 J Clin Aesthet Dermatol. 2022;15(1):21–26.
J Clin Aesthet Dermatol. 2022;15(1):21–26.
by Thomas J. Stephens, PhD; Sheryl Berkowitz, MS; Tess Marshall, ND; Sophia Kogan, MD; and Isabelle Raymond, PhD
Dr. Stephens is with SGS Stephens, Inc., in Richardson, Texas. Ms. Berkowitz and Drs. Marshall, Kogan, and Raymond are with Nutraceutical Wellness LLC in New York, New York.
FUNDING: This study was sponsored by Nutraceutical Wellness, LLC, in New York, New York.
DISCLOSURES: Ms. Berkowitz and Drs. Marshall, Kogan, and Raymond are employees of Nutraceutical Wellness, LLC.
ABSTRACT: Objective. The goal of this study was to assess the perceived efficacy of a standardized nutraceutical to improve hair growth and quality in men and women of various ethnicities with self-perceived hair thinning.
Methods. This prospective, single-blind study enrolled healthy men aged 20 to 55 years (n=47) and premenopausal women aged 20 to 45 years (n=51) with self-perceived, mild-to-moderate hair thinning and included African American, Asian, Hispanic Caucasian and Non-Hispanic Caucasian participants. The nutraceutical supplement (Nutrafol® Men or Women Capsules, Nutraceutical Wellness Inc., New York, New York) was taken daily for six months. Subjects were evaluated in the clinic at baseline and Weeks 12 and 24 with two self-assessments at Weeks 4 and 8. Study endpoints were standardized digital imaging and investigator rated assessments. Self-assessment questionnaires rated hair growth, hair satisfaction, and lifestyle factors.
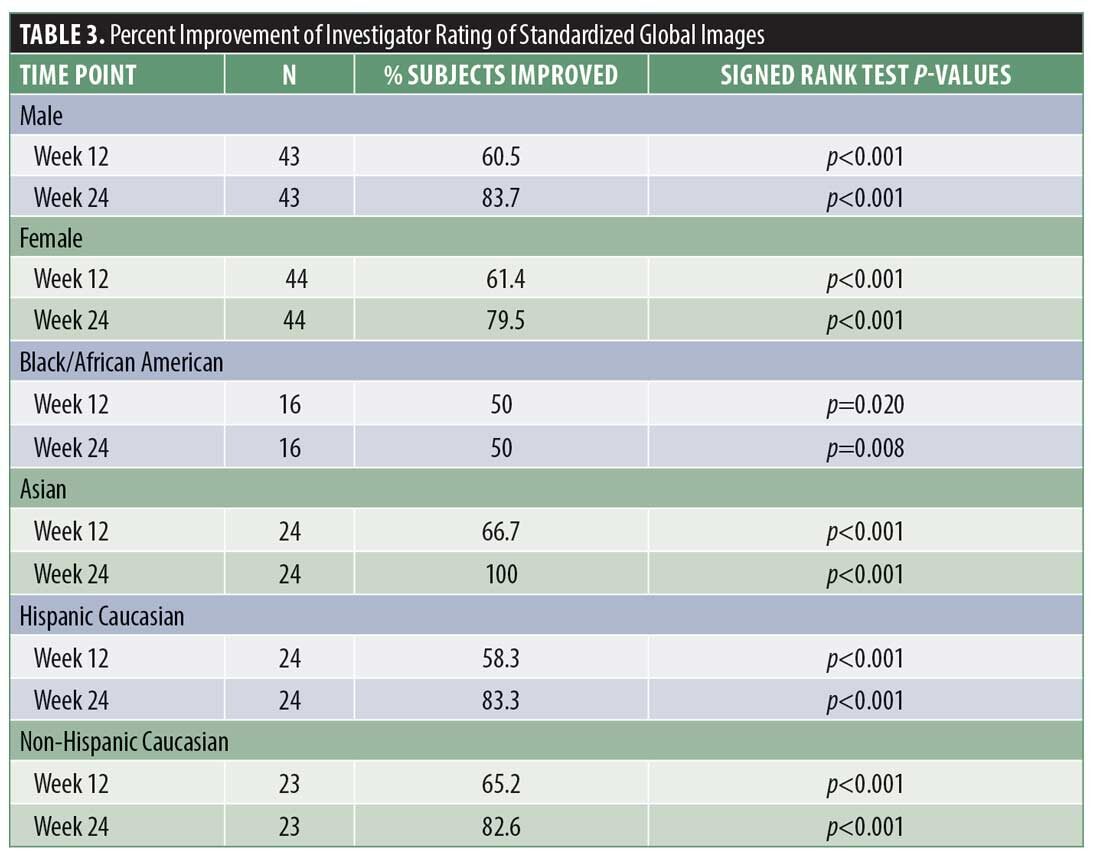
Results. Investigator ratings for baseline hair growth, coverage, density, and volume were significant at Weeks 12 and 24 for all subjects (for each, p<0.001). These significant improvements were seen in 83.7 percent of men and 79.5 percent of women at Week 24. Results were similar across ethnic subgroups with significant benefit at Weeks 12 and 24 (for each, p<0.05). All subjects reported significant improvements in baseline hair appearance/quality, volume/fullness, scalp coverage, thickness, and shedding at Weeks 4, 8, 12 and 24 (for each, p<0.01).
Conclusion. A standardized nutraceutical supplement improved visible hair growth with less notable shedding based on subjects’ and investigators’ overall perception of treatment benefit for men and women of various ethnic backgrounds.
Keywords: Nutraceutical supplement, hair thinning, men, women, race, ethnicity
Hair loss is a common condition affecting both men and women, and it has recently become recognized as a multi-factorial condition resulting from multiple causes.1–5 Hair loss is chronic and progressive, affecting at least 40 percent of men (progressing up to 70% in later life) and 50 percent of women by age 50, increasing in incidence after menopause.2, 6–9 Although the diagnosis of androgenetic alopecia (AGA) is most common in both sexes, the medical community now refers to it as male pattern hair loss (MPHL) and female pattern hair loss (FPHL) to underscore the significant differences in clinical presentation and pathophysiology between men and women.2,10–13 It is known that androgen-driven mechanisms and genetics play a more significant role in hair loss among men. Although dihydrotestosterone (DHT) and 5alpha-reductase (which converts testosterone to DHT) are known to be elevated in the scalps of both men and women with MPHL/FPHL, men have 3 to 4.5 times more total 5alpha-reductase and significantly less aromatase in hair follicles.14 The number and sensitivity of androgen receptors are enhanced in balding scalps in specific special patterns in men versus women, and similar to 5alpha-reductase are 40 percent more numerous in affected males.14 The effect of androgens is well studied and is presumed to be secondary to downstream autocrine and paracrine factors that are secreted after binding to the androgen receptor;15 however, recent science increasingly supports the contribution of other intrinsic and extrinsic factors, such as environmental aggressors, oxidative stress, aging, mediators of psycho-emotional stress, diet, genetic predispositions, and downstream inflammation.2,4,5,13,16–21
Sex differences in hair loss further expand among various racial and ethnic groups. For example, there are differences in straight and curly hair, including follicle shape and density and growth rates.22–25 There are also epidemiological differences in the types of hair loss more commonly seen in different ethnic groups and races. In the United States, for example, the prevalence of alopecia areata is higher among African Americans and lower among Asians compared to Whites26 and higher among Hispanic compared to non-Hispanic White women.27 MPHL and FPHL are common among Asian men and women,23 with a reported incidence of up to 73 percent among the general population;28 however, androgenetic alopecia occurs in Japanese men approximately 10 years slower than European men.23
Cultural differences may also be involved in hair loss. For example, women in China experience frontal pattern hair loss more often than Western women, possibly because they often style their hair in a ponytail.29 People with African hair types are prone to hair loss secondary to certain cultural styling practices, such as tight braiding that can cause traction alopecia and scarring.30,31 Harsh chemicals that are used to relax and straighten hair also contribute to hair loss, particularly among African American women.24 This population is also at increased risk of centrifugal cicatricial alopecia (CCCA), marked by inflammation and progressive destruction of follicles through scarring.25,32
Although not life-threatening, treating hair loss is important, as it can have detrimental psychosocial effects resulting in symptoms of depression33 and diminished quality of life among both sexes.7,34–36 While women appear to be affected more due to societal expectations,37,38 psychological stress can be more significant in younger men as well.39 Regardless of etiology, hair loss can have a significant negative impact on self-esteem40 and represent a substantial financial burden on individuals seeking treatment.41
Therapeutic options are limited and have been developed to address singular targets, as exemplified by androgen-inhibiting therapeutics like the 5alpha-reductase inhibitor finasteride, one of only two United States Food and Drug Administration (FDA)-approved drugs for MPHL. It is not approved for use in women due to its teratogenic potential.42 The vasodilator minoxidil is FDA-approved for use in both men and women.43 Both of these available treatments may be associated with unpleasant side effects and/or difficulty of application.3,5,44–46 Other hormonal therapies, such as spironolactone and cyproterone acetate, are sometimes used off-label in women (generally post-child-bearing).47,48 Additional therapies include lasers49 and photobiomodulation,50 microneedling,51 platelet-rich plasma,52 and nutraceuticals.53,54 Among the limited available treatment options,55 nutraceuticals are emerging as a safe and effective means to support growth and address some forms of hair thinning secondary to their multi-targeted action.3
Novel hair growth supplements comprised of standardized nutraceuticals were developed to target the multiple underlying factors involved in hair thinning in men and women, including DHT, stress mediators, oxidative damage, inflammation, aging, and hormonal fluctuation to improve hair growth and quality. These nutraceutical formulations contain a patented Synergen Complex®, a blend of standardized clinically-tested phytoactive ingredients, including curcumin, Sensoril® ashwagandha, saw palmetto, tocotrienol/tocopherol complex and hydrolyzed marine collagen, as well as vitamins, minerals, and other botanical and non-botanical ingredients that make up the entire supplement. The difference between formulas is in the dosages of ingredients, adjusted for the needs of men versus women, including the men’s formula containing a higher clinically effective dosage of saw palmetto to address increased amounts of androgen hormones to prevent the conversion of testosterone to DHT. Two previously published, randomized, double-blind placebo-controlled trials evaluating the women’s nutraceutical formulation demonstrated a significant increase in terminal and vellus hairs and significant improvements in overall hair growth and quality in women with thinning hair of childbearing and post-childbearing years.4,54
The objective of this study was to evaluate the efficacy of a nutraceutical oral supplement when used over the course of 24 weeks in both men and women of diverse ethnicities with self-perceived hair thinning.
Methods
Study participants. Ninety-eight subjects with self-perceived mild-to-moderate hair thinning were enrolled in the study. Eligible subjects included healthy men aged 20 to 55 years (n=47) or healthy premenopausal women, aged 20 to 45 years (n=51). Stratified random subject sampling according to race/ethnicity sub-groups (African American, Asian, Hispanic Caucasian and Non-Hispanic Caucasian) was implemented to ensure overall group diversity. Female subjects with child-bearing potential provided a negative urine pregnancy test prior to participation. Subjects agreed to follow the study requirements and maintain consistent hair product use, grooming, and color treatment frequency for the duration of the study. Subjects were excluded if they were nursing or pregnant, had a sensitivity or allergy to fish or other ingredients, or used any medication or supplement known to prevent or promote hair growth within the prior three months. Detailed selection criteria are listed in Table 1.
Procedure. This single-blind, single-arm, prospective study consisted of three clinic visits at baseline, Week 12, and Week 24 and two self-assessment evaluations at Week 4 and Week 8. Assessments at each clinic visit included standardized digital imaging (Nikon D300 DSLR Camera with 60 mm lens; Nikon Corporation, Tokyo, Japan) of the full scalp, superior scalp, scalp vertex, and/or temple area. The investigator rated subject images for overall treatment benefit, defined as a composite of overall hair growth, coverage, density and volume using a seven-point Likert scale ranging from 1 (Entirely Disagree) to 7 (Entirely Agree) compared to baseline.
For male subjects, an area of interest (AOI) was selected on the scalp by the investigator and evaluated separately for improvement in hair growth, overall volume/thickness/fullness and appearance of coverage using a more detailed nine-point Likert scale. Self-assessment questionnaires using five- and seven-point scales (Strongly Disagree to Strongly Agree) were performed at each visit to rate hair growth and quality and improvements in skin and nails. Hair satisfaction and lifestyle factors, such as mood and confidence, were also evaluated.
Test material. The test material subjects received was a standardized nutraceutical supplement (Nutrafol® Women or Men Capsules, Nutraceutical Wellness Inc., New York, New York). Subjects were instructed to take four of their assigned capsules once daily with a meal. Subjects maintained daily diaries which were reviewed for treatment adherence throughout the course of the study. Subjects could be discontinued from the study if the investigator determined that their nonadherence could affect the outcome of the study.
Ethics. This study was initiated following the approval by an IRB (IntegReview IRB, Austin, Texas) and conducted in accordance with 21 Code of Federal Regulations (CFR) 50.25 and the accepted standards for Good Clinical Practices (SGS Stephens, Inc., Richardson, Texas). Each subject provided signed informed consent and a photo release form approved by the IRB before participating in any study-related activities.
Statistical analysis. Descriptive statistics, including mean, median, standard deviation (SD), frequency, and percentage values were conducted for the per-protocol population, defined as all subjects who received treatment and completed the study in accordance with the protocol. Investigator ratings of standardized photographs and the subject self-assessment questionnaire results were analyzed with a Wilcoxon signed-rank test. All statistical tests were two-sided with alpha=0.05 performed using commercial statistical software (SAS software version 9.4; SAS Statistical Institute, Cary, North Carolina).
Results
Demographics. The 87 subjects (88.8%) completing the study were male (n=43; 50.6%) and female (n=44; 49.4%) with a mean (SD) age of 39.3 (8.3) years (range, 23 to 55 years). Additional demographic and clinical characteristics are summarized in Table 2.
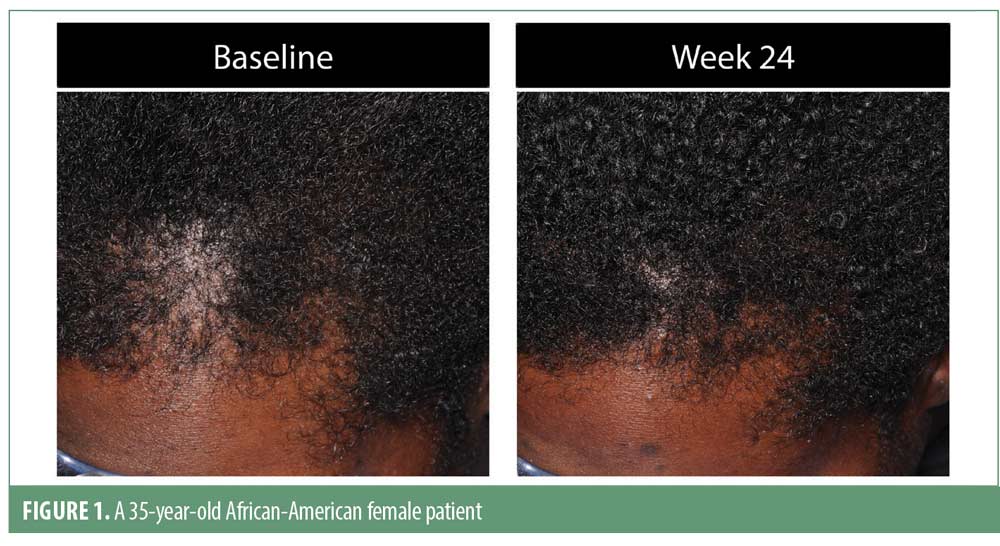
Investigator rating assessments. The investigator ratings for baseline overall growth, coverage, density, and volume were significant at Weeks 12 and 24 for all subjects (for each, p<0.001). Investigator ratings of significant improvement was seen in 83.7 percent of men and 79.5 percent of women for the composite endpoint at Week 24. Results were similar when analyzed by ethnic subgroup with significant overall treatment benefit compared to baseline at Weeks 12 and 24 (for each, p<0.05). Overall treatment results are summarized in Table 3. Improvement in hair growth for four representative subjects are shown in Figures 1 to 4.
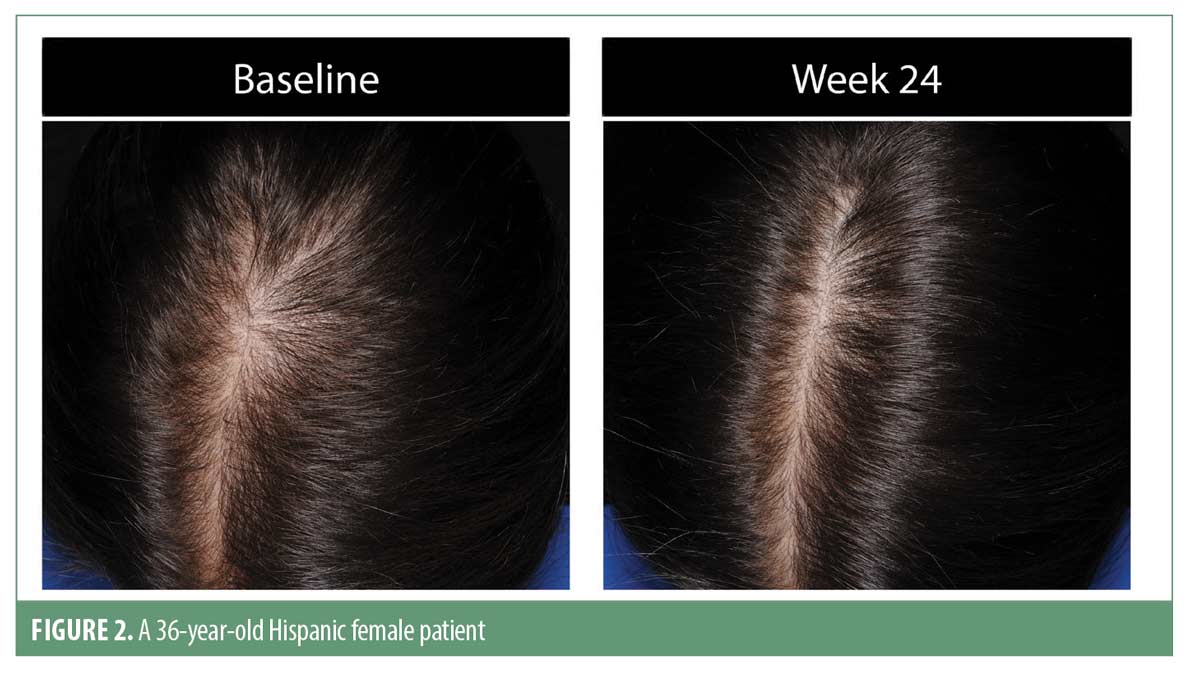
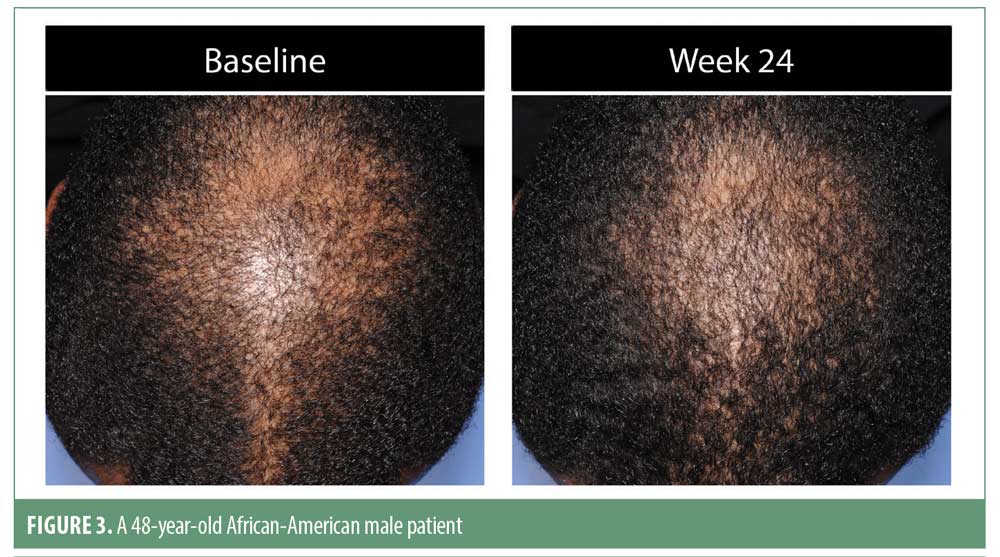
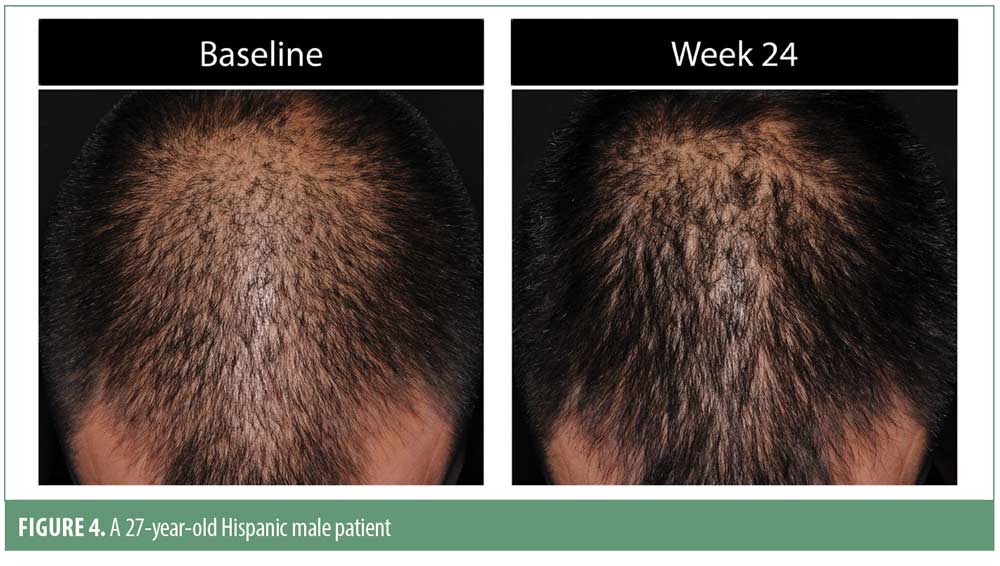
Analysis of the male areas of interest (AOI) showed significant improvements for all measures, including hair growth, overall volume/thickness/fullness, and appearance of coverage at both timepoints versus baseline (for each, p<0.001). An AOI ethnicity subgroup analysis demonstrated significant improvements for hair growth at Week 24 among African Americans, for hair growth and appearance of coverage at Week 24 among Asian men, and for hair growth at Weeks 12 and 24 and overall volume/thickness/fullness at Week 24 among non-Hispanic men (for each, p<0.05). Male Hispanic subjects demonstrated progressive improvement in overall hair volume/thickness/fullness but did not achieve statistical significance.
Self-assessment questionnaires. Results from the subject questionnaires were consistent with the investigator assessments. All subjects reported significant improvements in baseline appearance/quality, volume/fullness, coverage of the scalp, thickness, and lack of shedding at Weeks 4, 8, 12 and 24 (for each, p<0.01). Notably, 69.8 percent of male subjects and 79.5 percent of female subjects reported improvement in overall appearance. Results for the self-assessments were similar when analyzed by ethnic subgroups. Most satisfaction parameters significantly improved as early as Week 4 and continued to improve through Week 24.
Safety. There were no unanticipated adverse events (AEs) and no serious treatment-emergent AEs. One possibly related report of mild diarrhea occurred in one subject and resolved without intervention.
Discussion
Although hair loss is a common condition affecting both men and women, significant differences in hair loss exist between sexes and also between various ethnic groups. The results of this six-month study showed that the daily administration of this men’s and women’s nutraceutical supplement for hair thinning was effective across sexes and ethnicities.
The investigator noted improvement in hair parameters in more than 50 percent of subjects after 12 weeks and in 80 to 100 percent among Asian, Hispanic, Caucasian, and non-Hispanic Caucasian subjects. Indeed, investigator ratings of significant improvement was seen in 83.7 percent of men and 79.5 percent in women for the composite endpoint at Week 24 compared to baseline and these results were consistent for male patients of all ethnic subgroups. Analysis of the male AOI showed significant improvements for all subgroups as measures although the non-Hispanic men subgroup had more measures reaching statistical significance.
These differences may exist secondary to significant diversity in hair growth measures globally across races.22,23 African hair has lower density and a slower growth rate, Asian hair showed a thicker hair diameter with faster growth while Caucasian hair showed higher total hair density. One study found mean hair density ranged from 169 to 178/cm2 among Americans of Hispanic descent, 148 to 160/cm2 among African American and 214 to 230/cm2 among Caucasian individuals.56 While the full etiology of many types of hair loss remains elusive, and while the influence of causal factors of hair loss may be different across sexes and ethnicities, research has shown that in all cases it is multi-factorial.2,25 Nutraceuticals providing multi-modal biological activity against molecular, hormonal, inflammatory, stress, and environmental factors offer a unique therapeutic value. Further, nutraceuticals that are sex and age specific provide a tailored solution to these unique multi-factorial needs.
As hair loss can have detrimental psychosocial effects and a negative impact on quality of life, an individual’s perception of hair improvement is an important factor to consider when evaluating the overall benefits of an intervention. Over two-thirds of subjects reported improvement in overall appearance of their hair, with most satisfaction parameters significantly improving in as early as four weeks. Both investigator and subjective assessments showed that the standardized nutraceutical supplements administered during this study improved the growth and quality of hair in both men and women and across racial and ethnic groups. These results are similar and corroborate those of previous studies demonstrating the beneficial effects of these supplements in healthy premenopausal, as well as peri- and post-menopausal women.4,54
Limitations. The primary limitations of this study were the open-label study design and including only subjective assessments for hair growth and quality. African American subjects were slightly under-represented compared to the other sub-groups.
Conclusion
The results of this study demonstrate the daily administration of a standardized nutraceutical is effective in improving visible hair growth, volume/thickness/fullness, and coverage with less noticeable hair shedding for men and women of various ethnic backgrounds. Many self-assessment parameters continued to improve at Week 24 suggesting additional improvement may occur with continued product use.
Acknowledgments
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt of Apothekon, Inc., during the preparation of this manuscript.
References
- Phillips TG, Slomiany WP, Allison R. Hair loss: common causes and treatment. Am Fam Physician. 2017;96:371-8.
- Sadick NS, Callender VD, Kircik LH, Kogan S. New Insight Into the Pathophysiology of Hair Loss Trigger a Paradigm Shift in the Treatment Approach. J Drugs Dermatol. 2017;16(11):s135-s40.
- Farris PK, Rogers N, McMichael A, Kogan S. A Novel Multi-Targeting Approach to Treating Hair Loss, Using Standardized Nutraceuticals. Journal of drugs in dermatology : JDD. 2017;16(11):s141-s8.
- Ablon G, Kogan S. A randomized, double-blind, placebo-controlled study of a nutraceutical supplement for promoting hair growth in perimenopausal, menopausal, and postmenopausal women with thinning hair. J Drugs Dermatol. 2021;20:55-61.
- Ablon G, Kogan S. A Six-Month, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of a Nutraceutical Supplement for Promoting Hair Growth in Women With Self-Perceived Thinning Hair. Journal of drugs in dermatology : JDD. 2018;17(5):558-65.
- Birch MP, Lashen H, Agarwal S, Messenger AG. Female pattern hair loss, sebum excretion and the end-organ response to androgens. Br J Dermatol. 2006;154(1):85-9.
- Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29(4):568-75.
- El-Domyati M, Attia S, Saleh F, Abdel-Wahab H. Androgenetic alopecia in males: a histopathological and ultrastructural study. J Cosmet Dermatol. 2009;8(2):83-91.
- Ho CH, Sood T, Zito PM. Androgenetic Alopecia.StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020.
- Paus R, Arck P, Tiede S. (Neuro-)endocrinology of epithelial hair follicle stem cells. Mol Cell Endocrinol. 2008;288(1-2):38-51.
- Trueb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37(8-9):981-90.
- Gatherwright J, Liu MT, Amirlak B, Gliniak C, Totonchi A, Guyuron B. The contribution of endogenous and exogenous factors to male alopecia: a study of identical twins. Plast Reconstr Surg. 2013;131(5):794e-801e.
- Gatherwright J, Liu MT, Gliniak C, Totonchi A, Guyuron B. The contribution of endogenous and exogenous factors to female alopecia: a study of identical twins. Plast Reconstr Surg. 2012;130(6):1219-26.
- Banka N, Bunagan MJ, Shapiro J. Pattern hair loss in men: diagnosis and medical treatment. Dermatol Clin. 2013;31(1):129-40.
- Leiros GJ, Ceruti JM, Castellanos ML, Kusinsky AG, Balana ME. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol Cell Endocrinol. 2017;439:26-34.
- Magro CM, Rossi A, Poe J, Manhas-Bhutani S, Sadick N. The role of inflammation and immunity in the pathogenesis of androgenetic alopecia. Journal of drugs in dermatology: JDD. 2011;10(12):1404-11.
- Ito T. Hair follicle is a target of stress hormone and autoimmune reactions. J Dermatol Sci. 2010;60(2):67-73.
- Peters EMJ, Müller Y, Snaga W, Fliege H, Reißhauer A, Schmidt-Rose T, et al. Hair and stress: a pilot study of hair and cytokine balance alteration in healthy young women under major exam stress. PLoS One. 2017;12:e0175904.
- Ramos PM, Brianezi G, Martins AC, da Silva MG, Marques ME, Miot HA. Apoptosis in follicles of individuals with female pattern hair loss is associated with perifollicular microinflammation. Int J Cosmet Sci. 2016;38(6):651-4.
- Mahe YF, Michelet JF, Billoni N, Jarrousse F, Buan B, Commo S, et al. Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39(8):576-84.
- Breitkopf T, Leung G, Yu M, Wang E, McElwee KJ. The basic science of hair biology: what are the causal mechanisms for the disordered hair follicle? Dermatol Clin. 2013;31(1):1-19.
- Loussouarn G, Lozano I, Panhard S, Collaudin C, El Rawadi C, Genain G. Diversity in human hair growth, diameter, colour and shape. An in vivo study on young adults from 24 different ethnic groups observed in the five continents. Eur J Dermatol. 2016;26:144-54.
- Leerunyakul K, Suchonwanit P. Asian hair: a review of structures, properties, and distinctive disorders. Clin Cosmet Investig Dermatol. 2020;13:309-18.
- Tanus A, Oliveira CC, Villarreal DJ, Sanchez FA, Dias MF. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-65.
- Burgess C, Roberts W, Downie J, Kera M, Kogan S, Belpulsi D. A Closer Look at a Multi-Targeted Approach to Hair Loss in African American Women. Journal of drugs in dermatology : JDD. 2020;19(1):95-8.
- Lee H, Jung SJ, Patel AB, Thompson JM, Qureshi A, Cho E. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-70.
- Thompson JM, Park MK, Qureshi AA, Cho E. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-50.
- Lee WS, Lee HJ. Characteristics of androgenetic alopecia in Asian. Ann Dermatol. 2012;24:243-52.
- Goren A, Wei L, Tan Y, Kovacevic M, McCoy J, Lotti T. Frontal pattern hair loss among Chinese women is frequently associated with ponytail hairstyle. Dermatol Ther. 2019;32:e12784.
- Molamodi K, Fajuyigbe D, Sewraj P, Gichuri J, Sijako B, Galliano A, et al. Quantifying the impact of braiding and combing on the integrity of natural African hair. International journal of cosmetic science. 2021;Feb 19:Epub ahead of print.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-59.
- McMichael AJ. Hair and scalp disorders in ethnic populations. Dermatol Clin. 2003;21(4):629-44.
- Schmitt JV, Ribeiro CF, Souza FH, Siqueira EB, Bebber FR. Hair loss perception and symptoms of depression in female outpatients attending a general dermatology clinic. An Bras Dermatol. 2012;87:412-7.
- Rencz F, Gulácsi L, Péntek M, Wikonkál N, Baji P, Brodszky V. Alopecia areata and health-related quality of life: a systematic review and meta-analysis. Br J Dermatol. 2016;175:561-71.
- Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15:137-39.
- Hirsso P, Rajala U, Laakso M, Hiltunen L, Härkönen P, Keinänen-Kiukaanniemi S. Health-related quality of life and physical well-being among a 63-year-old cohort of women with androgenetic alopecia; a Finnish population-based study. Health Qual Life Outcomes. 2005;3:49.
- Tas B, Belli H, Kulacaoglu F, Altuntas M. The tendency towards the development of psychosexual disorders in androgenetic alopecia according to the different stages of hair loss: a cross-sectional study. An Bras Dermatol. 2018;93:185-90.
- Cash TF. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999;141:398-405.
- Cash TF. The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol. 1992;26:926-31.
- Hunt N, McHale S. The psychological impact of alopecia. BMJ. 2005;331(7522):951-3.
- Li SJ, Mostaghimi A, Tkachenko E, Huang KP. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-4.
- Propecia® (finasteride) tablets for oral use [Prescribing Information 2013]. Merck Sharp & Dohme Corp., Whitehouse Station, NJ.
- Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019.;13:2777-86.
- Kiguradze T, Temps WH, Yarnold PR, Cashy J, Brannigan RE, Nardone B, et al. Persistent erectile dysfunction in men exposed to the 5alpha-reductase inhibitors, finasteride, or dutasteride. PeerJ. 2017;5:e3020.
- Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5alpha-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8(3):872-84.
- Gur S KP, Hellstron WJ. Effects of 5-alpha reductase inhibitors on erectile function, sexual desire, and ejaculation. Expert Opin Drug Saf 2014;12(1):10.
- Ahluwalia J, Fabi SG. The psychological and aesthetic impact of age-related hair changes in females. J Cosmet Dermatol. 2019;18:1161-9.
- Fabbrocini G, Cantelli M, Masarà A, Annunziata MC, Marasca C, Cacciapuoti S. Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol. 2018;4:203-11.
- Alhattab MK, Al Abdullah MJ, Al-Janabi MH, Aljanaby WA, Alwakeel HA. The effect of 1540-nm fractional erbium-glass laser in the treatment of androgenic alopecia. J Cosmet Dermatol. 2020;19:878-83.
- Torres AE, Lim HW. Photobiomodulation for the management of hair loss. Photodermatol Photoimmunol Photomed. 2020;Dec 30:Epub ahead of print.
- Aggarwal K, Gupta S, Jangra RS, Mahendra A, Yadav A, Sharma A. Dermoscopic assessment of microneedling alone versus microneedling with platelet-rich plasma in cases of male pattern alopecia: a split-head comparative study. Int J Trichology. 2020;12:156-63.
- Okita AL, Steiner D, Berbert Ferreira S, Müller Ramos P, Ferreira W, Silveira R, et al. Treatment of male-pattern alopecia with platelet-rich plasma. Skin Appendage Disord. 2020;6:97-101.
- Bassino E, Gasparri F, Munaron L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: a review. Int J Mol Sci. 2020;21:523.
- Ablon G, Kogan S. A six-month, randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of a nutraceutical supplement for promoting hair growth in women with self-perceived thinning hair. J Drugs Dermatol. 2018;17:558-65.
- Rousso DE, Kim SW. A review of medical and surgical treatment options for androgenetic alopecia. JAMA Facial Plast Surg. 2014;16:444-50.
- Birnbaum MR MB, Shapiro J, Ye K, Reid SD,. Evaluation of hair density in different ethnicities in a healthy American population using quantitative trichoscopic analysis. Skin Appendage Disord. 2018;4:304-7.

