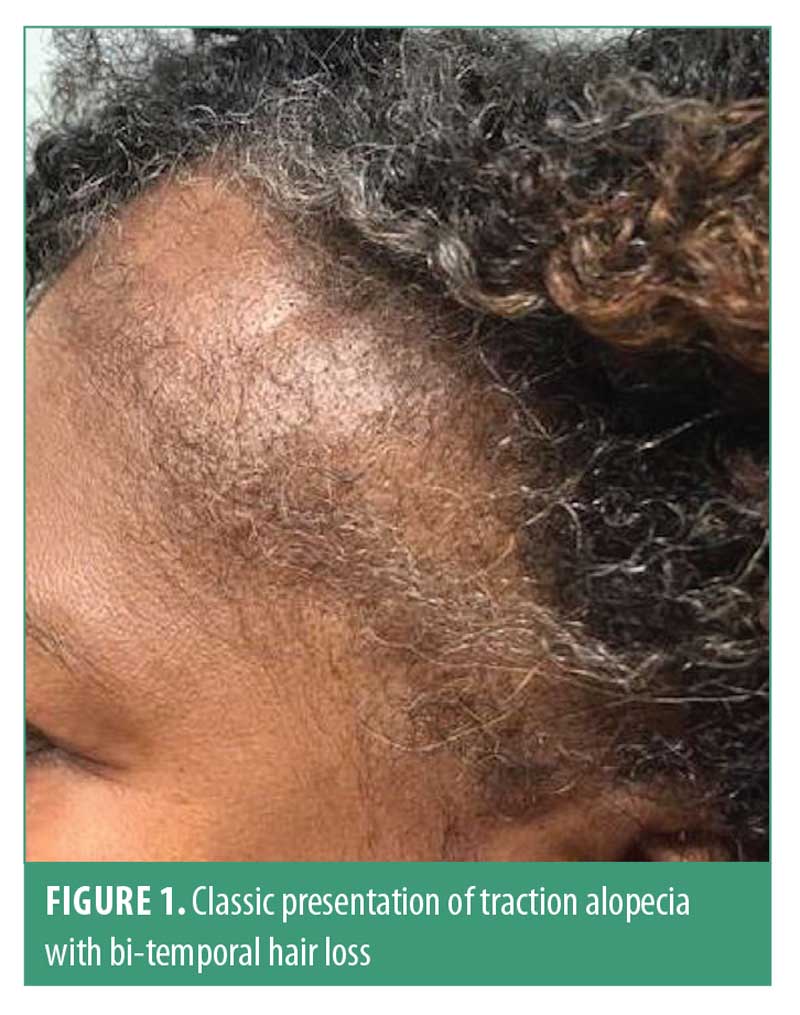
Dermatoscopic signs of traction alopecia include reduced hair density with/without the absence of follicular openings and numerous hair casts, especially at the periphery of the patch.10 Hair casts are a sign of ongoing traction and are present in more than 80 percent of patients with tight hairstyles.11–13 Less than 20-percent hair variability may be present.10 Other dermatoscopic findings include black dots, yellow dots, atypical red vessels, and broken hairs.14
Clinical-pathological correlation is essential in traction alopecia. Increased numbers of telogen and catagen hairs, trichomalacia, and normal number of terminal hairs characterize early stages of traction alopecia. The decrease in terminal hairs, which are replaced by fibrous tracts, vellus hairs, and retained sebaceous glands, is seen in the late stages.15 Transverse sections may aid in the quantitative assessment of vellus hairs, which can help to differentiate from primary cicatricial alopecia.16
Early education on appropriate hairstyling (i.e., use loose hairstyles, avoid tension) is essential to any treatment plan and may help to prevent late-stage disease. Oral antibiotics and intralesional triamcinolone are recommended by some authors to decrease the perifollicular inflammation in early stages.7 Topical 2% minoxidil has shown promising results in a brief case report, although, in regular practice, several authors have recommended minoxidil 5%.7,8,17 In longstanding disease, surgical hair restoration may be considered.18
Telogen effluvium. Telogen effluvium is a nonscarring condition with abnormal hair cycling, which causes excessive loss of telogen hairs. Common causes of telogen effluvium include malnutrition, thyroid disease, iron deficiency, drugs, chronic renal failure, early androgenetic alopecia, telogen gravidarium, and other systemic conditions that have a physiologic impact on the body. Patients should be evaluated for female pattern hair loss (FPHL) given its clinical similarity to telogen effluvium.
Detailed clinical history is essential for diagnosis; chronology of disease onset and duration of hair loss is important.19 Usually, acute telogen effluvium is most likely to be related to a specific trigger; however, in up to 33 percent of cases, it is possible that no trigger will be identified.20 If the insult is prolonged or recurrent, then the shedding is considered progressive, persisting over six months and leading to chronic telogen effluvium (CTE).
Hair shedding is the main complaint in TE; individuals usually report a high hair density prior to onset. Clinical examination usually reveals bi-temporal recession, without involvement of the central scalp (Figure 2). Hair pull test is strongly positive in active TE; however, negative pull test should not exclude telogen effluvium.21
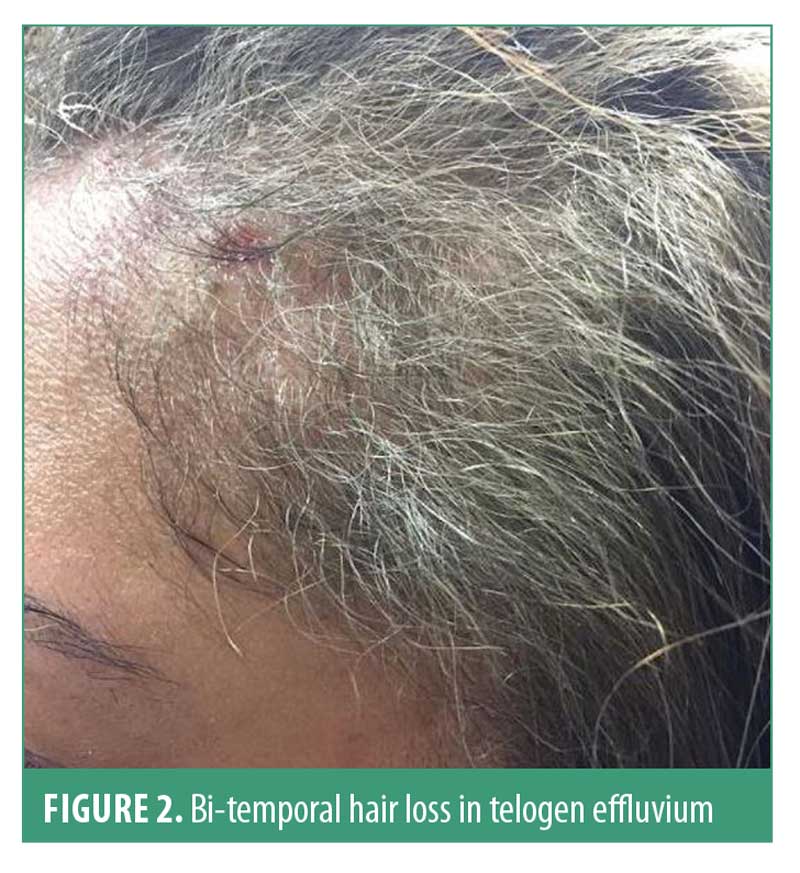
Dermoscopy has a limited diagnostic value in telogen effluvium. The most common findings include empty hair follicles, single hair pilosebaceous units, and peripilar sign. However, these findings are nonspecific for telogen effluvium. Of note, there are no trichoscopic differences between the frontal and occipital scalp, unlike in FPHL.22
Usually, history-taking and clinical examination are sufficient for diagnosis; however, scalp biopsy may be necessary. Multiple horizontal-sectioned scalp biopsies taken from the vertex are more accurate than a single biopsy.23 The anagen:telogen ratio is 8:1 (vs. 14:1 in the normal scalp). The normal total number of hairs and the terminal:vellus hairs ratio is, on average, 8:1 (vs. 1.9:1 in FPHL).19,23 However, it is important to note that the coexistence of telogen effluvium and FPHL has been reported.24
To treat telogen effluvium, providers must address the underlying cause. Secondarily, patients should be educated on the appropriate timeline for improvement. Hair shedding can take 3 to 6 months to cease once the trigger for the condition passes, after which time, hair regrowth can take 3 to 6 more months and significant regrowth can take 12 to 18 months to occur.25 Topical minoxidil is recommended, as it is known to prolong the anagen phase.26 A recent study demonstrated significant improvement in hair loss among 36 women with CTE treated with oral minoxidil (0.25–5 mg daily).27
FPHL. FPHL is a nonscarring progressive condition, caused by the miniaturization of hair follicles and the conversion of terminal hairs to vellus hairs. These hair follicles have a short anagen phase, resulting in short and fine hair shafts.28
Three different patterns of FPHL can be observed. First is the classic diffuse thinning of the midline scalp, which can be classified using the Ludwig and Sinclair scale. Second, the “Christmas tree pattern” described by Olsen,30 is characterized by frontal midline recession and thinning of the central part of the scalp. Finally, the third pattern presents as hair thinning associated with bi-temporal recession, which resembles the classic male pattern hair loss (MPHL) presentation (Figure 3).28 This later pattern is less frequent but should be differentiated from other types of bi-temporal alopecia. The hair pull test is usually negative; however, it may be positive in the frontal scalp as compared with in the occipital scalp due to the shortened hair cycle.
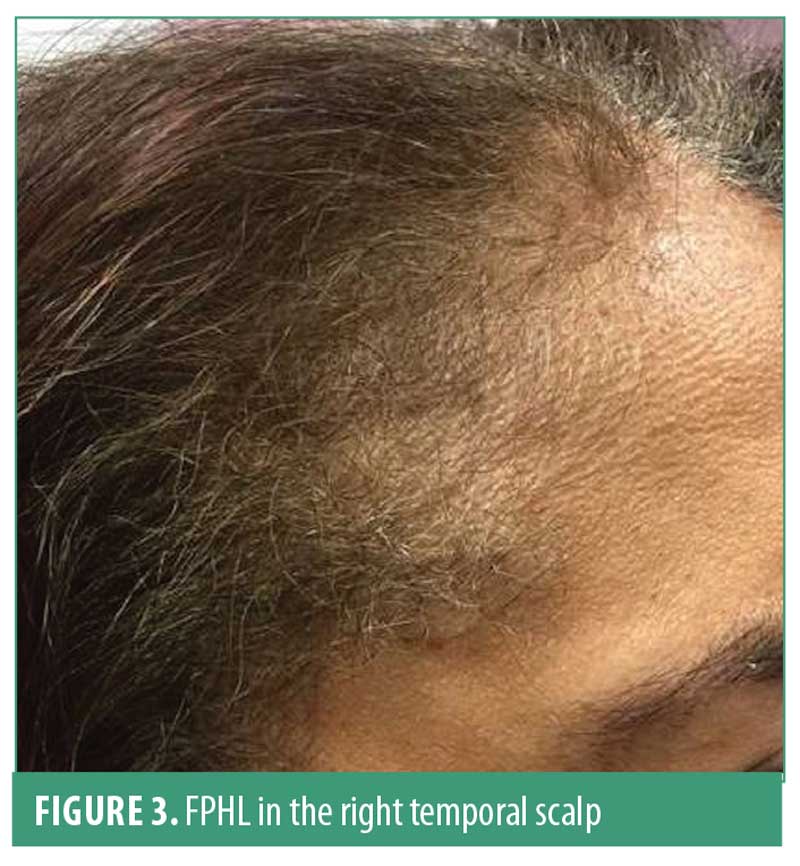
Miniaturization is the most characteristic sign of FPHL. Peripilar sign has been reported in previous studies and reflects the presence of perifollicular inflammation. Yellow dots, which represent empty follicular ostia filled with sebum and keratin, have also been described.30,31
A previous study aimed at standardizing the dermatoscopic patterns of 131 female patients with FPHL established a trichoscopic, diagnostic tool with 98-percent specificity.22 Two major criteria or one major and two minor criteria are used to confirm the diagnosis of FPHL. Major criteria include more than four yellow dots, a lower average of hair thickness in the frontal scalp relative to in the occipital scalp, and greater than 10% of hairs (< 0.003 mm) in the frontal scalp being thin. Meanwhile, minor criteria include single-hair pilosebaceous units, vellus hairs, and perifollicular discoloration. In 2013, Herskovitz et al29 reported that the presence of more than six vellus hairs in the frontal scalp (20× magnification) should be a marker for early FPHL, despite the absence of other signs.
Histopathologic findings of FPHL include a decrease in terminal hairs and anagen hairs and an increase in vellus-like hairs, telogen hairs, and fibrous residual tracts. Mild perifollicular inflammation may be present. In horizontal sections, one may observe diversity in hair diameter, which reflects follicle miniaturization.32
The only medication approved by the United States Food and Drug Administration for FPHL is minoxidil 2% solution or 5% foam. A previous study reported that the effects of minoxidil 5% foam were similar to those of 2% solution twice daily in women.33 However, several treatment alternatives are effective with off-label use for this condition. Spironolactone is used off-label for FPHL at a standard dose of 100 to 200mg/day.34,35 In one study, finasteride (2.5mg given orally) was shown to be effective in 62 percent of patients with FPHL.36 Several randomized controlled trials reported some efficacy of low-level light therapy for FPHL.37–39 A recent open-label study reported improvement in hair loss severity among 100 women with FPHL on low-dose oral minoxidil (0.25mg) and low dose spironolactone (25mg).40
Alopecia areata. Alopecia areata is an autoimmune disorder characterized by temporary, abrupt nonscarring hair loss, which affects two percent of the population at some point in their lifetime.41 More than 70 percent of individuals experience disease onset before 40 years of age and early-onset alopecia areata is generally characterized as a more severe subtype.42,43
The most common clinical presentation is well-defined patchy alopecia, although any hair-bearing area can be affected. Alopecia areata incognita is a rare presentation of this condition, which typically presents as a diffuse distribution with abrupt and intense hair loss. Skin in the involved area is smooth, sometimes slightly erythematous; temporal and retroauricular areas are commonly affected44 (Figure 4). Regrowth usually presents with normal hair thickness and color; however, initially, fine hair and nonpigmented hair may be observed. A minority of patients may progress to full scalp hair loss (alopecia totalis) or hair loss of all hair-bearing areas (alopecia universalis). Hair pull test is generally positive in the edges of the patches and is a marker of a dystrophic anagen phase.45,46 Up to 10 to 15 percent of individuals can have nail involvement, such as fine nail pitting and trachyonychia.47 Patients with severe disease have a higher risk of nail abnormalities relative to those with limited patchy hair loss.48
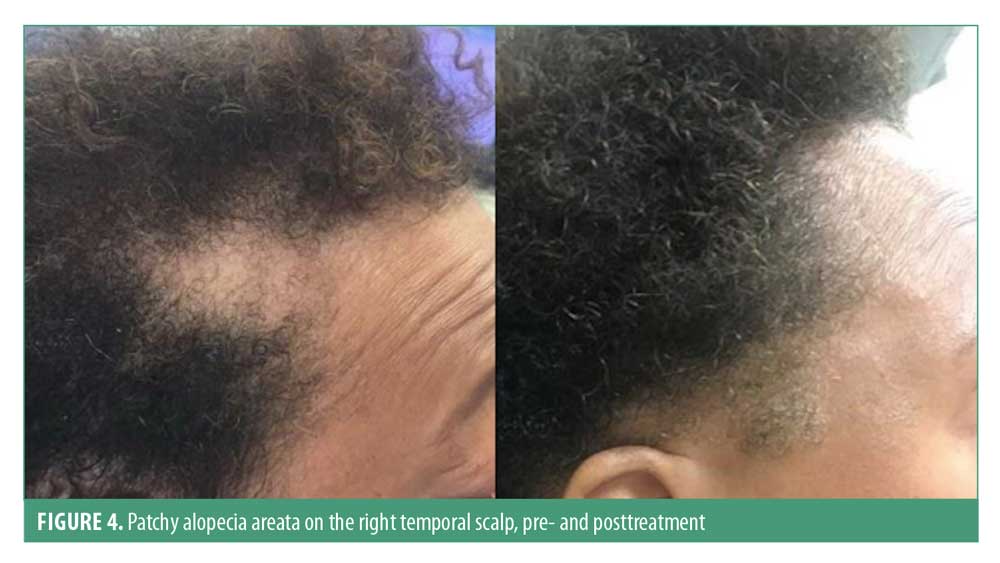
Dermoscopy is a useful tool in alopecia areata and can aid in differentiating from other hair loss disorders. The most common features are yellow dots (present in 64%–94% of individuals), black dots (44%–70%), exclamation mark hairs (30%–40%), broken hairs (45%–58%), vellus hairs (33%–72%), and tapered hairs (12%–42%).49
Trichoscopy is useful in identifying active disease. Features of active disease include black dots, exclamation mark hairs, and broken hairs. Yellow dots and vellus hairs are present in severe, late-stage disease.50–52
AA is a dynamic disease and histopathologic findings will vary with the disease stage. Horizontal and vertical sections are essential for an assessment. In early alopecia areata, anagen hairs show an inflammatory infiltrate of CD4+ and CD8+ T-cells, mainly around and within the hair bulb. Skin biopsies from the edges of active alopecia areata have shown increased number of follicles in the catagen and early telogen phases, as well as miniaturization.53 In chronic alopecia areata, follicles below the level of the sebaceous glands are miniaturized and can have telogen or anagen characteristics. Pigmentary incontinence in the follicular dermal papillae and degeneration of the hair bulb epithelium may be present.53,54
There are no treatments approved by the Food and Drug Administration for alopecia areata. A recent meta-analysis reviewed 17 studies and included 540 participants with alopecia areata.55 The authors assessed a range of topical and oral interventions and, overall, none showed significant treatment benefits in terms of hair growth when compared to the placebo. Despite the lack of evidence, authors recommend management depending on patient’s age and disease severity and duration. Topical therapy (e.g., corticosteroids, anthralin) are preferred for patients younger than 10 years old. Intralesional triamcinolone acetonide (2.5–10 mg/mL) is a common practice in children and adults. Oral mini-pulse therapy of steroids and other immunosuppressants such as methotrexate are reported in the literature.
Diphenylcyclopropenone is the contact sensitizer of choice in alopecia areata.46 Other recent therapies include topical and oral janus-kinase inhibitors, such as tofacitinib and ruxolitinib.56–58
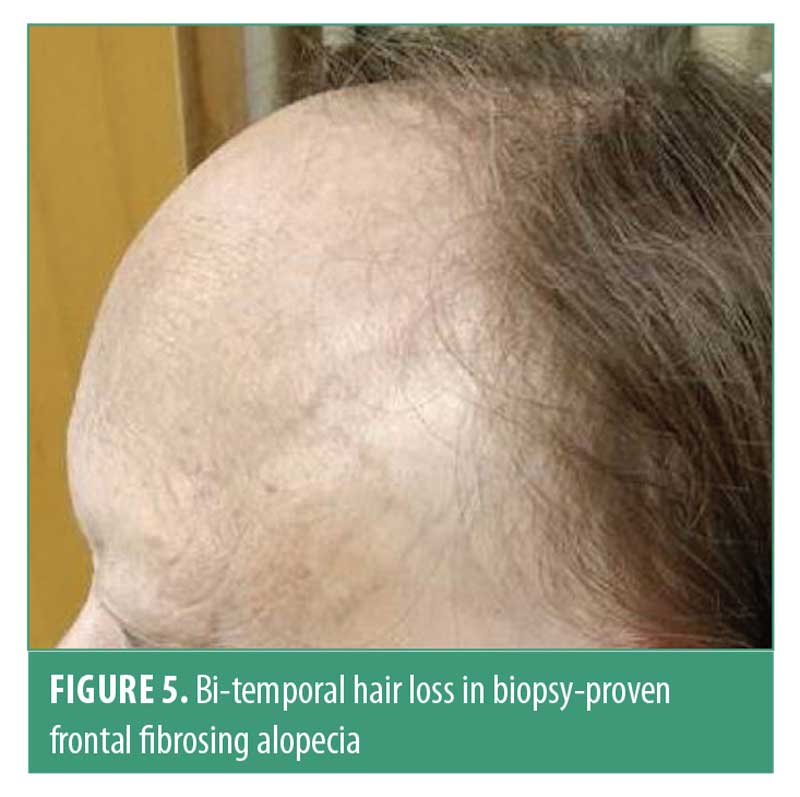
Frontal fibrosing alopecia. Frontal fibrosing alopecia (FFA) is a variant of lichen planopilaris (LPP) characterized by a progressive scarring affecting the frontotemporal hairline.59 Some authors suggest it may be a distinct entity, which shares histopathologic findings with LPP.60
FFA is clinically characterized by a progressive band of hair loss affecting the frontal and/or temporal hairline (Figure 5). Involvement of the eyebrows is common. Unique areas of involvement in men are sideburns and facial hair.61 The pseudo-fringe sign is characterized by more pronounced hair loss in the temporal area with sparing of the hairline; this should be distinguished from the fringe sign seen in TA.62 Perifollicular erythema and white scale are markers of disease activity.61 Hair shedding varies by patient and hair pull test is positive for anagen hairs.45
Recently, diagnostic criteria for FFA were proposed; the presence of two major criteria or one major and two minor criteria are necessary for diagnosis. Major criteria include predominant involvement of scarring hair loss of the frontal and/or temporal hairline and histopathologic features consistent with FFA/LPP, while minor criteria include involvement of other sites (i.e., eyebrows, sideburns, facial hair, eyelashes), associated lichen planus at other body sites, noninflammatory facial papules, and preceding symptoms at areas of involvement (pruritus or pain).61 Eyebrow involvement can be present in 73 to 81 percent of patients; therefore, some authors suggest this should be considered a major criterion. Trichoscopic findings have been shown to aid in the diagnosis of FFA; therefore, they are considered as minor criteria by some authors.60
Since FFA is considered to exist within the spectrum of LPP, both diseases share trichoscopic features.63 The most common findings in FFA include the absence of follicular openings and mild perifollicular scale.50,64,65 The presence of lonely hairs and an absence of vellus hairs in the frontal hairline have been reported as potential clues for FFA.63,65 Perifollicular erythema may occasionally be present as well as single-hair openings at the hair-bearing margin.63 An ivory-white to ivory-beige background has been reported.65 Pink-gray and gray dots are features that may be observed in the lateral eyebrows.49,66
Histopathologic findings include prominent perifollicular fibrosis and lymphocytic inflammatory infiltrates in a lichenoid pattern with spared interfollicular epidermis. On horizontal sections, prominent onion-skin fibrosis with peri-infundibular and peri-isthmus lymphocytic infiltrates may be observed. External outer root sheath shows vacuolar degeneration of the basal layer and mild keratinocyte degeneration.59,61 Eosinophilic necrosis of cells on the external root sheath and sparing of the interfollicular epidermis are distinctive of FFA.67 In FFA, loss of sebaceous glands is an early finding, which helps to differentiate from late-stage TA.15
Similar to other hair loss conditions, interpreting the efficacy of therapeutic options is difficult in FFA due to the lack of well-designed randomized controlled trials. However, combination therapies appear to be the standard therapy for FFA. Intralesional corticosteroids seem to be the first-line therapy, decreasing symptoms and achieving arrest of hairline recession.68,69 Oral antibiotics such as doxycycline (100mg twice daily) have shown efficacy.70 Hydroxychloroquine (200mg twice daily) has demonstrated variable efficacy in multiple case series.70–72 Medications such as finasteride (2.5–5mg daily) and dutasteride (0.5mg daily) resulted in disease stability and mild regrowth in 33 to 70 percent of patients with FFA.71,73–76
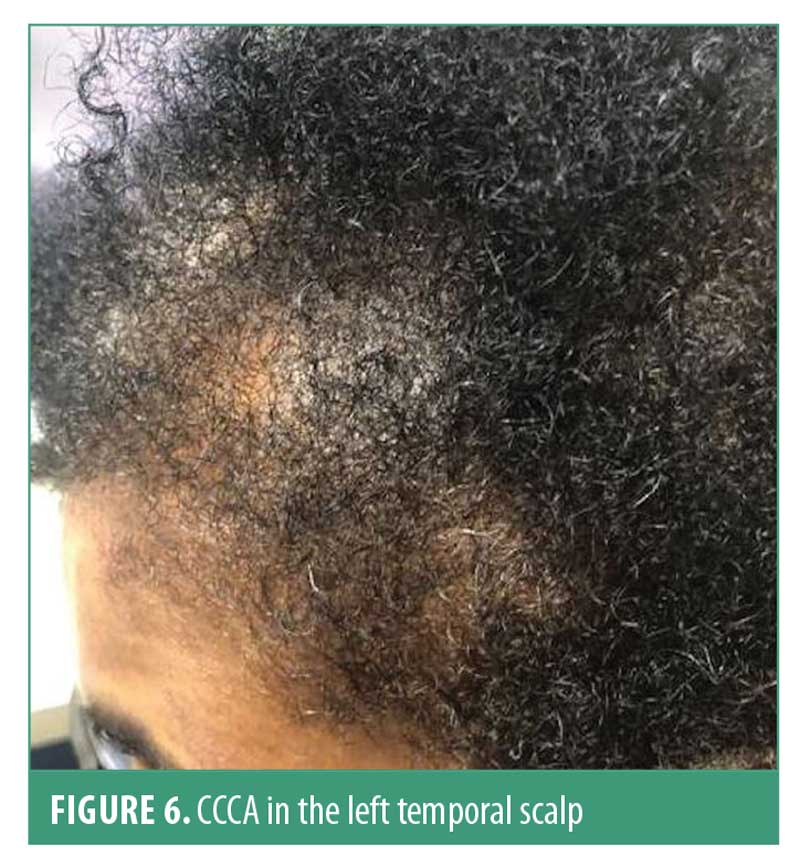
Central centrifugal cicatricial alopecia. Central centrifugal cicatricial alopecia (CCCA) is the most common cause of scarring hair loss in African-American women.77,78 Disease onset can present in the late second or third decade of life; however, many patients seek treatment once their hair loss has progressed. The etiology remains unclear; however, hair care practices such as hot combs, chemical relaxers, and traction styles have been implicated as contributors to CCCA.7,77,79 Genetic factors, such as PADI13 gene mutations, are also thought to play a role in disease susceptibility and progression.79,80
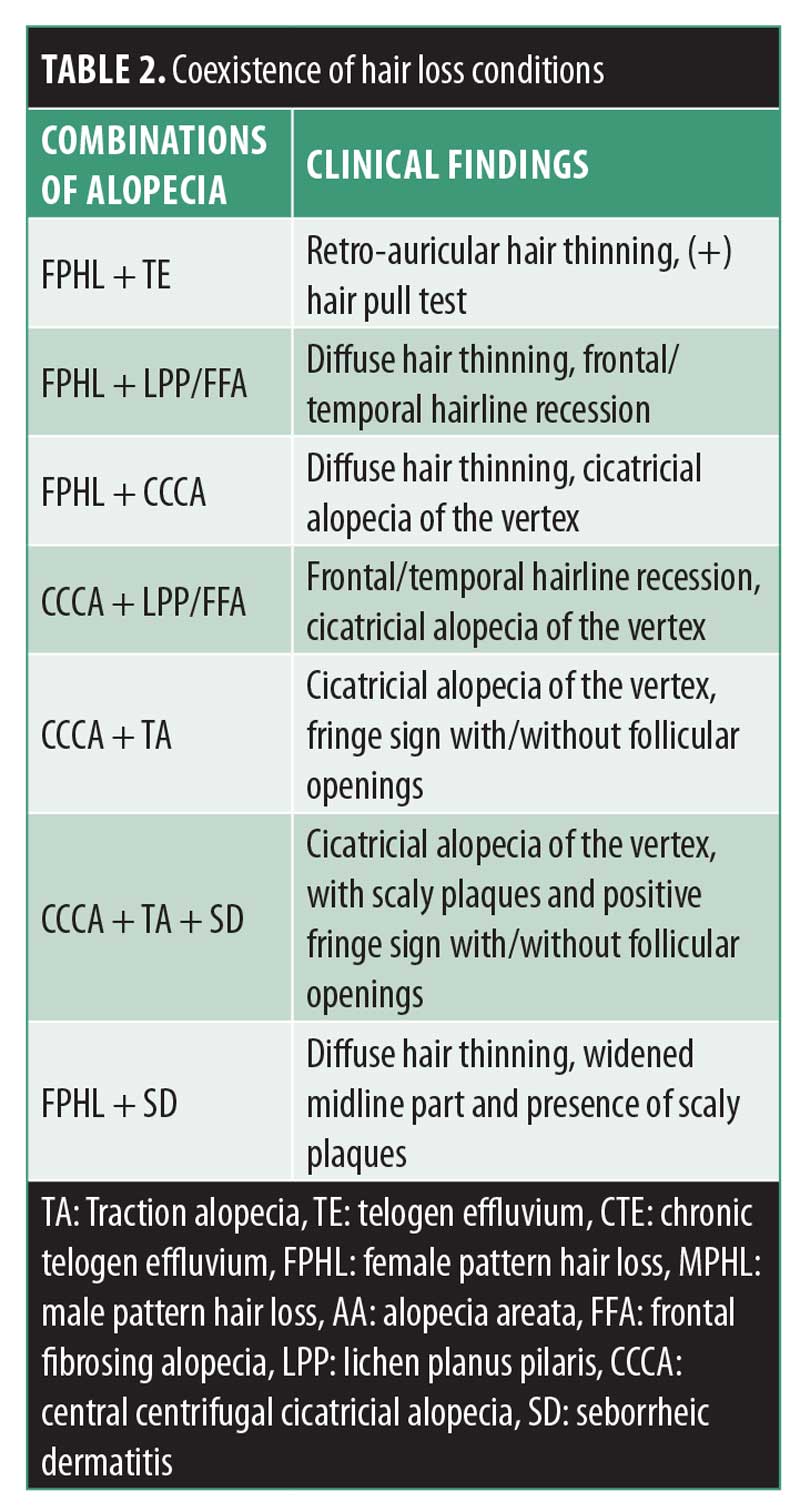
Hair breakage can be the first sign of early CCCA before hair loss is even perceived.81 The hair loss typically starts in the vertex and slowly progresses centrifugally. However, CCCA can also present as irregular patchy alopecia involving the temporal and/or occipital scalp.82 Follicular pustules can be seen in early disease, along with erythema and peripilar scale. Symptoms that present in CCCA include tenderness and pruritus. Early signs (e.g., hair breakage) and symptoms (e.g., tenderness, pruritus) suspicious for CCCA should be evaluated early on to optimize treatment. The hair pull test is usually negative.78,83 CCCA can coexist with other nonscarring hair loss conditions (Figure 6).
Dermatoscopic findings reported in 51 women with CCCA revealed that peripilar white-gray halo was present in 94 percent of patients, with 94-percent sensitivity and 100-percent specificity.84,85 This finding corresponds histopathologically to lamellar fibrosis surrounding the outer root sheath.84 Other dermatoscopic features include honeycomb pigmented network (present in 96% of patients), terminal hairs (100%), vellus hairs (94%), pinpoint white dots in the interfollicular scalp (76%), white patches (67%), perifollicular erythema (61%), and peripilar and interfollicular scale (46%).84,85
Histological features include decreased terminal hair follicles, increased and widened fibrous tracts, and peri-infundibular lymphocytic infiltrates. Premature desquamation of the inner root sheath is seen in early disease.81 In late stages, one may observe the loss of sebaceous glands and hair follicles with prominent fibrosis of the dermis.15,81,84
The main goal when treating CCCA is to halt disease progression. Providers should encourage patients to modify hair-grooming practices, especially if they trigger active disease.7 Topical corticosteroids, calcineurin inhibitors, and intralesional triamcinolone acetonide have been reported to decrease the inflammatory process. Oral antibiotics such as tetracycline can slow disease activity. Topical minoxidil may also be helpful in CCCA.86
Seborrheic dermatitis. Seborrheic dermatitis is a chronic, relapsing skin condition affecting 1 to 3 percent of immunocompetent adults.87 Disease onset is polyphasic, occurring in infants within the first three months of life, in adolescents and young adults, and again in patients older than 50 years.87 The literature has a litany of pseudonyms for the condition (e.g., dandruff, sebopsoriasis, seborrheic eczema, and pityriasis capitis), which underscores the complexity of the disease process.88 Its etiology is thought to be multifactorial, with both endogenous (hormone levels, Malassezia infection, immune response, neurogenic factors) and exogenous (drugs, cold weather, stress) factors at play.89
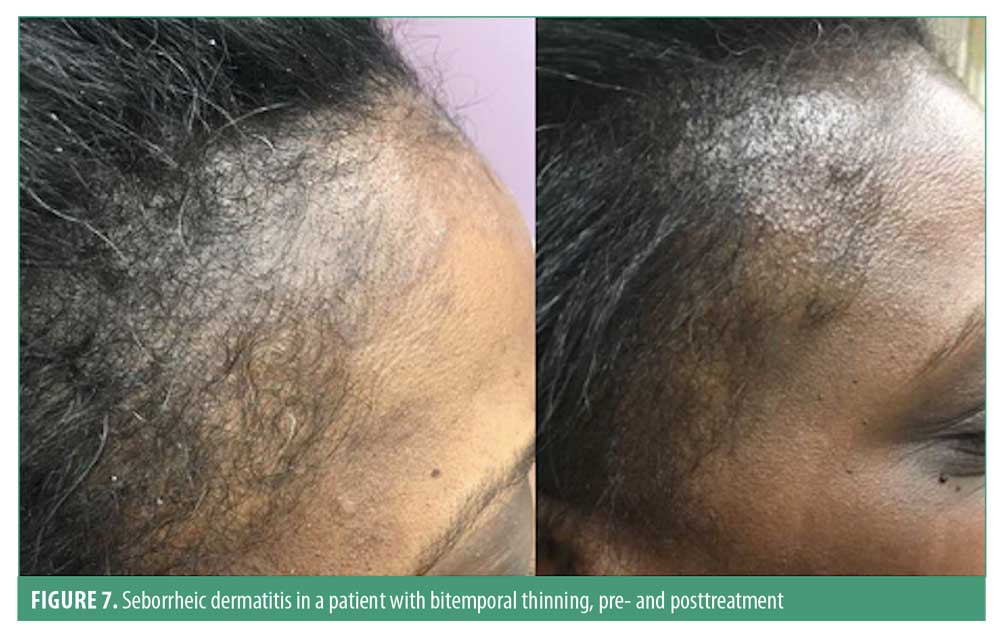
Clinically, seborrheic dermatitis presents as erythematous plaques with large, greasy scales in adults; however, the disease may also present with fine scale without significant erythema in some patients. These lesions typically appear in areas of increased sebaceous activity such as the scalp, hairline, eyebrow, glabella, nasolabial folds, ears, upper chest, back, axillae, umbilicus, and groin.89 In adults, the lesions on the scalp typically present as symmetric, mild desquamation to honey-colored crusts, distributed along the frontal, temporal, parietal, and occipital regions of the scalp. These lesions may extend unto the forehead as a scaly border known as corona seborrheica (Figure 7).90 In infants, the lesions are reddish-yellow plaques covered by thick, greasy scales on the vertex of the scalp occurring in most patients by three months of age.90 Pruritus is a common complaint among adult patients with seborrheic dermatitis, especially in those with scalp involvement. Hair loss is often associated with seborrheic dermatitis.
Common trichoscopic findings of seborrheic dermatitis include arborizing red lines, hidden hairs, perifollicular white scale, twisted red loops, atypical red vessels, structureless red areas, glomerular vessels, yellow dots, perifollicular pigmentation, signet ring vessels, comma vessels, honeycomb pigment pattern, and brown dots. In a study of 112 patients with seborrheic dermatitis, arborizing red lines and comma vessels were the most distinguishable findings via video-dermatoscopy when comparing seborrheic dermatitis to alopecia and scalp psoriasis.91 In a similar study involving 41 patients with seborrheic dermatitis, arborizing vessels and atypical red vessels in the absence of red dots and globules were common findings.92,93
Histologic features of seborrheic dermatitis are the classic “spongiform” appearance with associated edema, increased epidermal proliferation, and focal parakeratosis, as seen in seborrheic dermatitis associated with acquired immune deficiency syndrome.88 An enhanced neutrophil presence at the margins of follicular ostia is also notable.94
Despite its prevalence in the general population, the pathogenesis of seborrheic dermatitis remains poorly understand. There are three popular theories in regard to its pathogenesis. The first theory postulates that Malassezia yeast, which is native to the skin flora, is responsible for seborrheic dermatitis given patients’ responsiveness to antifungal therapy. The second theory postulates that seborrheic dermatitis is primarily an inflammatory disorder with increased cell turnover, scale production, and inflammation in the epidermis.87 The third theory postulates that a failure to suppress an inflammatory response to a surface yeast leads to an immune reaction and, thus, the development of seborrheic dermatitis.87
Although seborrheic dermatitis has no cure, there are a variety of options available to treat symptoms effectively. The goal of care is to control acute flares and maintain remission with long-term therapy. Patient’s age is critical to assess prior to medication selection. Mainstay treatments for seborrheic dermatitis include topical antifungals (ketoconazole), topical steroids (hydrocortisone), topical calcineurin inhibitors (tacrolimus, pimecrolimus), and keratolytics (selenium sulfide, tar, lithium succinate). In cases of refractory and/or widespread seborrheic dermatitis, intralesional steroids and oral therapies, such as itraconazole, may be prescribed.87
Conclusion
Bi-temporal alopecia is a challenging condition, due to the broad differential diagnosis. Clinical and dermatoscopic characteristics to be considered are summarized in Table 1. The coexistence of different types of hair loss can be present; the most common conditions are presented in Table 2. Preventing further hair loss is essential in all types of hair loss, especially in those patients with scarring conditions; therefore, prompt diagnosis is essential. Detailed medical history, grooming practices, dermatoscopic features, and histopathology can aid in the diagnosis of bi-temporal hair loss.
References
- Van der Donk J, Hunfeld JA, Passchier J, et al. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Soc Sci Med. 1994;38(1):1 59–163.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15(2):137–139.
- Nusbaum BP, Fuentefria S. Naturally occurring female hairline patterns. Dermatol Surg. 2009;35(6):907–913.
- Reid EE, Haley AC, Borovicka JH, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2012;66(3):e97–e102.
- Hjorth N. Traumatic marginal alopecia. a special type: alopecia groenlandica. Br J Dermatol. 1;69(9):319–322.
- Krsti? A, Konstantinovi? S, Žuni? M, et al. Traction alopecia due to traditional hair styles. In: Orfanos CE, Montagna W, editors. Hair research. Berlin, Germany: Springer; 1981: 390–391.
- Khumalo NP, Jessop S, Gumedze F, Ehrlich R. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59(3):432–438.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17(2):164–176.
- Samrao A, Price VH, Zedek D, Mirmirani P. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17(11):1.
- Ntuen E, Stein SL. Hairpin-induced alopecia: case reports and a review of the literature. Cutis. 2010;85(5):242–245.
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163(6): 1353–1355.
- Scott MJ, Roenigk HH. Hair casts: classification, staining characteristics, and differential diagnosis. J Am Acad Dermatol. 1983;8(1): 27–32.
- Zhu WY, Xia MY, Wu JH, Do DA. Hair casts: a clinical and electron microscopic study. Pediatr Dermatol. 1;7(4)270–274.
- Zhang W. Epidemiological and aetiological studies on hair casts. Clin Exp Dermatol. 1995;20(3):202–207.
- Shim WH, Jwa SW, Song M, et al. Dermoscopic approach to a small round to oval hairless patch on the scalp. Ann Dermatol. 2014;26(2): 214–220.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28(7):333–342.
- Donovan JC, Mirmirani P. Transversely sectioned biopsies in the diagnosis of end-stage traction alopecia. Dermatol Online J. 2013;19(4).
- Khumalo NP, Ngwanya RM. Traction alopecia: 2% topical minoxidil shows promise. Report of two cases. J Eur Acad Dermatol Venereol. 2007;21(3):433–434.
- Özçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29(4):325–327.
- Whiting DA. Chronic telogen effluvium: increased scalp hair shedding in middle-aged women. J Am Acad Dermatol. 1996;35(6): 899–906.
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129(3):356–363.
- Harrison S, Sinclair R. Telogen effluvium. Clin Exp Dermatol. 2002;27(5):389–395.
- Rakowska A, Slowinska M, Kowalska-Oledzka E, et al. Dermoscopy in female androgenic alopecia: method standardization and diagnostic criteria. Int J Trichology. 2009;1(2):123.
- Sinclair R, Jolley D, Mallari R, Magee J. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51(2):189–199.
- Rudnicka L, Olszewska M, Rakowska A. Atlas of trichoscopy: dermoscopy in hair and scalp disease. London: Springer; 2012.
- Bergfeld WF, Mulinari-Brenner F. Shedding: how to manage a common cause of hair loss. Cleve Clin J Med. 2001;68(3):256–261.
- Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venereol Leprol. 2013;79(5):591–603.
- Perera E, Sinclair R. Treatment of chronic telogen effluvium with oral minoxidil: a retrospective study. F1000Res. 2017;6:1650.
- Herskovitz I, De Sousa IC, Tosti A. Vellus hairs in the frontal scalp in early female pattern hair loss. Int J Trichology. 2013;5(3):118–120.
- Olsen EA. Androgenetic alopecia. In: Disorders of hair growth: diagnosis and treatment. New York, New York: McGrawHill; 1994: 257–283.
- Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: how it may help the clinician. Dermatol Clin. 2013;31(1):29–41.
- Tosti A, Torres F. Dermoscopy in the diagnosis of hair and scalp disorders. Actas Dermosifiliogr. 2009;100 Suppl 1:114–119.
- Camacho-Martínez FM. Hair loss in women. Semin Cutan Med Surg. 2009;28(1):19–32.
- Blume-Peytavi U, Hillmann K, Dietz E, et al. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011;65(6):1126–1134.
- Rushton DH, Futterweit WA, Kingsley DH, et al. Quantitative assessment of spironolactone treatment in women with diffuse androgen-dependent alopecia. J Soc Cosmet Chem. 1991;42:317–325.
- Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010;28(3):611–618.
- Iorizzo M, Vincenzi C, Voudouris S, et al. Finasteride treatment of female pattern hair loss. Arch Dermatol. 2006;142(3):298–302.
- Darwin E, Heyes A, Hirt PA, Wikramanayake TC, Jimenez JJ. Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med Sci. 2018;33(2):425–434.
- Munck A, Gavazzoni MF, Trüeb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6(2):45–49.
- Kim H, Choi JW, Kim JY, et al. Low level light therapy for androgenetic alopecia: a 24 week, randomized, double blind, sham device–controlled multicenter trial. Dermatol Surg. 2013;39(8):1177–1183.
- Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57(1):104–109.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70(7):628–633.
- Gip L, Lodin A, Molin L. Alopecia areata. A follow-up investigation of outpatient material. Acta Derm Venereol. 1968;49(2):180–188.
- Mirzoyev SA, Schrum AG, Davis MD, Torgerson RR. Lifetime incidence risk of Alopecia Areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990–2009. J Invest Dermatol. 2014;134(4):1141.
- Pratt CH, King Jr. LE, Messenger AG, et al. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011.
- Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part I. History and clinical examination. J Am Acad Dermatol. 2014;71(3):415.e1–415.e15.
- Trüeb RM, Dias MF. Alopecia Areata: a comprehensive review of pathogenesis and management. Clin Rev Allergy Immunol. 2018;54(1):68–87.
- Gandhi V, Baruah MC, Bhattacharaya SN. Nail changes in alopecia areata: incidence and pattern. Indian J Dermatol Venereol Leprol. 2003;69(2):114–114.
- Sharma VK, Dawn G, Muralidhar S, Kumar B. Nail changes in 1000 Indian patients with alopecia areata. J Eur Acad Dermatol Venereol. 1998;10(2):189–190.
- Rudnicka L, Olszewska M, Rakowska A, editors. Atlas of trichoscopy: dermoscopy in hair and scalp disease. Berlin, Germany: Springer; 2012.
- Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int J Dermatol. 2008;47(7):688–693.
- Lacarrubba F, Dall’Oglio F, Nasca MR, Micali G. Videodermatoscopy enhances diagnostic capability in some forms of hair loss. Am J Clin Dermatol. 2004;5(3):205–208.
- Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011 Jul;56(4):407–411.
- Messenger AG, Slater DN, Bleehen SS. Alopecia areata: alterations in the hair growth cycle and correlation with the follicular pathology. Br J Dermatol. 1986;114(3):337–347.
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139(12):1555–1559.
- Delamere FM, Sladden MJ, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008;(2):CD004413.
- Liu LY, Craiglow BG, King BA. Tofacitinib 2% ointment, a topical janus kinase inhibitor, for the treatment of alopecia areata: a pilot study of 10 patients. J Am Acad Dermatol. 2018;78(2):403–404.e1.
- Bayart CB, DeNiro KL, Brichta L, et al. Topical Janus kinase inhibitors for the treatment of pediatric alopecia areata. J Am Acad Dermatol. 2017;77(1):167–170.
- Vandiver A, Girardi N, Alhariri J, Garza LA. Two cases of alopecia areata treated with ruxolitinib: a discussion of ideal dosing and laboratory monitoring. Int J Dermatol. 2017;56(8): 833–835.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130(6): 770–774.
- Vañó-Galván S, Saceda-Corralo D, Moreno-Arrones ÓM, Camacho-Martinez FM. Updated diagnostic criteria for frontal fibrosing alopecia. J Am Acad Dermatol. 2018;78(1):e21–e22.
- Tolkachjov SN, Chaudhry HM, Camilleri MJ, Torgerson RR. Frontal fibrosing alopecia among men: A clinicopathologic study of 7 cases. J Am Acad Dermatol. 2017;77(4):683–690.
- Pirmez R, Duque Estrada B, Abraham LS, et al. It’s not all traction: the pseudo “fringe sign” in frontal fibrosing alopecia. Br J Dermatol. 2015;173(5):1336–1338.
- Rakowska A, Slowinska M, Kowalska-Oledzka E, et al. Trichoscopy of cicatricial alopecia. J Drugs Dermatol. 2012;11(6):753–758.
- Tosti A, Miteva M, Torres F. Lonely hair: a clue to the diagnosis of frontal fibrosing alopecia. Arch Dermatol. 2011;147(10):1240.
- Lacarrubba F, Micali G, Tosti A. Absence of vellus hair in the hairline: a videodermatoscopic feature of frontal fibrosing alopecia. Br J Dermatol. 2013;169(2):473–474.
- Torres F, Tosti A. Trichoscopy: an update. G Ital Dermatol Venereol. 2014;149(1):83–91.
- Poblet E, Jiménez F, Pascual A, Piqué E. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45(4):375–380.
- Banka N, Mubki T, Bunagan MJ, et al. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long term follow up. Int J Dermatol. 2014;53(11):1324–1330.
- Dlova N, Goh CL, Tosti A. Familial frontal fibrosing alopecia. Br J Dermatol. 2013;168(1):220–222.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163(6):1296–1300.
- Ladizinski B, Bazakas A, Selim MA, Olsen EA. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68(5):749–755.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67(5):955–961.
- Tosti A, Piraccini BM, Iorizzo M, Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52(1): 55–60.
- Vanó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70(4):670–678.
- Georgala S, Katoulis AC, Befon A, et al. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61(1):157–158.
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60(4):660–668.
- Whiting DA, Olsen EA. Central centrifugal cicatricial alopecia. Dermatol Ther. 2008;21(4):268–278.
- LoPresti P, Papa CM, Kligman AM. Hot comb alopecia. Arch Dermatol. 1968;98(3):234–8 77.
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380(9):833–841.
- Callender VD, Wright DR, Davis EC, Sperling LC. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148(9):1047–1052.
- Miteva M, Tosti A. Central centrifugal cicatricial alopecia presenting with irregular patchy alopecia on the lateral and posterior scalp. Skin Appendage Disord. 2015;1(1):1–5.
- Olsen EA. Female pattern hair loss and its relationship to permanent/cicatricial alopecia: a new perspective. J Investig Dermatol Symp Proc. 2005;10(3):217–221.
- Miteva M, Tosti A. Dermatoscopic features of central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2014;71(3):443–449.
- Miteva M, Tosti A. Dermoscopy guided scalp biopsy in cicatricial alopecia. J Eur Acad Dermatol Venereol. 2013;27(10):1299–1303.
- Eginli A, Dothard E, Bagayoko CW, et al. A retrospective review of treatment results for patients with central centrifugal cicatrical alopecia. J Drugs Dermatol. 2017;16(4): 317–320.
- Quéreux G. Dermatite séborrhéique. EMC – Dermatologie-Cosmetologie. 2205;2(30: 147–159.
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31(4):343–351.
- Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18(1):13–26; quiz 19–20.
- Kibar <, Aktan S, Bilgin M. Dermoscopic findings in scalp psoriasis and seborrheic dermatitis; two new signs; signet ring vessel and hidden hair. Indian J Dermatol. 2015;60(1):41–45.
- Schwartz RA, Janusz CA, Janniger CK. Seborrheic dermatitis: an overview. Am Fam Physician. 2006;74(1):125–130.
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol 2015;3(2):10.13188/2373-1044.1000019.
- Kim GW, Jung HJ, Ko HC, et al. Dermatoscopy can be useful in differentiating scalp psoriasis from seborrheic dermatitis. Br J Dermatol. 2011;164(3):652–656 .
- Mokos ZB, Kralj M, Basta-Juzbaši? A, Juki? IL. Seborrheic dermatitis: an update. Acta Dermatovenerol Croat. 2012;20(2):98-104.

