 J Clin Aesthet Dermatol. 2022;15(1):27–29.
J Clin Aesthet Dermatol. 2022;15(1):27–29.
by Vlatka Agnetta, MD; Sarah Williamson, BA; Elizabeth Bisbee, MD; Abel Torres, MD, JD, MBA; Leah Hooey, DO; Kiran Motaparthi, MD, and Sailesh Konda, MD
Dr. Agnetta, Dr. Bisbee, Dr. Torres, Dr. Hooey, Dr. Motaparthi and Dr. Konda are with the Department of Dermatology at University of Florida College of Medicine in Gainesville, Florida. Ms. Williamson is with the University of Florida College of Medicine in Gainesville, Florida.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. Mohs micrographic surgery (MMS) is the gold standard treatment for non-melanoma skin cancer (NMSC). However, NMSC recurrence may occur in a small proportion of patients. The aim of this study was to identify histopathologic features seen on the final stage of previous MMS, which may increase the risk of NMSC recurrence.
Methods. This was a single-institution retrospective study of 39 recurrent basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), which were treated with MMS. Slides from the final stage of previous MMS were reviewed by two board-certified dermatopathologists for the following histopathologic features: perineural inflammation, dense inflammation, mucin, ruptured follicle, actinic keratosis, and missing tissue.
Results. Twenty recurrent BCCs and 19 recurrent SCCs were included. Histopathologic features identified on the final stage of previous MMS included missing tissue from the epidermis, dermis, and/or subcutis (69%), actinic keratosis (51%), perineural inflammation (10%), and dense inflammation (8%). Ruptured follicle was present in one BCC case, and mucin was not identified in any cases.
Limitations. Limitations include retrospective study design, small number of recurrent cases, single institution, and lack of a control group consisting of NMSC cases which did not recur after MMS.
Conclusion. Mohs surgeons should carefully evaluate NMSC frozen sections for the presence of missing tissue, actinic keratosis, perineural inflammation, and dense inflammation as these histopathologic features may be associated with tumor recurrence. It is of paramount importance to acquire high quality frozen sections for thorough margin evaluation.
Keywords: non-melanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, Mohs micrographic surgery, recurrence
Mohs micrographic surgery (MMS) is a tissue-sparing procedure performed with the purpose of achieving complete clearance of cutaneous malignancies. MMS offers the highest cure rates for non-melanoma skin cancer (NMSC) and is considered the gold standard treatment for basal cell carcinoma (BCC)1 and squamous cell carcinoma (SCC)2 in high-risk locations. The five-year cure rates with MMS for previously untreated BCC and SCC are 99 percent3 and 97 percent4, respectively.
Regardless, NMSC may recur after MMS. Several studies previously examined histopathologic characteristics associated with NMSC recurrence. Zabielinski et al conducted a retrospective study to evaluate the MMS slides of 22 locally recurrent NMSC cases and identified residual tumor, dense inflammation at the final margin, missing epidermal or dermal tissue, and presence of actinic keratoses as possible causes of tumor recurrence5. Additional studies have also identified missing tissue6-8, local inflammation6, and aggressive tumor subtypes7 as potential factors contributing to local recurrence of cutaneous malignancies after MMS.
Identification of high-risk histopathologic features during MMS may help predict and minimize risk of NMSC recurrence. The purpose of this study was two-fold. First, to identify if, aside from residual tumor, additional histopathologic characteristics, such as mucin, ruptured follicle(s), and perineural inflammation, could potentially be implicated in NMSC recurrence and second, to externally validate the findings of previous studies with a larger sample size. The additional histopathologic features investigated in this study were included because mucin deposition often surrounds BCC, ruptured follicle can histopathologically resemble BCC, and perineural inflammation may mask adjacent tumor.
Methods
A single-institution retrospective chart review was performed at two regional sites of the University of Florida Department of Dermatology in Gainesville and Jacksonville, Florida. Following Institutional Review Board approval, the MMS database was searched between June 2014 and January 2020 for recurrent NMSCs treated again with MMS. A total of 265 recurrent cases were identified out of 8227 total cases performed. Fifty-five of these tumors were treated previously with MMS at the University of Florida between 2012 and 2019. All of the 55 recurrent cases were BCCs or SCCs and were treated by nine different American College of Mohs Surgery fellowship-trained Mohs surgeons, of which two currently practice in our department. All of the recurrences occurred within five years of the initial MMS.
Data collected from the electronic medical record included current age, gender, tumor location, tumor type, date of previous MMS, total number of stages performed during the previous MMS, and date of subsequent MMS to treat the recurrence. Slides obtained from the last stage of the previous MMS procedure were reviewed independently by two board-certified dermatopathologists who were blinded to original tumor characteristics. Any discrepancies in histopathologic evaluation were reviewed together by the two dermatopathologists to reach a consensus.
A total of 16 cases were excluded: six cases could not be reliably classified as true recurrences upon further chart review, which included detailed evaluation of clinical photos and pathology reports. Local tumor recurrence was defined as “a tumor with comparable histology, with contiguity to the surgical scar after treatment, and arising within the area of the previously treated tumor” based on criteria by the American College of Mohs Surgery Registry and Outcomes Committee.9 Ten additional cases (7 SCC and 3 BCC) were excluded due to the presence of residual tumor on the final stage. The process for subject inclusion is depicted in Figure 1.
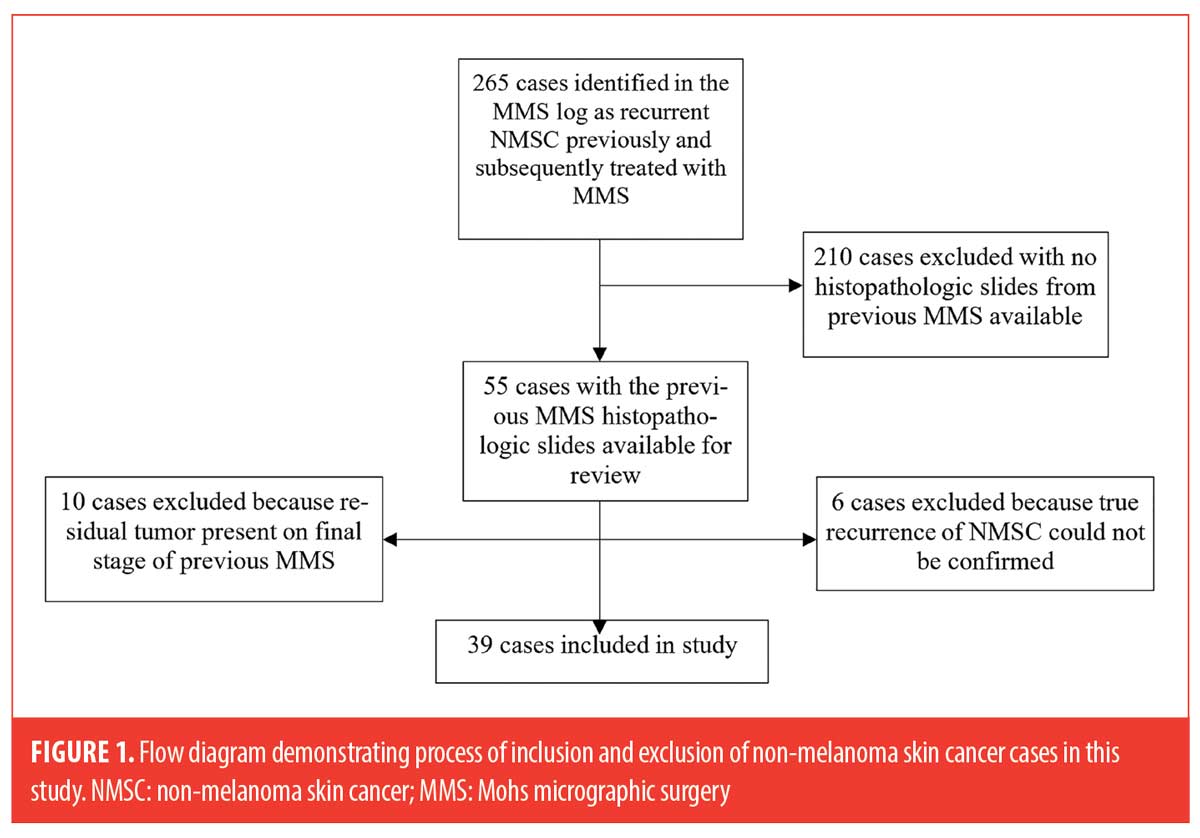
The remaining 39 cases included in the study were then examined for the following high-risk histopathologic features: perineural inflammation, dense inflammation, mucin, ruptured follicle(s), actinic keratosis, and/or missing tissue (epidermal, dermal, or subcutis). For cases in which missing tissue was identified and more than one MMS stage was performed, MMS maps were reviewed and compared to the MMS slides to determine if the examined sections were missing tissue.
Results
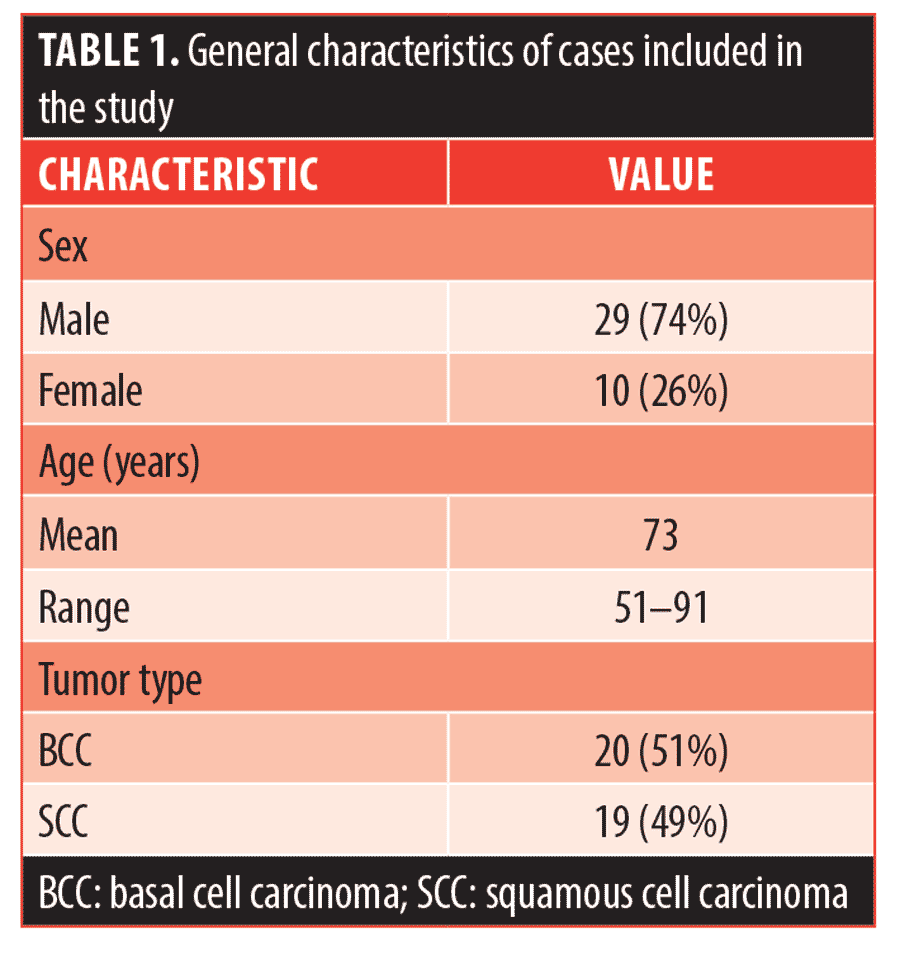
General characteristics of the included cases are summarized in Table 1. The patients’ mean age was 73 years (range 51–91) with 74 percent of the cases in men. There was an approximately equal number of recurrences of BCCs (n=20, 51%) and SCCs (n=19, 49%). The average number of stages performed during the previous MMS was 1.67 for all cases, 1.75 for BCC cases, and 1.53 for SCC cases. The mean time interval between the previous and subsequent MMS was 19 months (range 3–58 months). Ninety percent of the recurrent cases were NMSC of the head and neck (n=35), and 10 percent were on the trunk and extremities (n=4).
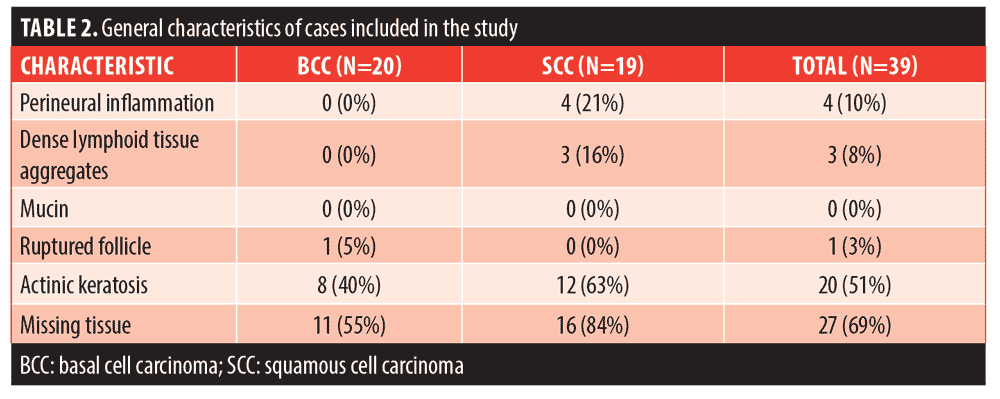
The histopathologic evaluation of the tissue samples obtained from the final stage of the previous MMS is summarized in Table 2. Of the cases, 41 percent had more than one high-risk histopathologic feature present (n=16), while 15 percent (n=6) had none of the listed histopathologic features. The most common feature identified was missing tissue from the epidermis, dermis, and/or subcutis, which was identified in 69 percent of the total cases (n=27). Presence of actinic keratosis was the second most common characteristic, identified in 51 percent of the total cases (n=20). However, only 31 percent of actinic keratoses were found in the setting of SCC (n=12), and 8 of the 12 cases were also associated with missing histopathologic tissue. Perineural inflammation was identified exclusively in cases of SCC (n=4, 21% of SCC cases), and 3 of the 4 cases also had missing tissue. Dense inflammation was only identified in SCC cases, (n=3, 16%) but all of these cases had at least one additional histopathologic feature present (actinic keratosis or missing tissue). Ruptured follicle was present in 1 case of BCC in addition to missing tissue. No mucin aggregates were identified in the examined cases.
Discussion
While MMS offers the highest cure rate for high-risk NMSC, one to three percent of cases are reported to have recurrences.3,4 Our findings suggest that several key histopathologic features present on the final stage of previous MMS may be associated with recurrence of NMSC. This investigation replicates the work of several prior studies5-8 but differs in that it includes a larger sample size of recurrent NMSC cases and evaluates the following additional histopathologic characteristics on the final stage of previous MMS: perineural inflammation, mucin, and ruptured follicle.
In our study, missing tissue from the epidermis, dermis, and/or subcutis on the final stage of the previous MMS was observed in a greater percentage (69%) of recurrent NMSC cases compared to prior investigations including Zabielinski et al5 (23%), Hruza6 (30%), Campbell et al7 (21%), and Lee et al8 (27%). The retrospective nature of the study made it difficult to definitively determine whether tissue was actually missing or if there was an artifact due to a tissue tear or tissue processing. Regardless, it appears that inability to visualize and evaluate a complete tissue specimen during MMS may lead to a higher chance of NMSC recurrence.
Areas of dense inflammation may mask residual tumor cells. A prior study investigated areas of dense inflammation in cases of primary BCC treated with MMS and determined that no histopathologic evidence of remaining BCC was present in the areas of dense inflammation.10 Another study by Alam et al determined that the presence of histopathologic inflammation in frozen sections modestly predicted adjacent NMSC in the subsequent stage; however, the absence of inflammation strongly predicted the absence of additional NMSC.11 In our study, areas of dense inflammation were noted in 8 percent of the cases (n=3), which were all recurrent SCC. This proportion was comparable to previous studies by Hruza6 (7%) and Campbell et al7 (10.5%). While Zabielinski et al5 reported a higher proportion of cases with dense inflammation (27%), all were also cases of SCC. We additionally evaluated perineural inflammation as a separate histopathologic characteristic from dense inflammation. Ten percent of reviewed cases had perineural inflammation, and all were SCCs.
Actinic keratoses were noted in approximately half (51%) of recurrent NMSC cases, including 63 percent of SCC cases and 40 percent of BCC cases. Zabielinski et al5 reported actinic keratosis in only one case of recurrent SCC. While a ruptured follicle was present in only one case of recurrent BCC, the final MMS stage demonstrated missing subcutis as well. Therefore, it was difficult to conclude if a ruptured follicle is a risk factor for tumor recurrence. Presence of mucin was not found to be associated with tumor recurrence in our study.
Although we excluded cases from our study that had residual tumor present on the final slide of the previous MMS procedure, other studies found 18-26% of recurrent cases were associated with residual tumor.5-8 These percentages are similar to the percentage of excluded cases with residual tumor in our study (20.6%).
Conclusion
Mohs surgeons should carefully evaluate NMSC frozen sections for the presence of missing tissue. This confirms what would be obvious to some and validates data from previous studies. In addition, actinic keratosis, perineural inflammation, and dense inflammation are histopathologic features that seem to predict tumor recurrence, especially with respect to SCC. Limitations of this study include a small sample size and lack of a control group consisting of NMSC cases treated with MMS with no recurrence for comparison. We could not make any conclusion as to the importance of these histopathologic characteristics on the interval between recurrences. However, it is interesting that recurrences in a large cohort of patients treated by nine different Mohs surgeons had a mean time interval of 19 months, which approximates a prior study by Campbell et al.7 Further studies with a matched control group and larger sample size may be helpful to better ascertain the significance of an association between each of these variables and tumor recurrence.
References
- Kauvar ANB, Cronin Jr T, Roenigk R, et al. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-71.
- Kauvar ANB, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-40.
- Rowe DE, Carroll RJ, Day Jr CL. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15(3):315-28.
- Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53(2):253-60.
- Zabielinski M, Leithauser L, Godsey T, Gloster Jr HM. Laboratory errors leading to nonmelanoma skin cancer recurrence after Mohs micrographic surgery. Dermatol Surg. 2015;41(8):913-6.
- Hruza GJ. Mohs micrographic surgery local recurrences. J Dermatol Surg Oncol. 1994;20(9):573-7.
- Campbell T, Armstrong AW, Schupp CW, et al. Surgeon error and slide quality during Mohs micrographic surgery: is there a relationship with tumor recurrence? J Am Acad Dermatol. 2013;69(1):105-11.
- Lee KC, Higgins 2nd HW, Dufresne Jr RG. Tumor recurrence after Mohs micrographic surgery. J Am Acad Dermatol. 2014;70(2):385-6.
- Leitenberger JJ, Rogers H, Chapman JC, et al. Defining recurrence in nonmelanoma skin cancer after Mohs micrographic surgery: report of the American College of Mohs Surgery Registry and Outcomes Committee. J Am Acad Dermatol. 2016;75(5):1022-1031.
- Katz KH, Helm KF, Billingsley EM, Maloney ME. Dense inflammation does not mask residual primary basal cell carcinoma during Mohs micrographic surgery. J Am Acad Dermatol. 2001;45(2):231-8.
- Alam M, Khan M, Veledar E, et al. Correlation of inflammation in frozen sections with site of nonmelanoma skin cancer. JAMA Dermatol. 2016;152(2):173-6.

