J Clin Aesthet Dermatol. 2019;12(1):28–31
 by Andrew Duncanson, DO; Michael Kopstein, DO; and Stanley Skopit, DO, MSE, FAOCD, FAAD
by Andrew Duncanson, DO; Michael Kopstein, DO; and Stanley Skopit, DO, MSE, FAOCD, FAAD
Drs. Duncanson and Skopit are with the Larkin Community Hospital/LECOM Dermatology Residency Training Program in Miami, Florida. Dr. Kopstein is with the Sky Ridge Medical Center/RVUCOM Internal Medicine Residency Training Program in Lone Tree, Colorado.
Funding: No funding was received for this study.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: We report the case of a 47-year-old woman who presented to the emergency department with a 9.7cm × 8.3cm melanoma on her foot, which impeded her ability to walk. The patient had been treating the fungating mass with black salve after seeking treatment from a homeopathic physician when first noticing a mole increasing in size several years previously. Additionally, we examine published reports of giant melanoma in the literature and investigate the possible maleficence of black salve treatment.
Keywords: Melanoma, homeopathic, black salve, giant melanoma
Melanoma is among the deadliest forms of skin cancer and accounts for 90 percent of skin cancer deaths.1 In the United States, the incidence rate of melanoma is 20 to 30 per 100,000 people.1 The majority of lesions are initially detected during self-examination, with patients self-detecting 44 percent of melanomas, followed by physicians detecting 25.3 percent and partners detecting 18.6 percent.2 There are four main subtypes of melanoma: superficial spreading, nodular, in situ/lentigo maligna, and acral lentiginous. Acral lentiginous melanoma, in particular, is aptly named, as it occurs on the hands and feet and sometimes involves the nail unit. In the initial intraepidermal phase, there is irregular, poorly circumscribed pigmentation, followed by a nodular, invasive growth pattern. In studying the histopathological subtype of cutaneous melanoma of the foot, Nam et al3 found that 80.8 percent of cases are classified as acral lentiginous melanoma. Early diagnosis and treatment is key to long-term survival. Roughly 90 percent of melanomas are diagnosed as primary tumors without evidence of metastasis and have a 10-year survival rate of 75 to 85 percent.1
Ten-year patient survival rate for tumors with regional lymph node metastasis on presentation is much worse, at roughly 20 to 40 percent.4 Untreated patients with distant metastasis have a median survival of about 6 to 9 months.1
Factors that indicate a poor prognosis include increased Breslow’s depth, histologically recognized ulcerations, an elevated serum lactate dehydrogenase level, and high mitotic rate.1,4 Cutaneous melanoma is usually diagnosed prior to the lesion reaching a large diameter. Seidenari et al5 found a mean horizontal diameter of 13.26mm for lesions diagnosed on the trunk and limbs. Large skin melanomas are rare and are usually the result of long-term neglect of a growing skin lesion. Several giant melanomas have been documented in the literature.6–10 In these cases of advanced melanoma, it is very difficult, if not impossible, to determine an original histological type.7 From our review of the literature, it appears there are only three reports of giant cutaneous melanoma involving the foot.7,10
Topical herbal remedies are a growing industry aided in large part by the ease of access and the powerful marketing capabilities of the internet. It appears that many patients strongly believe these natural remedies to be effective and harmless.
Black salve is an escharotic that commonly consists of a combination of zinc chloride, dimethyl sulfoxide, and a bloodroot extract called Sanguinaria canadensis.11–13 S. canadensis is a flowering plant native to North America, commonly referred to as bloodroot, redroot, or Indian paint. At low doses, sanguinaria has been found to cause oxidative deoxyribonucleic acid (DNA) damage and apoptosis. However, at the higher doses found in black salve products, it kills both cancerous and normal cells.11
Black salve has been used for more than 1,000 years as an herbal remedy.14 The use of S. canadensis in the medical literature dates back to 1869 in Cook’s The Physiomedical Dispensatory, where he detailed four patients who died after drinking bloodroot tincture.15,16 A variant of black salve was used in the 1940s by Dr. Frederic Mohs as a tissue fixative in his micrographic tissue technique. However, this technique was largely supplanted by the fresh tissue technique, due to technical and other advantages.11
There are numerous reports in the literature detailing the danger and negative patient outcomes associated with black salve treatment, including disease progression and a need for corrective cosmetic surgery.12,15,16 There are no randomized, clinical trials that have examined the efficacy or safety of black salve products for the treatment of melanoma. Starting in 2008, the United States Food and Drug Administration (FDA) began warning many companies not to claim that black salve cures cancer. Despite this, a simple Internet search reveals numerous websites selling black salve products and making false, unsubstantiated claims. These sites frequently imply dangers associated with the conventional medical management of skin lesions and often refer to the work of Mohs as medical justification for the safety and efficacy of black salve.
Case Presentation
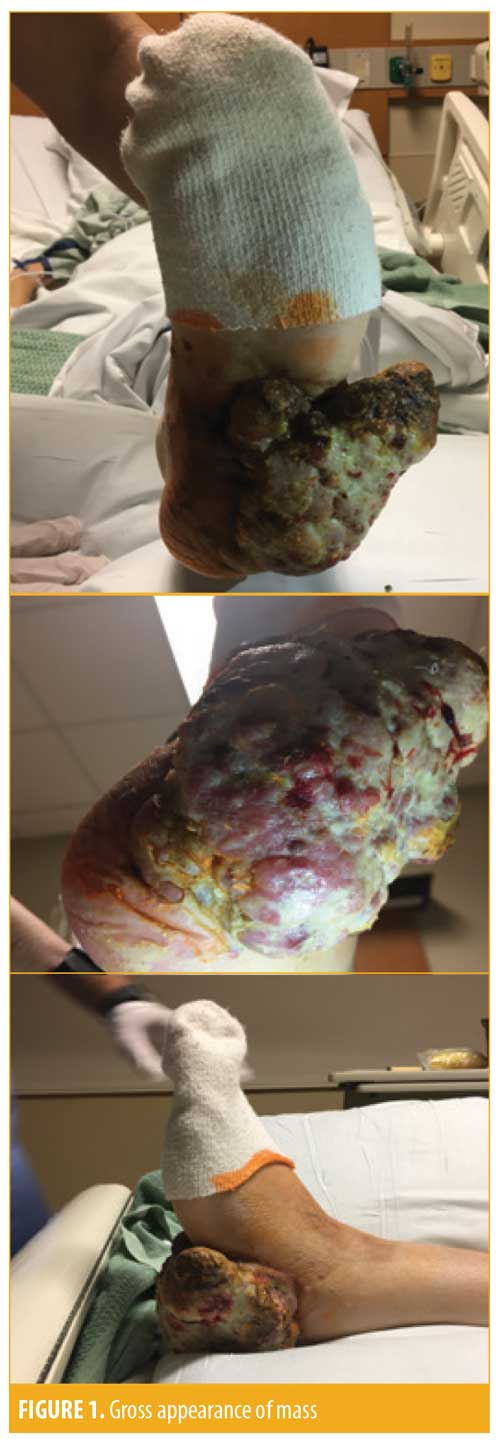 We present the case of a 47-year-old Caucasian woman who visited the emergency department with a growth on the plantar surface of her left heel that she stated had been increasing in size for at least one year. She denied any personal or family history of skin cancer. She was accompanied by her mother and brother to the emergency department. They stated that she lived alone and had refused to see a doctor since she was a child. Her family reported that they had brought her to the hospital after noticing a foul odor coming from her foot and discovering the growth that had been wrapped and hidden.
We present the case of a 47-year-old Caucasian woman who visited the emergency department with a growth on the plantar surface of her left heel that she stated had been increasing in size for at least one year. She denied any personal or family history of skin cancer. She was accompanied by her mother and brother to the emergency department. They stated that she lived alone and had refused to see a doctor since she was a child. Her family reported that they had brought her to the hospital after noticing a foul odor coming from her foot and discovering the growth that had been wrapped and hidden.
Upon further questioning, the patient stated that she had noticed a “coin-sized” mole on her foot, which had been present since birth, had begun growing in size several years ago. She attributed this to normal aging. When she first noted the lesion increasing in size, she sought treatment from a homeopathic physician who recommended using a black salve ointment. She was told that the black salve would cause the lesion to ulcerate, and she believed the treatment was working when she noticed the lesion ulcerating along with a foul smell. Of note, she also stated that her past medical history was positive for a self-diagnosed “groin hernia,” which she described as a softball-sized inguinal mass. She reported that the mass had developed one month prior to the presentation but had self-resolved after several days. She denied any past medical or surgical history and denied excess sunlight exposure or tanning.
Physical examination revealed a fungating mass measuring approximately 10cm×8cm on the plantar surface of the left foot. It was not painful to palpation and did not have gross drainage. However, a noticeably foul odor was present upon removing the bandaging. The patient also had painful, enlarged inguinal lymph nodes on the right side. Laboratory studies were essentially within normal limits, with the exception of an elevated lactate dehydrogenase level of 7,031U/L (normal level: 140–280U/L).
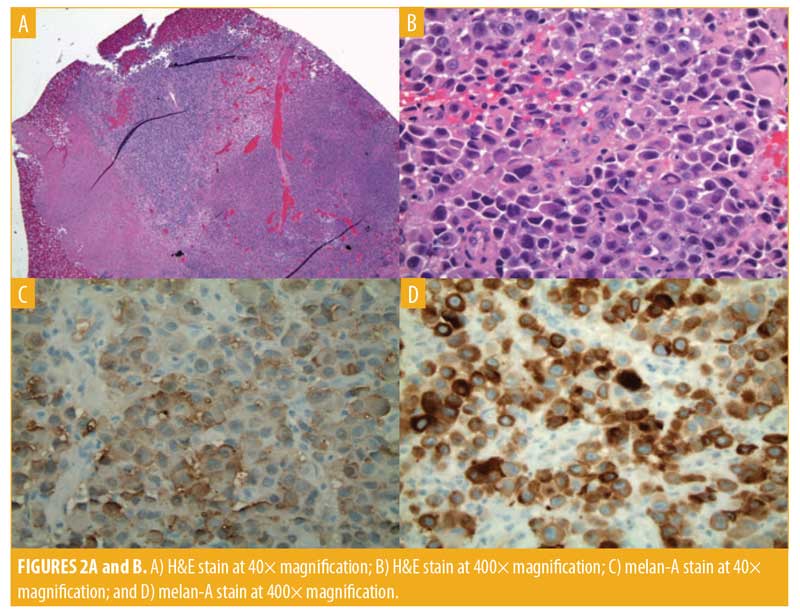
An incisional biopsy was performed. Morphology on hematoxylin and eosin (H&E) was consistent with melanoma. The histopathology report described sections of ulcerated, necrotic tissue. One mitotic figure was visualized from the sample, which appeared epithelioid in morphology. However, as noted above, this was taken from a small section of the tumor, and additional morphological features elsewhere within the tissue could not be excluded. The tissue stained positive for melan-A and S100 and negative for pancytokeratin.
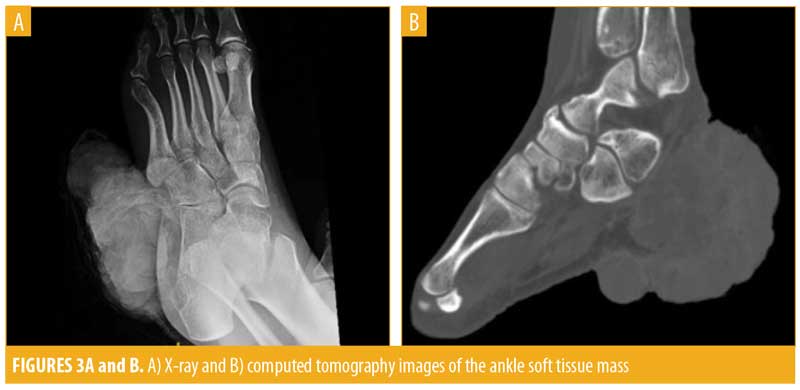
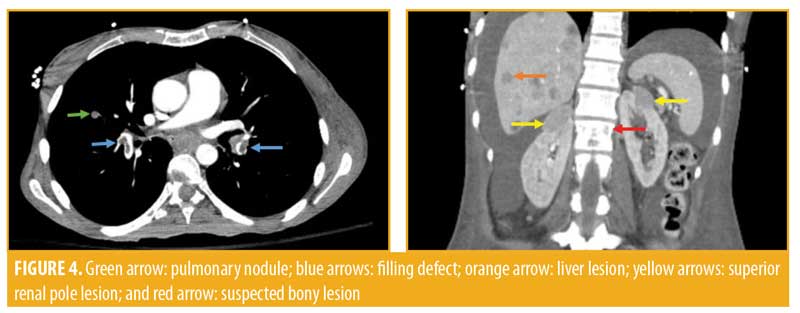
Computed tomography (CT) imaging of the left lower extremity revealed a large mass arising from the lateral aspect of the hindfoot extending to the dermis measuring 9.7cm×8.3cm, with further extension along the plantar heel and medial hindfoot. No bony involvement was observed. Imaging of the chest revealed diffuse pulmonary nodules, bilateral pulmonary emboli, bilateral supraclavicular adenopathy, and bilateral pleural effusions. Abdominal and pelvic scans showed innumerable small liver lesions, bilateral adrenal masses up to 2.4cm in size, and a mass in the superior pole of the left kidney measuring 2.1cm. A large retroperitoneal mass was visualized measuring 11.4cm × 6.0cm, which was anteriorly displacing the aorta and inferior vena cava and was presumed to be an enlarged lymph node. Diffuse adenopathy extended along the left iliac chain and a contiguous mass lesion extended from the femoral canal anteriorly. A subtle lucency was noted in the left L1 vertebral body. These findings were perceived to be indicative of Stage IV diffuse metastatic disease with T4bN3M1c staging.
The patient met severe sepsis criteria on presentation and was empirically started on intranveous vancomycin and piperacillin-tazobactam for the treatment of the soft tissue mass superinfection. However, she later refused antibiotic treatment, as she believed that the intravenous medicines were worsening her illness. She was transitioned to oral trimethoprim/sulfamethoxazole on the third hospital day and completed 10 days of antibiotic therapy. She was also started on anticoagulation with enoxaparin due to her bilateral pulmonary emboli, but subsequently refused treatment as well as further laboratory work.
Oncology was consulted to recommend treatment going forward, and a multidisciplinary discussion with members of the hematology/oncology, podiatry, surgery, and internal medicine teams was held to discuss the optimal treatment plan moving forward. Palliative surgery was not recommended given her poor nutritional status and the unlikely prospect of a surgical wound healing. The patient agreed to be started on intravenous pembrolizumab (Keytruda®, Merck & Co., Inc., Kenilworth, New Jersey), 2mg/kg every three weeks, and oral aipxaban (Eliquis®, Bristol-Myers Squibb Co., Princeton, New Jersey; Pfizer, Inc., New York, New York), 2.5mg twice daily, on the seventh hospital day.
On Day 19, the patient displayed decreased renal function. Nephrology was consulted with a concern for tumor lysis syndrome. However, the differential diagnosis also included drug toxicity secondary to pembrolizumab. A renal ultrasound ruled out hydronephrosis and obstruction. The patient was also hyponatremic (sodium: 126mEq/L) and hyperkalemic (potassium: 5.6mEq/L) secondary to adrenal failure, which was consistent with suspected adrenal metastasis on imaging. At this point, she was started on steroids (1g of intravenous solumedrol daily for 3 doses followed by 40mg of oral prednisone) and daily allopurinol. The patient also had uric acid level of 18.9mg/dL and received three doses of intravenous rasburicase, resulting in improvement to her serum uric acid. Her clinical status continued to worsen due to disease progression and multi-organ failure. She was not a candidate for the second dose of pembrolizumab after three weeks. She became encephalopathic secondary to hepatic and renal failure and was transitioned to palliative care. The patient died 32 days after emergency department presentation and admission.
Discussion
Several management options for our patient were discussed by an interdisciplinary team. There is currently no consensus in the literature about the optimal management for metastatic melanoma. Most commonly, patients are treated with chemotherapy and/or immunotherapy. There have been many recent advances in the treatment of advanced melanoma with the advent of BRAF inhibitors in combination with MEK inhibitors, programmed cell death protein 1 (PD-1) inhibitors (e.g., pembrolizumab), and immunotherapies, with more cancer targets currently being developed.However, response rates remain poor, with only moderate increases in median survival.1,6
Curative surgical resection was not a possibility in our patient due to the extent of disease present on imaging studies. Palliative surgical resection was considered for the patient, but it was decided that she was a poor surgical candidate and that resection would have a minimal or even negative impact on quality of life. The patient also voiced the desire not undergo any surgical procedures. We elected to treat the patient with a PD-1 antibody, pembrolizumab, which is approved for therapy of unresectable metastatic melanoma in the United States and Europe. Some studies have shown that PD-1 inhibitors can achieve a two-year survival rate of 50 percent.1 Unfortunately, after one dose, our patient quickly deteriorated to the point that she was no longer a candidate to continue receiving pembrolizumab.
It is rare in the United States for patients to present with grossly advanced giant melanoma as our patient did. Most commonly, the patient seeks treatment before the tumor enlarges. Massive melanomas are almost always the product of a combination of neglect and patient denial. Due to the size and advanced stage of our patient’s melanoma, it was not possible to determine the original histological subtype from the small incisional biopsy taken. We believe that our patient most likely had an acral lentiginous melanoma, based on her description of evolution and this being the most common type of melanoma of the foot. However, in such advanced cases, the histological type does not have prognostic significance.
In non-Caucasian people, the most common sites of melanoma are the hands, feet, and nails, and in Caucasian people, the most common site is the posterior legs in women and the back in men.3 Melanoma of the foot in a Caucasian women, such as in our patient, is fairly uncommon.3 Our review of the literature yielded only a handful of reports of giant primary cutaneous melanomas. Furthermore, we found only two papers describing a total of three cases of giant primary melanoma of the foot.7,10 Zou and Lam10 report two Chinese patients, a 35-year-old woman, and a 72-year-old men who both developed 8-cm melanomas of the foot. Bartoš and Štofová7 report a Slovakian patient with an 8cm×4cm melanoma of the foot, which had been growing for three years. Our patient’s tumor was 9.7cm×8.4cm, which makes it larger than any cutaneous melanoma reported in the currently available literature.
This case is unique in that our patient did not seek care from a traditional physician for several years after first noticing the changing lesion on her foot. However, this aligns with her history of refusing to see a doctor since childhood, instead turning to homeopathy for treatment. Use of the internet makes it easier for patients to search for alternative medicine and “natural” treatments for their skin lesions. Escharotic agents like black salve have a long history of being used to treat skin lesions and cancerous growths and have been used by Native Americans for centuries.13 A Google search produced a handful of websites selling variations of black salve products containing Sanguinaria under different names, including Cansema® (Alpha Omega Labs, Guayaquil, Ecuador) and HerbVeil8® (Altered States). They label black salve as a safe and natural cure for basal cell carcinoma, squamous cell carcinoma, and melanoma despite no randomized, clinical trials demonstrating efficacy or FDA approval.11–13,15
Leecy et al15 published a report of 16 case studies and a review of the literature in which black salve was applied to cutaneous lesions, finding that the majority of outcomes included dermal scarring, necrosis, ulceration, a need for reconstructive surgery, recurrence of cancer, and death. While it is true that treatment with an escharotic-like black salve can cause an inflammatory tissue response with scarring and fibrosis, there is no histological evidence to support the claim that black salve leads to selective destruction of neoplastic cells.15 There are only two case reports in the literature of attempting to treat melanoma with black salve. Both patients in the case reports progressed to metastatic melanoma.12,16
Lack of regulation of information published on the internet makes false, misleading information easily accessible to the public. This allows the social media user next door and alternative medicine websites professing the efficacy of black salve to promote these “natural” remedies without mention of the possible side effects, toxicity, and negative outcomes due to delayed medical care. It is possible that the homeopathic physician who treated our patient did not provide her with the likely diagnosis of melanoma or enough information to make an educated decision when she was instructed to treat the lesion with black salve. It is also feasible that the patient ignored advice to seek medical care and chose to solely treat with black salve. This case demonstrates the importance of educating the public and all health care providers about the “ABCDEs” of melanoma and the importance of early detection along with the potential dangers of treatment with natural remedies such as black salve.
Conclusion
Our patient’s late presentation with advanced metastatic massive (i.e., giant) cutaneous melanoma resulted in an unpreventable outcome. If the patient had presented when she first noticed the lesion increasing in size, instead of treating it homeopathically with black salve, her prognosis could have been much better. However, the patient exercised her personal right to seek the treatment of her choice. This case report emphasizes the importance of early recognition of melanoma and the potential dangers of homeopathic treatment with black salve. It is essential to increase regulation of escharotic agents like black salve and awareness among patients regarding the possible outcomes and dangers inherent with their use.
Acknowledgments
The authors wish to thank Dr. Michele McElroy (pathology) for her help and technical assistance.
References
- Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline—update 2016. Eur J Cancer. 2016;63:201–217.
- Heymann WR. Screening for melanoma. J Am Acad Dermatol. 2007;56(1):144–145.
- Nam KW, Bae YC, Nam SB, et al. Characteristics and treatment of cutaneous melanoma of the foot. Arch Plast Surg. 2016;43(1):59–65.
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36): 6199–6206.
- Seidenari S, Fabiano A, Al Jalbout S, et al. Relationship between histological and computer-based assessment of melanoma diameter and thickness in head versus trunk-limbs melanomas. Head Neck Oncol. 2013;5:32.
- Harting M, Tarrant W, Kovitz CA, et al. Massive nodular melanoma: a case report. Available at: http://escholarship.org/uc/item/08m9k707. Accessed April 3, 2018.
- Bartoš V, Štofová Z. Giant primary melanoma of the skin arising on the left foot. Our Dermatol Online. 2016;7(1):54–58.
- Grisham AD. Giant melanoma: novel problem, same approach. South Med J. 2010;103(11): 1161–1162.
- Ching JA, Gould L. Giant scalp melanoma: a case report and review of the literature. Eplasty. 2012;12:e51.
- Zou Y, Lam A. Giant primary plantar melanoma. J Eur Acad Dermatol Venereol. 2009;23(3):361.
- Garner-Wizard M. Re: efficacy and safety of the topical use of black salve. Available at: http://cms.herbalgram.org/herbstream/ima/Herbclip/index.html#param.wapp?sw_page=@@@@@@@@review%3Fufgp%3D511/061443-511.html. Accessed April 3, 2018.
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4(3):77–80.
- Black Salve.biz. Available at: blacksalve.biz. Accessed April 24, 2018.
- Krajewski A, Garg M, Chandawarkar RY. Topical herbal remedies: research opportunities for plastic surgeons. J Plast Reconstr Aesthet Surg. 2010;63(6):896–905.
- Leecy TN, Beer TW, Harvey NT, et al. Histopathological features associated with application of black salve to cutaneous lesions: a series of 16 cases and review of the literature. Pathology. 2013 Dec;45(7):670–674.
- Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16(10):1125–1127.

