 J Clin Aesthet Dermatol. 2021;14(2):34–40.
J Clin Aesthet Dermatol. 2021;14(2):34–40.
by Soheir Ghonemy, MD; Mohamed Mohamed Mahmoud Nasr, MD; Mohammad Soliman, MD; and Heba Allah Hosiney
All authors are with the Dermatology department, Faculty of Medicine, Zagazig University in Zagazig, Egypt.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background: Skin aging can be a contributing factor in the prediction, follow-up, and early diagnosis of some disorders related to the aging process, including degenerative cardiovascular diseases.
Objective: We sought to explore the association between a clinical skin aging score and risk of degenerative heart diseases.
Methods: This study included two groups; a case group consisting of 44 patients older than 30 years who were admitted to the cardiology department with degenerative heart disease and 44 age- and sex-matched healthy individuals to act as a control group. The skin aging score was calculated for all subjects.
Results: Regarding intrinsic skin aging parameters, there were highly significant differences between the two groups in uneven pigmentation, reduced fat tissue, benign skin tumors, fine wrinkles, and lax skin appearance. Concerning extrinsic skin aging parameters, there were significant differences between the two groups regarding pigment changes, changes in skin phenotype, yellowness, pseudoscars, cutis rhomboidalis nuchae, telangectasia, coarse wrinkles, and dryness.
Conclusion: Our study suggests that any individual with intrinsic skin aging score greater than eight points or total score of more than 15 points is at high risk for degenerative cardiovascular disease and should undergo periodic follow-up.
KEYWORDS: Skin aging, degenerative cardiovascular disease, SCINEXA score
Degenerative cardiovascular diseases are increasingly common problems that mostly remain asymptomatic until an advanced stage. Such late presentation limits treatment options and increases both morbidity and mortality rates.1
Some cardiovascular disorders are often associated with a variety of dermatologic manifestations. Frequently, these cutaneous signs can be used in the diagnosis of underlying cardiac disease. Therefore, using the skin as a mirror to the internal state of the body could offer a noninvasive means for the early prediction of different pathological problems.2
The present study aimed to discern the association between clinical skin aging score and degenerative cardiovascular diseases to be used in the future as a noninvasive screening method for populations at high risk of developing such a problem.
Methods
This comparative cross-sectional study included two groups; Group A consisted of 44 patients older than 30 years of age who were admitted to the cardiology department with degenerative heart disease based on echocardiography and coronary angiography, while Group B included 44 age- and sex-matched healthy individuals acting as a control group. In each group, the subjects were further stratified according to age into three subgroups: 1) those aged 30 to 49 years old; 2) those aged 50 to 69 years old; and 3) those aged 70 years or older. In total, there were six subgroups, including three subgroups of patients (A1, A2, and A3) and three subgroups of control participants (B1, B2, and B3).
Inclusion and exclusion criteria. Eligible patients with degenerative heart disease included those with mitral annular calcification, which is diagnosed by two-dimensional (2D) echocardiography;3 those with aortic sclerosis, which is diagnosed by 2D echocardiography;4 those with left ventricular diastolic dysfunction (LVDD), which is diagnosed by echocardiography;5 and those with coronary atherosclerosis diagnosed by coronary angiography.6 Patients younger than 30 years old, those with rheumatic heart diseases, those with congenital heart diseases, and those with acute coronary syndromes were excluded. Written informed consent was obtained from every patient after providing an explanation of the procedure and medical research. Our institution’s ethics committee approved the study.
Full history-taking was performed and a full clinical examination was completed, which included a general examination, cardiac examination, and calculation of the skin aging score.7 For the latter, each item was awarded a score of 0 to 3 points and the sum of these scores was calculated, with a maximum score of 15 points possible for the intrinsic items and that of 54 points possible for the extrinsic items; these two scores were then added to determine the total, final score.
Investigations. A standard transthoracic echocardiogram was performed using the Vivid E9 ultrasound system (GE Healthcare, Chicago, Illinois). Images were obtained using a 2.5-MHz transducer. M-mode, two-dimensional, and pulsed Doppler echocardiographic assessments were performed for all patients.8 In addition, coronary angiography was performed for patients with chronic stable angina according to the 2014 European Society of Cardiology’s guidelines for myocardial revascularization.8
Statistical analysis. Statistical analysis was performed using the Statistical Package for the Social Sciences version 16.0 for Windows (IBM Corporation, Armonk, New York).
Results
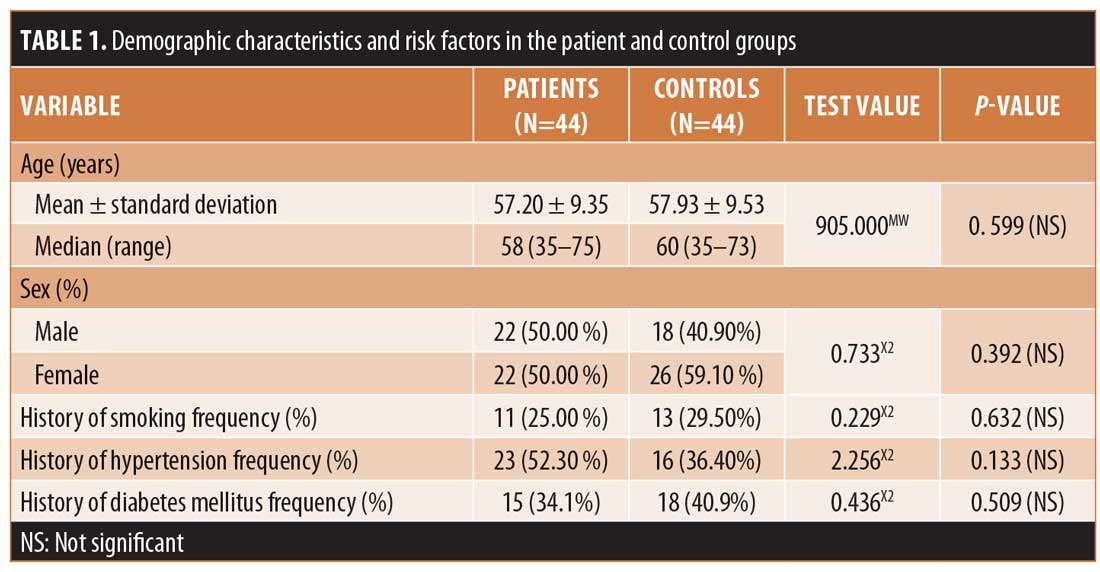
In this study, the 44 patients in Group A included 22 (50.00%) men and 22 (50.00%) women with an age range of 35 to 75 years and a mean age of 57.20±9.35 years. Meanwhile, the 44 subjects in control group included 18 (40.90%) men and 26 (59.10%) women with an age range of 35 to 73 years and a mean age of 57.93±9.53 years.
Regarding risk factors, in Group A, 23 (52.30%) participants were hypertensive, 15 (34.1%) participants were diabetic, and 11 (25.00%) participants were current smokers, while, in the control group, 16 (36.40%) participants were hypertensive, 18 (40.9%) participants were diabetic, and 13 (29.50%) participants were current smokers (Table 1).
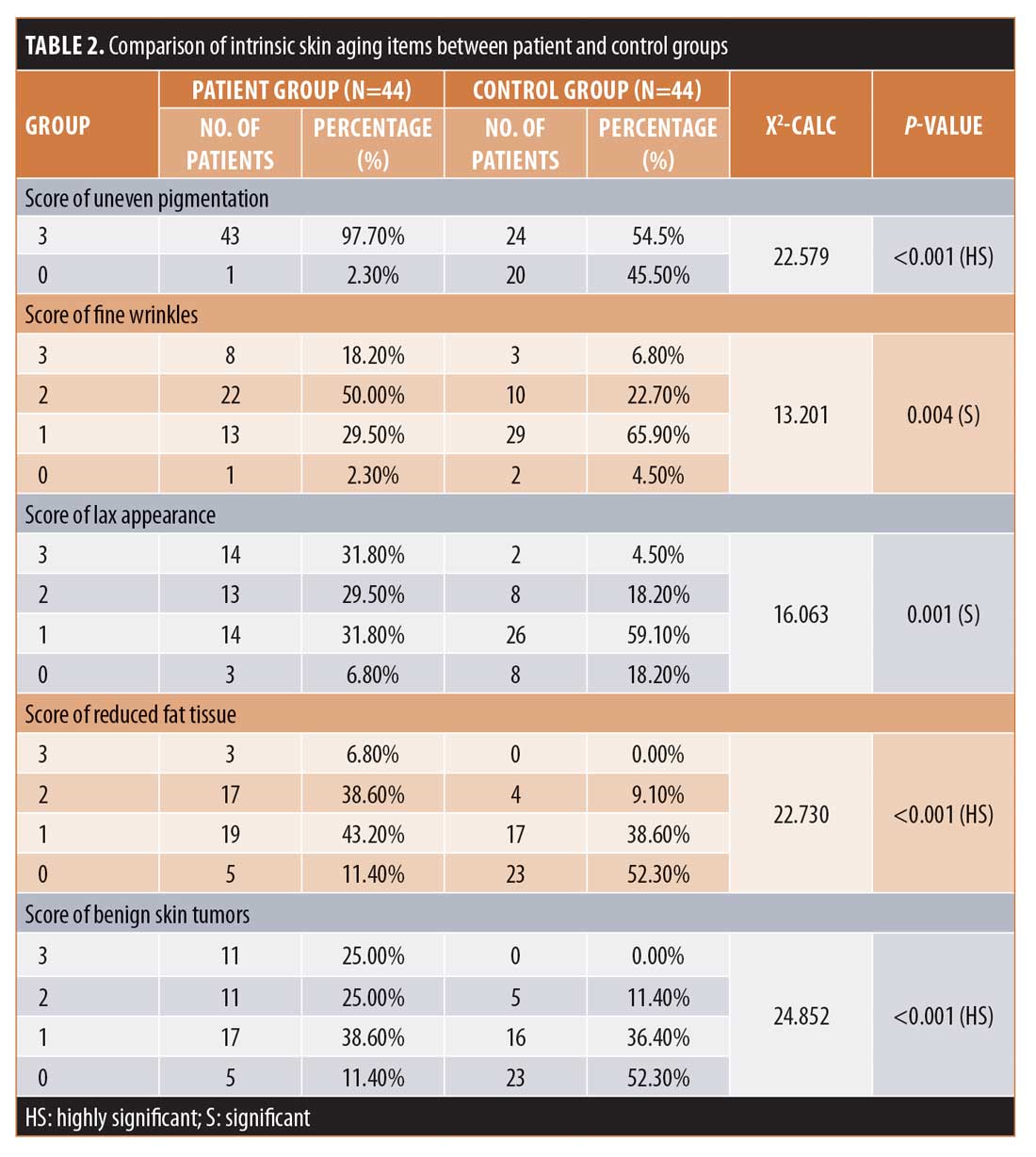
When assessing intrinsic skin aging parameters, there were highly statistically significant differences (p<0.001) noted between the two groups concerning uneven pigmentation, reduced fat tissue, and benign skin tumors and significant differences observed between the two groups regarding fine wrinkles and lax appearance.
Among the extrinsic skin aging parameters, there were highly statistically significant differences (p<0.001) between the two groups concerning coarse wrinkles and dryness and statistically significant differences (p<0.05) between the two groups regarding pigment change, change in skin phenotype, yellowness, pseudo-scars, cutis rhomboidalis nuchae, and telangectasia. Meanwhile, there were no statistically significant differences between the two groups concerning sun burn freckles, solar lentigines, elastosis, Favre-Racouchot, comedones, permanent erythema, actinic precancerosis, basal cell carcinoma, squamous cell carcinoma, or melanoma.
The mean intrinsic scores in Groups A and B were 9.66±2.42 points and 5.20±2.27 points (p<0.001) and the mean extrinsic scores in Groups A and B were 9.59 ± 4.32 points and 5.57±2.26 points (P<0.001). The mean total score in Group A was 19.23±6.10 points, while that in Group B was 10.77±3.65 points (p<0.001) (Table 2).
In age subgroup 1 (30–49 years), there were no statistically significant differences between the two groups in terms of intrinsic, extrinsic, and total scores. In age subgroup 2 (50–69 years), there were highly statistically significant differences between the two groups for intrinsic, extrinsic, and total scores. Finally, in age subgroup 3 (?70 years), there was no statistically significant difference in intrinsic scores between the two groups, but there were significant difference between the two groups for extrinsic and total scores (Table 3). 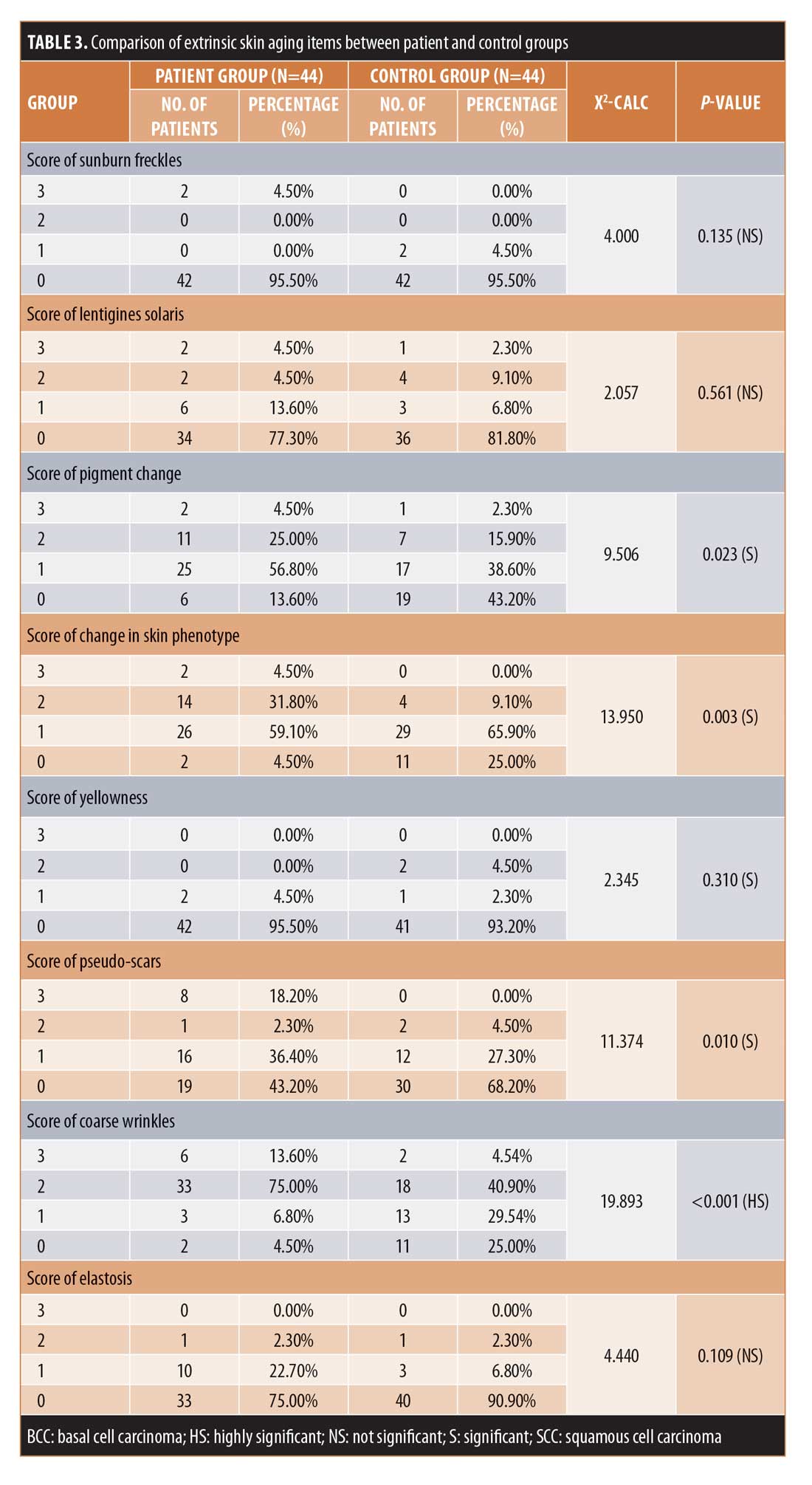
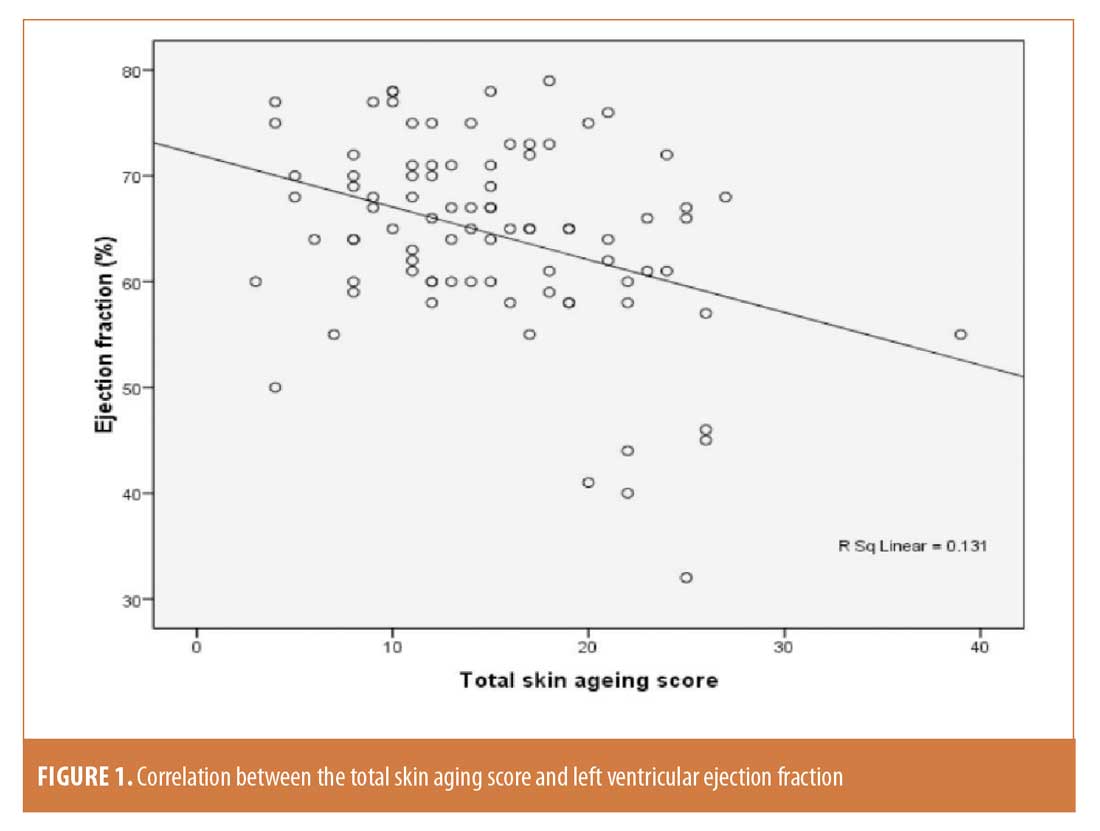
 There was a significant positive correlation between skin aging scores and coronary atherosclerosis. Also, there was a significant positive correlation between intrinsic skin aging score and left atrial diameter. On the other hand, there was a significant negative correlation between skin aging scores and systolic function by ejection fraction (Figure 1).
There was a significant positive correlation between skin aging scores and coronary atherosclerosis. Also, there was a significant positive correlation between intrinsic skin aging score and left atrial diameter. On the other hand, there was a significant negative correlation between skin aging scores and systolic function by ejection fraction (Figure 1).
There was also a highly statistically significant (p<0.001) positive correlation between skin aging scores and age but a significant (p<0.05) negative correlation between extrinsic skin aging scores and diabetes mellitus and a nonsignificant correlation of the other risk factors and sex with skin aging scores.
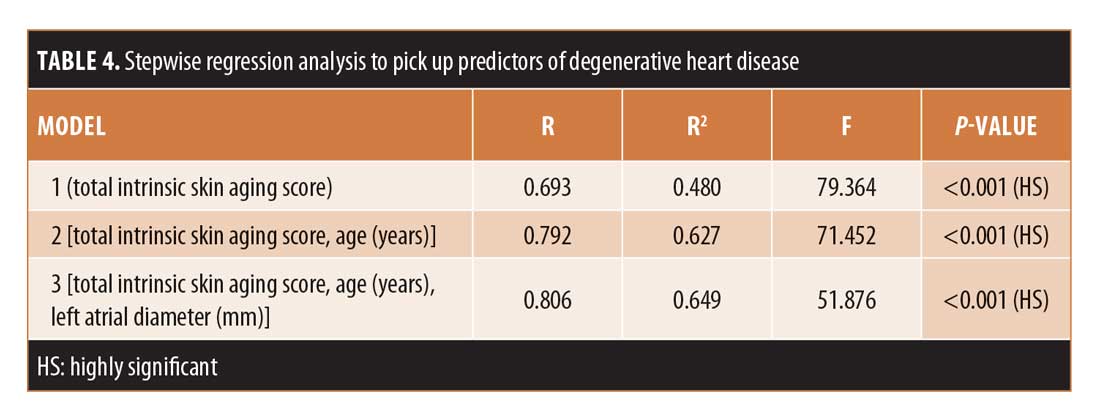
Stepwise regression analysis. Stepwise regression analysis was performed with total intrinsic skin aging score acting as the independent predictor of degenerative heart disease with a regression coefficient of 0.693 and p<0.001. Age and left atrial diameter were the copredictors with a total regression coefficient of 0.806 and p<0.001 (Table 4).
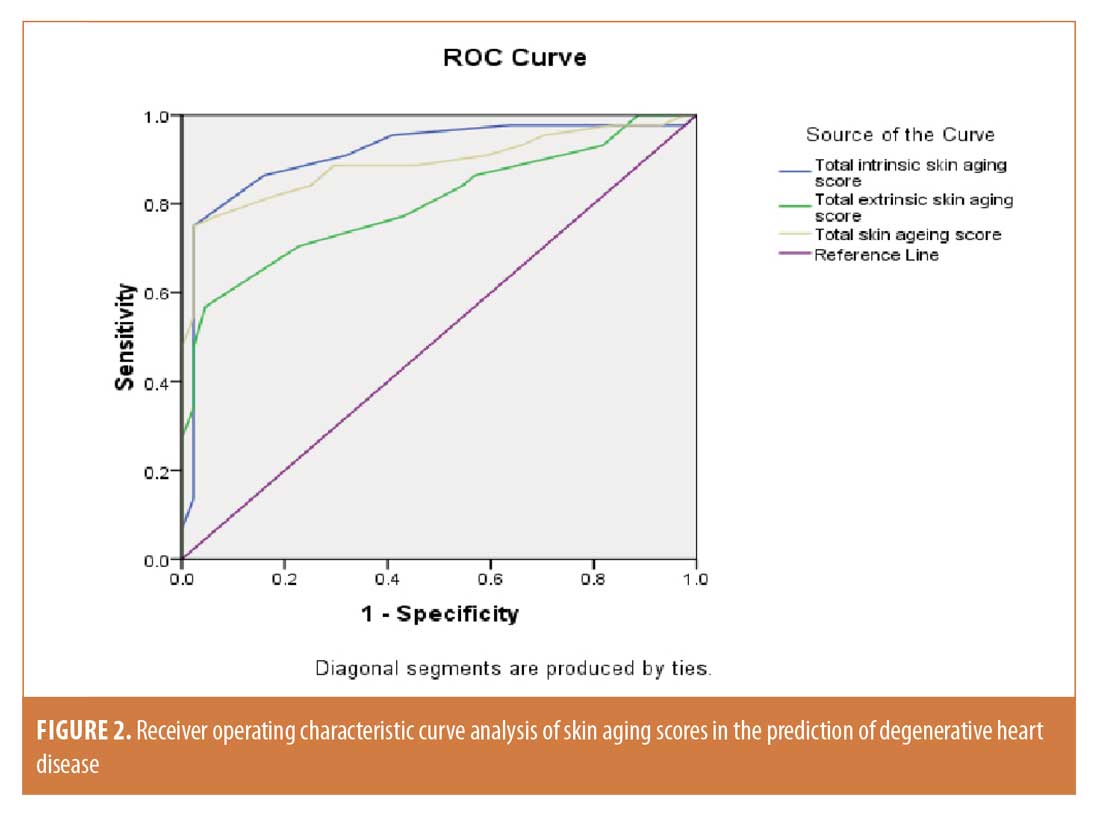
Receiver operating characteristic curve analysis. Receiver operating characteristic curve analysis was completed for skin aging scores. Concerning total intrinsic skin aging, the area under the curve was 0.913 (95% confidence interval: 0.847–0.980) and the best cutoff value was 8 with a sensitivity of 86.40% and specificity of 84.10%. For total extrinsic skin aging, the area under the curve was 0.801 (95% confidence interval: 0.707–0.894) and the best cutoff value was 8 with a sensitivity of 70.50% and specificity of 77.30%. Finally, regarding the total skin aging score, the area under the curve was 0.890 (95% confidence interval: 0.816–0.963) and the best cutoff value was 15 with a sensitivity of 81.80% and specificity of 81.80% (Figure 2).
 Discussion
Discussion
Degenerative cardiovascular diseases are increasingly common problems that mostly remain asymptomatic until reaching an advanced stage. Then, the patient presents with an irreversible problem or complication and treatment options are limited.9
Using skin as a mirror to the internal state of the body can act as a noninvasive tool for the early prediction of different pathological problems. Some cardiovascular disorders are often associated with a variety of dermatologic manifestations. Frequently, these cutaneous signs can be used in facilitating a diagnosis of the underlying cardiac disease.10
To date, few studies have described an association between some skin aging signs, such as wrinkles, graying of the hair, and facial pigmentation, and cardiovascular diseases or recommended using them as markers for some cardiovascular diseases.11,12
This study was conducted to discern the association between a comprehensive clinical score of intrinsic and extrinsic skin aging and certain degenerative cardiovascular diseases to use the described scoring system as a possible predictor of the risk of degenerative cardiovascular disease.
In our study, the prevalence of uneven pigmentation was higher in the patient group than the control group. This was in concordance with the results of Miyawaki et al,12 who studied facial pigmentation as a biomarker for carotid atherosclerosis. Facial pigmentation was found to be significantly correlated with carotid intima-media thickness (R=0.13; p=0.03).
Also, the association between facial pigmentation, which represents the perceived age more than the chronological age, and the development of degenerative cardiovascular diseases in the elderly was expressed by Kido et al,13 who concluded that perceived age could be a better predictor of mortality than chronological age and that carotid atherosclerosis is correlated well with perceived age.
Grades of facial lax appearance, reduced fat tissue, and benign skin tumors were significantly higher in the patient group than in the control group in our study. This was concordant with the findings of Blagosklonny,14 who proposed that atherosclerosis, hypertension, and seborrheic keratosis share the same underlying pathophysiology, which is aging. Regarding the total scores of intrinsic skin aging in our study, they were significantly higher in the patient group than in the control group. This was in agreement with the work of Roshdy et al,15 who reported that intrinsic skin aging scores of patients with degenerative advanced-degree atrioventricular block were higher than those of healthy age-matched subjects.
The association between intrinsic skin aging and degenerative cardiovascular diseases can be clarified by looking to the pathogenesis background that may be incriminated in the development of both disorders.
Advanced glycation end-products (AGEs) are developed by the glycation and oxidation of different structural proteins and play an important role in age-dependent changes. The only products that accumulate with age are N-(carboxymethyl) lysine (CML), N-(carboxymethyl) hydroxyl-lysine (CMhl), and pentosidine. Protein glycation was described to occur not only in the skin but also in organs such as the kidneys, blood vessels, and ocular lens. This may explain the link between skin aging and heart aging, suggesting that AGEs are an important factor in cardiac aging and fibrosis, whereas the receptor for AGE and transforming growth factor-?/Smad signaling pathway might be involved in the AGE-induced cardiac aging process.16–18
The link between connective tissue growth factor (CTGF) and heart diseases and aging as an emerging marker for tissue fibrosis can be explained by the fact that CTGF expression and protein are elevated in nearly all fibrotic tissues examined. Increased CTGF concentrations in blood and other biological fluids have also been reported for many fibrotic diseases.18–20 A positive correlation between the levels of CTGF and cardiovascular fibrotic diseases in the elderly population has been reported.20,21
Ling et al22 and Cieslik et al23 each reported that tissue fibrosis is a typical aging change that affects both the skin and heart. They have demonstrated that tissue fibrosis is a result of tissue repair using collagen fibers. The misrepair-accumulation theory has an aspect consistent with this scientific finding. A repair performed with collagen fibers is a manifestation of “misrepair” and the accumulation of such misrepairs will result in the progressive deposition of collagen fibers and, then, fibrosis. The accumulation of the repairing collagen fibers disorganizes gradually the structure of a tissue and reduces the functionality of an organ. The deposition of collagen fibers is not only a basis for fibrosis-associated diseases but also a basis for some typical aging changes such as wrinkle formation, senile hair-loss, and senile hair-whitening.
With the correlation between risk factors and the total intrinsic score, we found that there was a nonsignificant correlation between diabetes mellitus, hypertension, and smoking and the total score in the patient group, although Bernhard et al24 and Morita25 each concluded that smoking is a risk factor of premature skin aging.
On the other hand, extrinsic skin aging is mainly a photo-aging process driven by sun exposure; the reason for including it in our study was that Vierkotter and Krutmann,26 concluded that smoking and environmental pollution equally influence extrinsic skin aging, so smoking is a common risk factor for both degenerative cardiovascular diseases and skin aging.
Regarding extrinsic skin aging parameters, there were significant differences between the two groups in terms of pigment change, changes in the skin phenotype, yellowness, pseudo-scars, cutis rhomboidalis nuchae, and telangectasia as well as highly significant differences between the two groups regarding dryness and coarse wrinkles, consistent with the findings described by Ernster et al27 and Urbanska et al,28 who reported that the risk of facial wrinkling is higher in smokers than nonsmokers, regardless of the history of sun exposure. This was further confirmed by Helfrich et al,29 who studied the effects of smoking on photo-protected skin, and by Stallones,30 who described smoking as an important risk factor for degenerative cardiovascular disease. The link between the two effects is most probably due to free radical damage as well as other pathogeneses. Also, Aizen and Gilhar31 concluded in their study that smoking causes premature wrinkling and is one of the more potent risk factors for atherosclerosis.
On the other hand, there were no significant differences between patient and control group participants with regard to sunburn freckles, solar lentigines, elastosis, Favre-Racouchot, comedones, and permanent erythema, most likely because these changes are almost all related to the effects of ultraviolet radiation, which has no role in the pathogenesis of degenerative cardiovascular disease.32
Conclusion
Our study suggests that any individual with an intrinsic skin aging score greater than eight points or a total score greater than 15 points, especially in conjunction with a history of smoking, is at high risk for degenerative cardiovascular disease and should undergo periodic follow-up for diagnosis in early asymptomatic stages for proper management.In the stepwise regression analysis, the total intrinsic skin aging score was found to be the best predictor of degenerative heart disease, followed by age and left atrial diameter as copredictors.
References
- Yang H, Wang Y, Nolan M, et al. Community screening for nonischemic cardiomyopathy in asymptomatic subjects ? 65 years with stage B heart failure. Am J Cardiol. 2016;117(12):1959–1965.
- Nikolakis G, Makrantonaki E, Zouboulis CC. Skin mirrors human aging. Horm Mol Biol Clin Investig. 2013;16(1):13–28.
- Abramowitz Y, Jilaihawi H, Chakravarty T, et al. Mitral annulus calcification. J Am Coll Cardiol. 2015;66(17):1934–1941.
- Nightingale AK, Horowitz JD . Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart. 2005;91(11):1389–1393.
- Galderisi M. Diastolic dysfunction and diastolic heart failure: diagnostic, prognostic and therapeutic aspects. Cardiovasc Ultrasound. 2005;3:9.
- Deligonul U. Coronary angiography as a prognostic tool. Anadolu Kardiyol Derg. 2001;1(3):189–196.
- Vierkotter A, Ranft, U, Kramer, U, et al. The SCINEXA: a novel, validated score to simultaneously assess and differentiate between intrinsic and extrinsic skin ageing. J Dermatol Sci. 2009;53(3):207–211.
- Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr. 2003;16(10):1091–1110.
- Schnohr P, Lange P, Nyboe J, Appleyard M, Jensen G. Gray hair, baldness, and wrinkles in relation to myocardial infarction: the Copenhagen City Heart Study. Am Heart J.1995; 130(5): 1003–1010.
- Kolh P, Windecker S, Alfonso F, et al. ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46(4):517–592.
- Ionescu SD, Sandru V, Artenie R, et al. [Implications of some risk factors in degenerative valvular heart diseases]. Rev Med Chir Soc Med Nat Iasi. 2004;108(2): 311–313. [Article in Romanian].
- Miyawaki S, Kohara K, Kido T, et al. Facial pigmentation as a biomarker of carotid atherosclerosis in middle-aged to elderly healthy Japanese subjects. Skin Res Technol. 2016s;22(1):20–24.
- Kido M, Kohara K, Miyawaki S, et al. Perceived age of facial features is a significant diagnosis criterion for age-related carotid atherosclerosis in Japanese subjects: J-SHIPP study. Geriatr Gerontol Int. 2012;12(4): 733–740.
- Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. 2012; Am J Pathol. 181(4): 1142–1146.
- Roshdy HS, Soliman MH, El-Dosouky II, Ghonemy S. Skin aging parameters: a window to heart block. Clin Cardiol. 2017;41(1):51–56.
- Prasad C, Imrhan V, Marotta F, Juma S, Vijayagopal P. Lifestyle and advanced glycation end products (AGEs) burden: its relevance to healthy aging. Aging Dis. 2014;5(3):212–217.
- Luevano-Contreras C, Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2(12):1247–1265.
- Gkogkolou P, Bohm M. Advanced glycation end products: key players in skin aging?. Dermatoendocrinol. 2012;4(3):259–270.
- Behnes M, Brueckmann M, Lang S, et al. Connective tissue growth factor (CTGF/CCN2): diagnostic and prognostic value in acute heart failure. Clin Res Cardiol. 2014;103(2):107–162.
- Szabó Z, Magga J, Alakoski T, et al. Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure. Hypertension. 2014;63(6):1235–1240.
- Wu CK, Wang YC, Lee JK, et al. Connective tissue growth factor and cardiac diastolic dysfunction: human data from the Taiwan diastolic heart failure registry and molecular basis by cellular and animal models. Eur J Heart Fail. 2014;16(2):163–172.
- Posch MG, Schmidt G, Steinhoff L, et al. A promoter polymorphism -945C>G in the connective tissue growth factor in heart failure patients with mechanical circulatory support: a new marker for bridge to recovery?. Eur J Cardiothorac Surg. 2015;47(1):e29–e33.
- Ling LH, Kistler PM, Ellims AH, et al. Diffuse ventricular fibrosis in atrial fibrillation: noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol. 2012;60(23):2402–2408.
- Cieslik KA, Trial J, Crawford JR, et al. Adverse fibrosis in the aging heart depends on signaling between myeloid and mesenchymal cells; role of inflammatory fibroblasts. J Mol Cell Cardiol. 2014;70:56–63.
- Bernhard D, Moser C, Backovic A, Wick G. Cigarette smoke—an aging accelerator? Exp Gerontol. 2007;42(3):160–165.
- Morita A. Tobacco smoke causes premature skin aging. J Dermatol Sci. 2007;48(3):169–175.
- Vierkotter, A, Krutmann J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinol. 2012;4(3):227–231.
- Ernster VL, Grady D, Miike R, et al. Facial wrinkling in men and women, by smoking status. Am J Public Health. 1995;85(1):78–82.
- Urbanska M, Nowak G, Florek E. [Cigarette smoking and its influence on skin aging]. Przegl Lek. 2012;69(10):1111–1114. [Article in Polish].
- Helfrich YR, Maier LE, Cui Y, et al. Clinical, histologic, and molecular analysis of differences between erythematotelangiectatic rosacea and telangiectatic photoaging. JAMA Dermatol. 2015;151(8):825–836.
- Stallones RA. The association between tobacco smoking and coronary heart disease. Int J Epidemiol. 2015;44(3):735–743.
- Aizen E, Gilhar A. Smoking effect on skin wrinkling in the aged population. Int J Dermatol. 2001;40(7):431–433.
- Bartak P, Ettler K. Sun photoaging. Cas Lek Cesk. 2003;142(1):46–50.

