 J Clin Aesthet Dermatol. 2020;13(11):13–18
J Clin Aesthet Dermatol. 2020;13(11):13–18
by Monica Boen, MD; Marwan Alhaddad, MD; Douglas C. Wu, MD, PhD;and Mitchel P. Goldman, MD
All authors are with the Cosmetic Laser Dermatology in San Diego, California.
FUNDING: This study was funded by Topix Pharmaceuticals Inc.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. The neck is one of the most common areas affected by the aging process. A novel two product combination system composed of a serum and cream with hyaluronic acid and multiple strong antioxidants were investigated to determine their efficacy and safety in neck rejuvenation.
Objective. The objective of this prospective, randomized, double-blind, placebo-controlled clinical trial was to assess the efficacy and safety of a novel serum containing fractionated hyaluronic acid, peptides, and antioxidants for photodamage of the neck.
Methods. This was an institutional review board (IRB)-approved, randomized, double blind, placebo-controlled clinical trial involving 31 healthy subjects with moderate-to-severe neck wrinkling corresponding to at least a Grade 2 in wrinkles and score of 4 in elastosis on the Fitzpatrick-Goldman Wrinkle Scale. Twenty subjects were randomized to receive the active cream and serum system, while 11 subjects were randomized to receive the vehicles alone in serum and cream format for a course of two months.
Results. Both active and placebo cream and serum showed improvement of wrinkles, laxity, pigmentation, erythema, dryness, and texture of the skin, and high patient satisfaction scores. Histology of one of the active serums and cream samples revealed improvement in the quality of papillary dermal collagen and increase in the number of elastic fibers in the upper dermis after treatment.
Conclusion. Our prospective, randomized controlled trial showed that the novel serum and cream showed improvement in skin aging on the neck, was well-tolerated by patients, and had a high degree of patient satisfaction.
KEYWORDS: Aging neck, hyaluronic acid cream, antioxidant, neck rejuvenation
Skin photoaging is the result of multiple complex intrinsic and extrinsic factors. Intrinsic factors are due to a genetically induced process with largely unknown mechanisms, while ultraviolet radiation and environmental pollutants, particularly hydrocarbons, to be the most prominent extrinsic factors.1–3 Photoaged skin appears lax and dry with wrinkles, deep furrows, tactile roughness, pigment irregularity, erythema, atrophy, and telangiectasia.4 Currently, patients achieve a more youthful and revitalized appearance through a variety of facial rejuvenating procedures. However, these facial procedures might create an abrupt contrast between the rejuvenated face and other parts of the body, such as the neck.
Options currently available to improve the appearance of the neck include injectable dermal fillers, neurotoxins, chemical peels, photodynamic therapy, intense pulsed light, and a variety of lasers, including the 595nm pulse dye laser, 1927nm thulium laser, 755nm alexandrite laser, microfocused ultrasound with visualization (MFU-V), radiofrequency, non-ablative fractionated lasers, and ablative fractionated lasers alone or in combination.5–17 An effective antiaging topical product would offer complementary treatment for these cosmetic procedures, to help boost and maintain the results of these procedures. Little clinical data have been generated to support the efficacy of commercially available cosmeceuticals specifically design for the neck area.8,9,12,13,18
Hyaluronic acid (HA) is a major naturally occurring component of the dermis and epidermis.19 HA creates volume and turgor of the skin through water attraction into the extracellular matrix, binding up to 1000 times its volume in water, to promote tissue hydration.20 Moreover, studies have shown that HA increases the production of Collagen 1 and 3 through mechanical stretching of fibroblasts by the hydrating effect of HA on extracellular matrix.21, 22 Progressive reduction of HA with age represents one of the most prominent contributors to the morphology of photoaged skin.23
Oxidative stress is another factor for aging of the skin. Antioxidants, such as niacinamide, vitamin E, green tea, and other botanical extracts, have shown to prevent cellular or structural damage in the skin.24, 25 Another class of cosmeceuticals includes bioactive peptides, such as palmitoyl pentapeptide-4, which has been showed in vitro to simulate collagen and clinical studies have shown improvement in signs of aging (e.g., wrinkle and fine line reduction).26,27 As such, replenishing the dermis and epidermis through HA, antioxidants, and peptides is an intuitive approach to reverse the signs of photoaging and to prevent further deterioration.
In this study, the investigators assessed the efficacy and safety of a novel serum containing fractionated hyaluronic acid, peptides and antioxidants to determine if a combination of this novel serum and cream can improve photodamage on the neck.
Methods
This was a prospective, randomized, double-blind, placebo-controlled, institutional review board (IRB)-approved clinical trial that was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization. After obtaining informed consent, 31 subjects over the age of 18 with moderate-to-severe neck wrinkling corresponding to at least a Grade 2 in wrinkles and score of 4 in elastosis on the Fitzpatrick-Goldman wrinkle scale (Table 1) were enrolled. Subjects were excluded if they were pregnant or breastfeeding; had a microdermabrasion on their face or neck within the previous one month; had treatment with chemical peel, a non-ablative laser, light, radiofrequency, or ultrasound treatment on their face or neck within the previous three months; had a dermabrasion and/or ablative laser treatment on their neck within the previous six months; subjects using any topical imiquimod, 5-fluorouracil, or diclofenac on their neck within three months prior to or during the study period; had applied any topical products known to rejuvenate the skin, such as products containing vitamins A, C, or E derivatives, alpha-hydroxy acids, or salicylic acids within the previous two weeks or at any time during the trial; or had any pre-existing dermatological condition in the neck region.
Twenty subjects were randomized to receive the active cream and serum system (TPX) (Replenix Pure Hydration hyaluronic acid serum and Replenix Neckletage cream, Topix® in West Babylon, New York), while 11 subjects were randomized to receive the vehicles alone in serum and cream format. The test products were applied twice daily for a period of 60 days, with follow-up visits conducted at Day 30 and Day 60. Subjects were directed to wash the neck with a non-alpha hydroxy acid gentle cleanser, then to apply the hyaluronic acid serum or placebo serum to the entire neck and wait for this to dry, and then apply the cream or placebo cream to the entire neck. Subjects were requested to perform this procedure in the morning and the evening. In the morning, the final step was to apply a sunscreen containing zinc oxide with SPF 30 or higher. Baseline and follow-up photography were obtained utilizing the Canfield Vectra 3-dimensional imaging system (Canfield Scientific in Parsippany, New Jersey).
The first five subjects who volunteered for the histological evaluation underwent pre- and post-treatment biopsies. Prior to initiating the treatment, a punch biopsy of the neck was performed using a 2-mm circular blade. Sixty days later, a post-treatment biopsy was taken from the neck for comparison. After biopsy, samples were immediately transferred to 10% formalin in preparation for further histological processing. Pre- and post-treatment paraffin embedded tissue samples from the neck of five subjects were analyzed. The following stains were performed on every sample: Hematoxylin and Eosin (H&E), Masson’s Trichrome, Verhoeff van Gieson, and elastin stains. Pre- and post-treatment tissues were stained on the same slide, to minimize staining variability
Subjects, evaluating investigators, and evaluating dermatopathologist were blinded. Neck wrinkling was assessed by the blinded evaluator at baseline and at the last visit via the Fitzpatrick-Goldman wrinkle scale. The blinded evaluator also assessed percentage of improvement in rhytids, skin texture, laxity, dyschromia, and erythema at each follow-up visit based on a 6-point scale (1=worse, 2=1–25% improvement, 2=26–50% improvement, 3=51–75% improvement, 4=76–100% improvement). Assessments were performed in person and used previous photographs as comparison. Additionally, a clinician global aesthetic improvement scale was done at each follow-up visit on a 5-point scale (1=much improved, 2=improves, 3=no change, 4=worse, 5=much worse). At Day 30 and 60, subjects completed a questionnaire regarding the effectiveness and tolerability of the treatment regimen. Local tolerability including erythema, burning/stinging, dryness, peeling, and tenderness on the neck was assessed by the investigator using a 4-point scale (0=none, 1= mild, 2= moderate, and 3=severe)
Statistical analysis was conducted on an intent-to-treat basis (all randomized subjects with at least one follow-up visit were included). All statistical tests were two-tailed student’s t-test and interpreted at a five-percent significance level. Statistical evaluation was performed with Microsoft Excel 2013.
Results
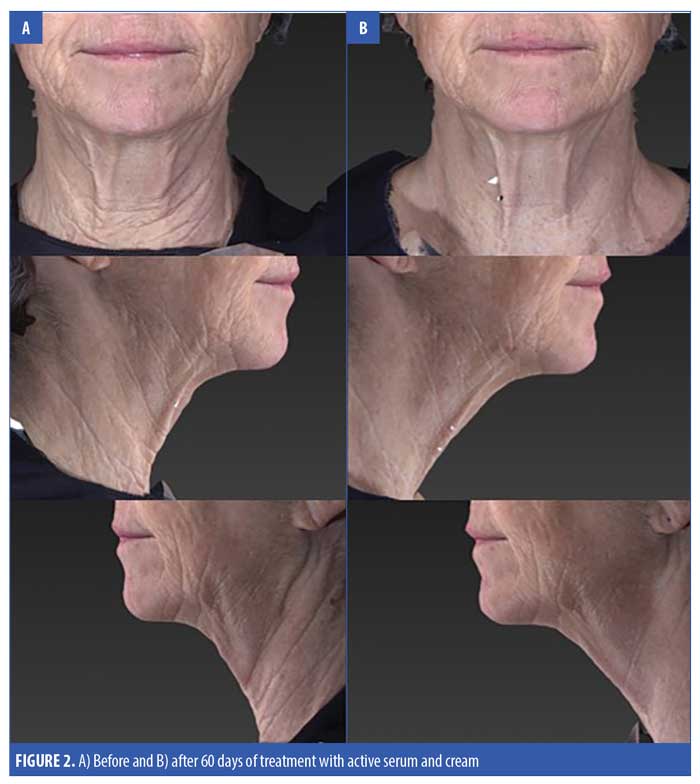
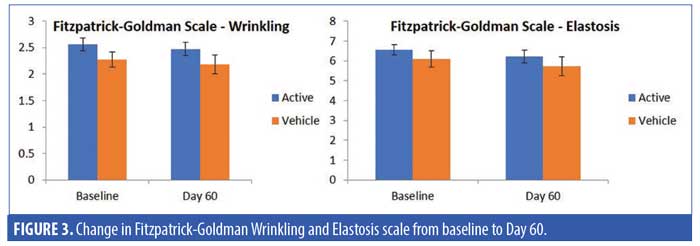
A total of 31 subjects, 30 female and one male, were enrolled in the study. The mean age of the subjects was 62.15 ± 7.18 (range 47–77) (Table 2). One patient who received the active product did not complete the final follow-up visit, otherwise, all the other 30 patients completed all the visits in the study. For the primary endpoints of analysis of wrinkling as evaluated by blinded clinical investigators, on Day 30, 55 percent of subjects applying active product had at least a 1- to 25-percent improvement in wrinkling. By Day 60, 74 percent of subjects receiving the active topical agents had at least a 1- to 25-percent improvement in wrinkling, and within this group, 16 percent had a 26- to 50-percent improvement (Figure 1). On Day 30, 50 percent of subjects using the active topical agent had a 1- to 25-percent improvement in laxity. By Day 60, 68 percent of these subjects noted a 1- to 25-percent improvement in skin laxity, and of these subjects, 11 percent had a 26- to 50-percent improvement. In terms of texture, by Day 30 and 60, 70 percent of patients treated with the active product had up to 25-percent improvement in texture of the neck, and by Day 60, 79 percent of patients had at up to 25-percent improvement in dryness of the neck (Figure 2). The vehicle topical agents had similar improvements with 91 percent of patients noticing at mild improvement (1–25%) in wrinkling and texture and 63 percent noting a mild improvement in skin laxity by Day 60 (Figures 3 and 4). There was no statistically significant difference in improvement of wrinkles, laxity, pigmentation, erythema, dryness, and texture of the skin between the active and vehicle topical agents.

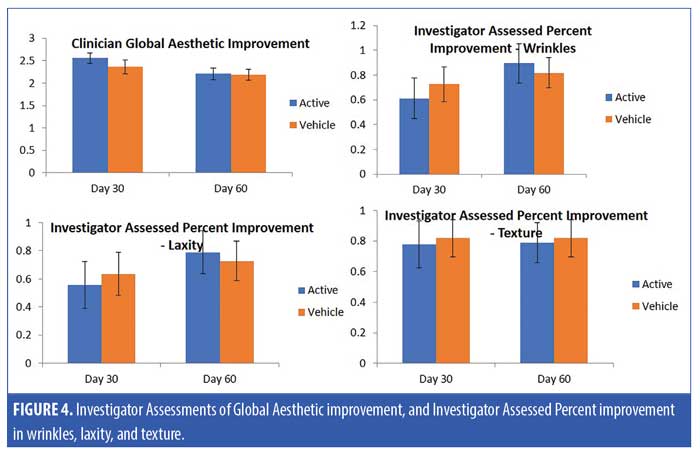
The clinician tolerability assessment showed that the active serum and cream were well tolerated without any adverse events. On subject questionnaire, 79 percent of subjects noted that the wrinkles on the neck were much reduced (mean of 1.05 ± 0.78, where 1=much reduced) and a majority of patients were very satisfied to satisfied with the active serum and cream (mean of 1.42 ± 0.96, where 1=satisfied and 2=very satisfied) on Day 60. All of the patients agreed that the products were convenient and easy to use on the neck. In addition, 79 percent of subjects who received the active product noted that the study products improved hydration of the skin on the neck, and 68 percent noted improved texture of the neck. All patients noted the study product well and reported no adverse effects.
Five subjects were randomized to have a 2mm neck punch biopsy on the neck before and 60 days after treatment, of which four patients received the active product and one received the vehicle. One of the biopsy samples from the subject applying the active topical products showed significant improvement in the quality of papillary dermal collagen, with some deposition of fine, new collagen fibers and significantly less dermal solar elastosis in the papillary dermis as assessed by a blinded dermatopathologist (Figure 5). Trichrome stain demonstrated the improvement in the quality of papillary dermal collagen and increase in the number of elastic fibers in the upper dermis after application of the active products (Figure 6). The remaining biopsy samples showed minimal to no changes.
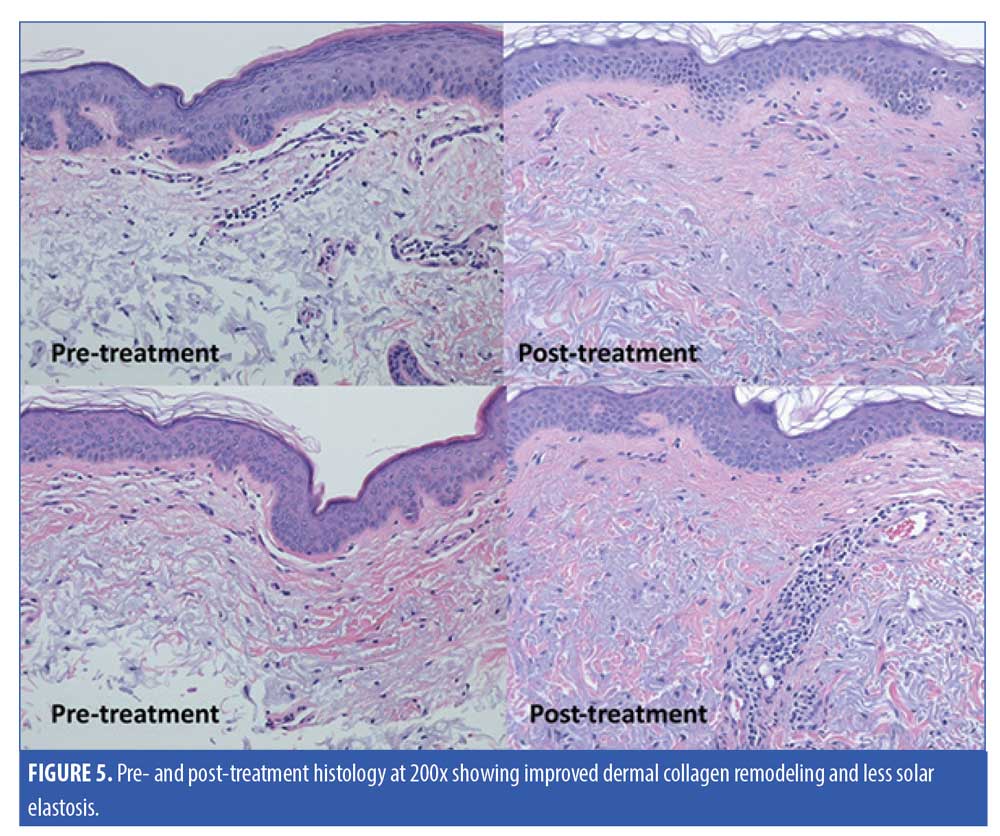
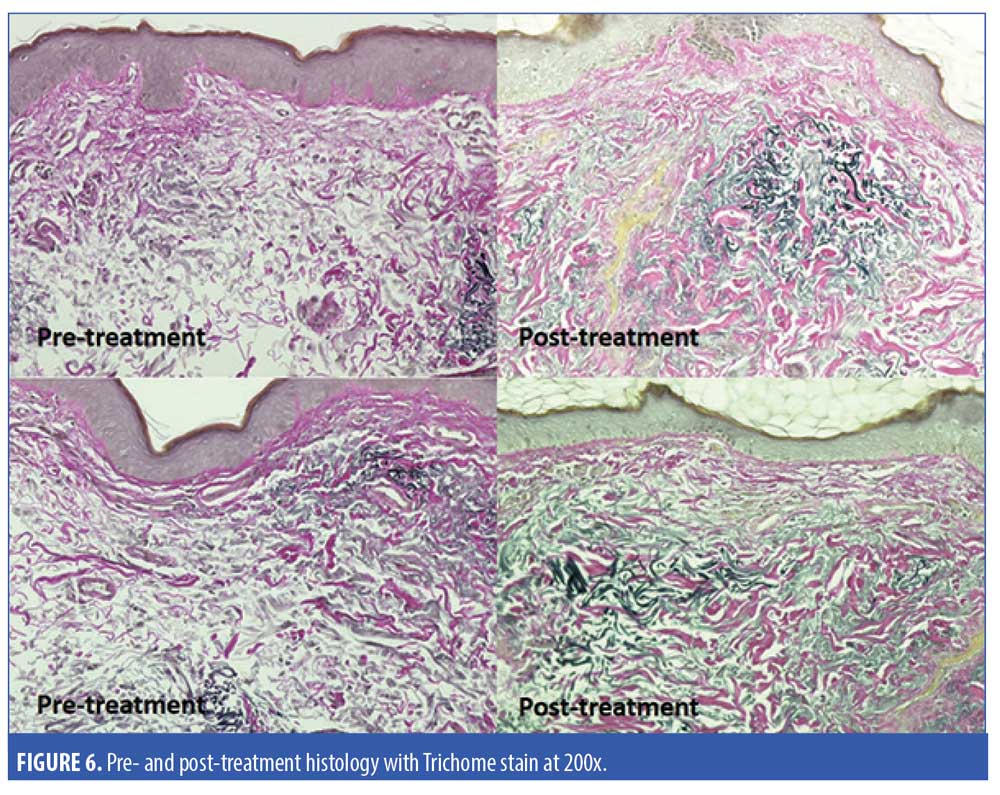
Discussion
Our study demonstrated an improvement in neck skin wrinkles, laxity, dyschromia, texture, and dryness in 63 percent of subjects who had applied the active serum and cream. Moreover, these products caused no adverse events, were well-tolerated by patients, and all patients stated that they were convenient and easy to use as part of their skin care regimen on the neck. One of the skin biopsies from the neck demonstrated improvement in solar elastosis, and new collagen and elastic fibers after treatment with the active serum and cream.
The active serum and cream in this study is a two-product combination system. The active serum is composed of hyaluronic acid in varying chain lengths of HA, from longer, higher molecular weight molecules, which very effectively bind moisture and nutrients to the skin, to fully fractionated HA right down to the single monomer, which is small enough (~360Da) to penetrate the skin easily and also acts as an antioxidant28. Lastly, the peptide component contains palmitoyl tripeptide-28 and palmitoyl pentapeptide-4, whose primary function is to support collagen production in combination with saccharomyces lysate. The active cream contains several powerful antioxidants, such as green tea polyphenols, niacinamide, apple stem cells, vitamin E, caffeine, and pea and botanical extracts.29, 30 This layering of antioxidants provides broad spectrum coverage across multiple oxidative pathways, providing greater protection. This unique serum and cream combination improve the hydration of the skin, but also targets free radicals and reactive oxygen species that are responsible for skin aging and have been well-studied in the literature. Some of the antioxidant agents, such as green tea and vitamin E, also have anti-inflammatory and anticarcinogenic properties.24, 25 The body does have its own innate antioxidant defense, but this reservoir can be depleted under repeated stressors.30 Thus, topical antioxidants can protect and restore the skin’s defense against oxidative damage.
While both the vehicle and placebo serum and cream showed an improvement in photodamage on the neck, it could be due to the hydrating non-active ingredients present in both agents. The carrier vehicles of both products comprise a delivery system for the active ingredients and have benefit to the skin in and of themselves as a consequence of the supplemental humectants: glycerin and sodium PCA, secondary antioxidants, and micronutrient sources, such as Leontopodium Alpinum Callus Culture Extract (a phyto-stem cell supplement). They also contain zinc and copper polycarboxylic acids (trace metals that are important co-factors for collagen production and regulation). The cream vehicle also contains biomimetic ceramides, a mixture of ceramides similar to the lipid profile found in healthy skin, which help maintain an effective barrier, preventing transepidermal water loss (TEWL) and allowing the skin to undergo homeostasis with regard to self-repair at a higher rate. These properties might have explained why both the active and placebo groups showed improvement in neck wrinkles and texture.
Furthermore, it is difficult to assess the improvement of a topical agent because these are usually subtle effects that might be difficult to capture; other studies have reported issues.28, 31,32 Additionally, the grading scale used to measure efficacy was not sensitive enough to detect differences between the active group and the placebo group. Our study duration was only 60 days, which might not have been long enough to assess the potential of the active topical ingredients to improve skin aging on the neck. Lastly, one of the limitations of the study was the small sample size.
Subject reported outcomes have become increasingly valued in clinical trials. Our study showed that the majority of subjects (19/30) noted that their neck felt slightly or more youthful, and most reported that the overall photodamage on their neck somewhat too much improved (20/30) by the end of the study. About 83 percent of the subjects (25/30) reported that they were slightly to very satisfied with the treatment, and 67 percent (20/30) noted that they would continue using the study products for skin pigmentation in the future. In summary, there was high patient satisfaction with the use of the topical serum and cream in our study.
Conclusion
Our prospective, randomized controlled trial showed that the novel serum and cream showed improvement in skin aging on the neck, was well-tolerated by patients, and had a high degree of satisfaction from patients, but was not statistically significantly better than the vehicle.
References
- Katsambas AD , Katoulis AC. Topical retinoids in the treatment of aging of the skin. Adv Exp Med Biol. 1999;455:477–482.
- Cadet J , Douki T. Oxidatively generated damage to DNA by UVA radiation in cells and human skin. J Invest Dermatol.
2011;131:1005–1007. - Burke KE , Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health. 2009;25:219–224.
- Peterson JD , Goldman MP. Rejuvenation of the aging chest: A review and our experience. Dermatol Surg. 2011;37:555–571.
- Vanaman M , Fabi SG. Decolletage: Regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136:276S–2781S.
- Fitzpatrick RE, Rostan EF , Marchell N. Collagen tightening induced by carbon dioxide laser versus erbium: YAG laser. Lasers Surg Med. 2000;27:395–403.
- Avram DK , Goldman MP. The safety and effectiveness of single-pass erbium: YAG laser in the treatment of mild to moderate photodamage. Dermatol Surg. 2004;30:1073–1076.
- Goldman MP, Weiss RA , Weiss MA. Intense pulsed light as a nonablative approach to photoaging. Dermatol Surg.
2005;31:1179–1187. - Fitzpatrick RE, Goldman MP , Sriprachya-Anunt S. Resurfacing of photodamaged skin on the neck with an UltraPulse((R)) carbon dioxide laser. Lasers Surg Med. 2001;28:145–149.
- Ratner D, Tse Y, Marchell N, Goldman MP, Fitzpatrick RE , Fader DJ. Cutaneous laser resurfacing. J Am Acad Dermatol. 1
999;41:365–389; quiz 90–92. - Manuskiatti W, Fitzpatrick RE , Goldman MP. Treatment of facial skin using combinations of CO2, Q-switched alexandrite, flashlamp-pumped pulsed dye, and Er:YAG lasers in the same treatment session. Dermatol Surg. 2000;26:114–120.
- Goldman MP , Marchell NL. Laser resurfacing of the neck with the combined CO2/Er:YAG laser. Dermatol Surg. 1999;25:923–925.
- Goldman MP. Techniques for erbium:YAG laser skin resurfacing: initial pearls from the first 100 patients. Dermatol Surg. 1997;23:1219–1221.
- Goldman MP , Manuskiatti W. Combined laser resurfacing with the 950-microsec pulsed CO2+ Er:YAG lasers. Dermatol Surg. 1999;25:160–163.
- Friedmann DP, Tzu JE, Kauvar AN , Goldman MP. Treatment of facial photodamage and rhytides using a novel 1,565nm non-ablative fractional erbium-doped fiber laser. Lasers Surg Med.
2016;48:174–180. - Wu DC, Fletcher L, Guiha I , Goldman MP. Evaluation of the safety and efficacy of the picosecond alexandrite laser with specialized lens array for treatment of the photoaging decolletage. Lasers Surg Med. 2016;48:188–192.
- Wu DC, Friedmann DP, Fabi SG, Goldman MP , Fitzpatrick RE. Comparison of intense pulsed light with 1,927-nm fractionated thulium fiber laser for the rejuvenation of the chest. Dermatol Surg. 2014;40:129–133.
- Fitzpatrick RE, Goldman MP, Satur NM , Tope WD. Pulsed carbon dioxide laser resurfacing of photo-aged facial skin. Arch Dermatol. 1996;132:395–402.
- Baumann L. Skin ageing and its treatment. J Pathol.
2007;211:241–251. - Matarasso SL, Carruthers JD, Jewell ML. Restylane Consensus G. Consensus recommendations for soft-tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg. 2006;117:3S–34S; discussion 5S–43S.
- Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143:155–163.
- Quan T, Wang F, Shao Y, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133:658–667.
- Ghersetich I, Lotti T, Campanile G, Grappone C , Dini G. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol. 1994;33:119–122.
- Elmets CA, Singh D, Tubesing K, et al. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44:425–432.
- Palmer DM , Kitchin JS. Oxidative damage, skin aging, antioxidants and a novel antioxidant rating system. J Drugs Dermatol. 2010;9:11–15.
- Reddy B, Jow T , Hantash BM. Bioactive oligopeptides in dermatology: Part I. Exp Dermatol. 2012;21:563–568.
- Robinson LR, Fitzgerald NC, Doughty DG, et al. Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin. Int J Cosmet Sci. 2005;27:155–160.
- Pavicic T, Gauglitz GG, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990–1000.
- Roh E, Kim JE, Kwon JY, et al. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit Rev Food Sci Nutr. 2017;57:1631–1637.
- Chen L, Hu JY , Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol. 2012;67:1013–1024.
- Saxena SJ, Duque D , Schirripa MJ. Assessment of a comprehensive anti-aging neck cream. J Drugs Dermatol. 2015;14:997–1002.
- Schlessinger J, Green B, Edison BL, Murphy L , Sabherwal Y. A firming neck cream containing N-acetyl glucosamine significantly improves signs of aging on the challenging neck and decolletage. J Drugs Dermatol. 2016;15:47–52.

