 J Clin Aesthet Dermatol. 2022;15(8):22–26.
J Clin Aesthet Dermatol. 2022;15(8):22–26.
by Ahmed Ibrahim Abd Elneam, MD; Ghadah Alhetheli, MD; Mohammed Saleh Al-Dhubaibi, MD; Ali Ismaeil Ali Abd Alrheam, MD;
and Ahmed El-sayed Hassan, MD
Dr. Abd Elneam AI is with the Department of Clinical Biochemistry, the Department of Basic Medical Sciences, and College of Medicine at Shaqra University in Dawadmi, Saudi Arabia; and Molecular Genetics and Enzymology Department, Human Genetics and Genome Research Institute in Cairo, Egypt; and the National Research Center in Cairo, Egypt. Dr. Ghadah Alhetheli is with the Division of Dermatology and Cutaneous Surgery and College of Medicine at Qassim University in Buraydah, Saudi Arabia. Dr. Mohammed Saleh Al-Dhubaibi is with the Departments of Dermatology and College of Medicine at Shaqra University in Dawadmi, Saudi Arabia. Dr. Ali Ismaeil Ali Abd Alrheam is with the Clinical Laboratory Science Department College of Applied Medical Sciences at Shaqra University in Dawadmi, Saudi Arabia. Dr. Ahmed El-Sayed Hassan is with the Department of Medical Physiology and Faculty of Medicine at Zagazig University in Zagazig, Egypt and the Departments of Basic Medical Science, Physiology unit, and College of Medicine at Shaqra University in Dawadmi, Saudi Arabia.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Psoriasis vulgaris is a chronic, relapsing, inflammatory disorder marked by an intensified immune response. The role of immunogenetics in psoriasis is still poorly understood; however, experts agree that its expression depends on proinflammatory cytokines. Forkhead box class O3A (FOXO3a), a transcription factor, plays a crucial role in intercellular regulation, oxidative stress, deoxyribonucleic acid (DNA) repair, and cell death.
Objective. The objective of this study was to investigate the role of FOXO3a genetic polymorphism as a risk factor for psoriasis vulgaris and assess its possible relationship with disease severity.
Methods. A comparative case-control study included 53 patients with psoriasis and 41 matched healthy controls. We measured serum FOXO3a levels and used the PCR-RFLEP technique to detect FOXO3a genetic polymorphism (rs13217795) in both groups.
Results. Our results revealed significantly higher serum FOXO3a levels in the psoriasis group compared to the control group (p≤0.001). Serum FOXO3a levels were significantly higher in patients with severe psoriasis than in those with mild-to-moderate disease. FOXO3a genotypes found homozygous mutant genotype (TT) was substantially more frequent in the psoriasis group than in the control group. Furthermore, the T allele was more frequent in the psoriasis group than in the control group.
Conclusion. The study indicates that rs13217795 polymorphism of the FOXO3a gene is strongly associated with susceptibility to psoriasis. Also, the serum level of FOXO3a is significantly higher in patients with severe psoriasis, compared to patients with mild-to-moderate psoriasis. This finding could be an area of future targeted therapy.
Keywords: Psoriasis vulgaris, Serum FOXO3a, FOXO3a gene.
Psoriasis is a chronic, relapsing, immune-mediated, inflammatory, cutaneous, systemic disorder affecting children and adults, with a strong genetic predisposition and autoimmune pathogenic traits. Psoriasis can impact patient quality of life.1 The pathogenesis is closely related to abnormal interactions among innate immunity e.g., (T cells, keratinocytes). Immune cells in patients with psoriasis release excess proinflammatory factors, leading to uncontrollable activation of the innate and adaptive immune system, such as the nuclear factor-κB (NF-kB) signaling pathway and the differentiation of T helper (Th) cells toward Th1 and/or Th17 cells.1
Psoriasis affects both sexes equally.2 The worldwide prevalence of psoriasis varies between two and three percent, making psoriasis a serious global problem with regional variations.2 In the United States, psoriasis affects approximately 3.2 percent of adults.3 It is less prevalent among Asians and some African groups but affects 11 percent of Caucasian and Scandinavian populations.4-7
Psoriasis has different clinical genotypes and phenotypes.8, 9 The clinical subtypes of psoriasis vary, including plaque, pustular, guttate, nail, inverse, and erythrodermic. According to recent studies, approximately 30 to 33 percent of patients with psoriasis develop psoriatic arthritis, which mostly appears after cutaneous manifestations but can proceed or coexist with cutaneous psoriasis.10
Psoriasis can develop at any time of life. However, the mean age of onset for psoriasis vulgaris is 33 years, with 75 percent of cases occurring before the age of 46 years.11 The age of onset seems to be slightly earlier in female patients than in male patients.12
Forkhead box class O (FOXO) proteins are a subclass of the FOXO family, including FOXO1, FOXO3a, FOXO4, and FOXO6.13 FOXO proteins play crucial roles in cellular processes, such as deoxyribonucleic acid (DNA) repair, apoptosis, cell-cycle regulation, and reactive oxygen species scavenger.14-16
FOXO3a, a transcription factor of the FOXO family, is one of the most abundant FOXO isoforms and is involved in several biological processes by regulating the expression of genes involved in cell-cycle progression, apoptosis, differentiation, metabolism, and or stress resistance.17
At present, there are limited studies focused on the role of FOXO3a gene polymorphism as a risk factor for psoriasis. Pektas et al13 found that rs4946936 polymorphism of FOXO3a was associated with early-onset psoriasis, and Gaoetal18 reported that one single-nucleotide polymorphisms (SNP) (rs3761548) to be associated with a risk of developing psoriasis. Several studies on the FOXO3a gene polymorphism have indicated an association between FOXO3a and other inflammatory and autoimmune diseases.19-21
Oxidative stress and inflammation play an essential role in the pathophysiology of psoriasis. Proinflammatory cytokines, such as nuclear factor-κB (NF-κB) Th1cytokines, tumor necrosis factor-alpha (TNF-α) and natural killer cells are involved in the pathophysiology of psoraisis.22
This study investigated FOXO3a genetic polymorphism as a risk factor for developing psoriasis vulgaris.
Methods
Subjects. We conducted this comparative case-control study in an outpatient dermatology clinic at Qassim University, Saudi Arabia, between December 2019 and February 2021.The study was conducted in accordance with the Declaration of Helsinki and was approved by the Local Ethical Committee. We obtained written informed consent from all the participants before enrollment and after explaining the aim and nature of the study.
Following the inclusion criteria of our study, we selected male and female patients at least 18 years of age with a clinically established diagnosis of psoriasis but with no systemic involvement. Patients were excluded if they were or had been taking any systemic, topical, or phototherapy treatment within eight weeks prior to the start of the study. Patients were also excluded if they were pregnant or lactating, had maliagnancies, active liver disease, renal disorders, infections, or a history of alcohol abuse.
Overall, we enrolled 53 patients with a clinically established diagnosis of psoriasis (24 male and 29 female) in the study. A certified dermatologist thoroughly examined and classified the patients based on the severity of their psoriasis as measured by the body surface area (BSA) affected by the disease. Patients had severe psoriasis vulgaris if greater than 10 percent of their BSA was affected, moderate if 5 to 10 percent of their BSA was affected, and mild if less than five percent of their BSA was affected.23-25
The control group included 41 age- and sex-matched healthy controls (22 male and 19 female), with no clinical evidence or family history of psoriasis or other autoimmune disorders. Both groups underwent complete clinical examinations, genetics studies, and biochemical tests.
Genomic DNA extraction. We collected venous blood samples on Na2EDTA as an anticoagulant. According to the manufacturer’s instructions for blood protocol, we purified genetic DNA from 200μl whole blood with the QIAamp® DNA BloodMini Kit (Qiagen, Hilden, Germany).
Detection of FOXO3a polymorphism using PCR-RFLP (polymerase chain reaction-restriction fragment length polymorphism). We amplified genomic DNA fragments by using the PCR method with a pair of oligonucleotide primers to determine the genotyping of FOXO3a polymorphism. The upstream of the primer sequence was 5′-ATG AGT GAA GAT GGA AGT AAG C -3′. We blasted the primers to the gene bank database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). We performed amplification using a 2X PCR master mix solution (iTaqTM) (iNtRON Biotechnology, Korea) in a total volume of 20μl. For PCR amplification, 35 cycles consisting of 30 seconds of denaturation at 95°C, 30 seconds of annealing at 62°C, extension for one minute at 72°C, and a final extension at 72°C for five minutes followed an initial denaturation at 95 °C for five minutes. The enzyme PagI (Thermo Scientific, Waltham, Massachusetts, USA) subjected amplification products to restriction digestion. We separated fragments on 2% agarose gel and ethidium bromide staining under ultraviolet light (UV) visualized bands. This reaction yielded one fragment of 667bp indicating a homozygous wild genotype (CC) or two 391 and 275bp fragments indicating a homozygous mutant genotype (TT). In contrast, the presence of 677,391 and 275 bp products showed heterozygous genotype (CT).26
Measurement of serum FOXO3a levels.We measured serum FOXO3a levels using a human enzyme-linked immunosorbent assay (ELISA) kit (Cusabio Biotech Co., Newark, New Jersey, USA) according to the manufacturer’s instructions. We calculated the levels of FOXO3a from a standard curve and expressed them in picograms per milliliter (pg/mL).14
Statistical analysis. We analyzed recorded data using the Statistical Package for Social Sciences, version 22.0 (SPSS Inc., Chicago, Illinois, USA). We performed an analysis of variance (ANOVA) between more than two means t-tests and chi-squares to analyze the mean and percentage differences. We considered P≤0.001 to be highly significant and deemed P≥0.05 to be insignificant. Furthermore, we expressed the quantitative data as a mean ± standard deviation (SD) and the qualitative data as frequency and percentage.
Results
Our study analyzed FOXO3a gene with polymorphism in 53 patients with psoriasis (24 male and 29 female) and 41 healthy controls (22 male and 19 female). Table 1 shows the demographic and clinical data of both the patients and control groups. We detected no statistically significant differences in the mean ages between the groups (P=0.421). As shown in Table 1, we found that the serum levels of FOXO3a were statistically significantly higher in the psoriasis group than in the healthy control group (P≤0.001).
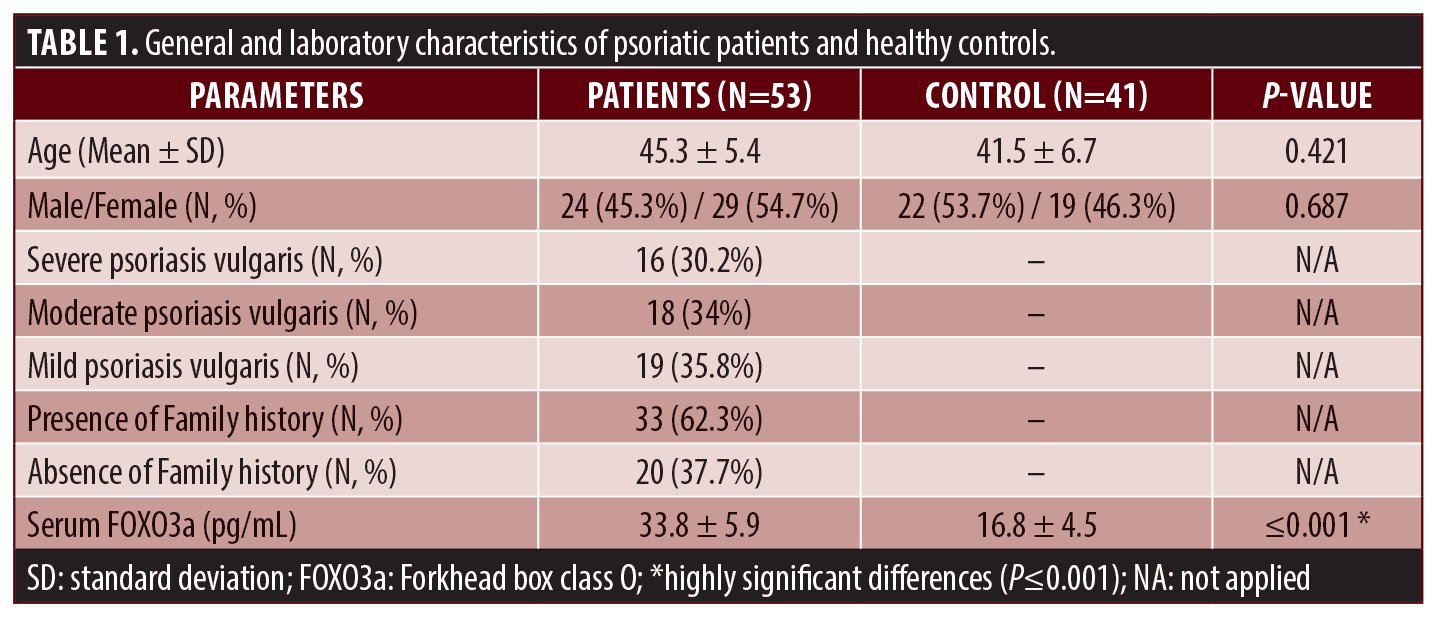
We amplified the specific segment of FOXO3a gene polymorphism (rs13217795) into 667bp and then digested it with PagI restriction enzyme, where 667bp indicated a CC. Two fragments of 391 and 275bp indicated TT. In contrast, the presence of 677,391 and 275bp products showed CT (Figures 1a,1b).
Comparison of FOXO3a gene polymorphism genotypes between patients and control groups showed that in the psoriasis group, TT was significantly more common (54.6%) (P≤0.001), followed by CC (28.3%), then CT (17.2%). In the healthy control group, CC was significantly more common (53.7%) (P≤0.001) followed by CT and TT genotypes (23.3% and 23%, respectively) (Table 2).
The allele frequency was significantly different between the psoriasis group and the healthy control groups (P≤0.001). The results indicated that the T allele was over-represented consistently in the psoriasis group compared to the control group (63.2% vs. 34.2%) (P≤0.001) (Table 3). The C allele levels were significantly higher in the healthy control group.
Our data indicated that patients with FOXO3a gene TT genotypes have statistically significantly higher serum FOXO3a levels (P≤0.001), as shown in Table 4.
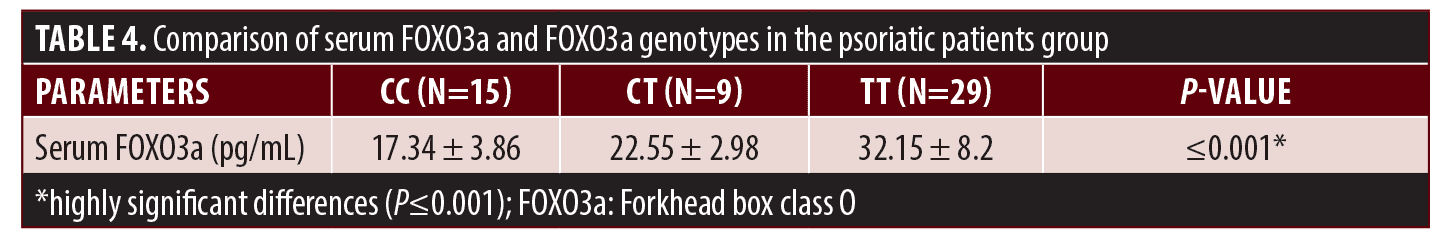
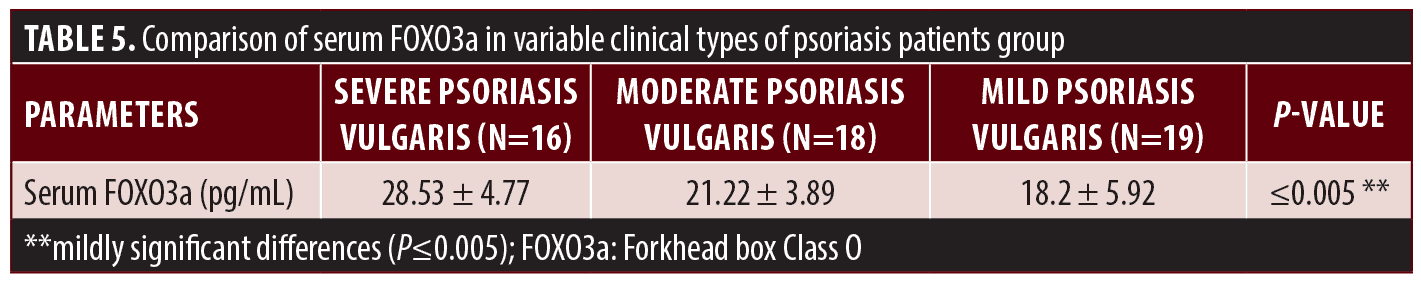
The comparison between serum levels of FOXO3a in variable clinical types of psoriasis indicated that the serum level of FOXO3a was significantly higher in patients with severe psoriasis vulgaris than in those with mild-to-moderate psoriasis vulgaris with (P≤0.005) as shown in Table 5.
Discussion
Clinical studies investigating the FOXO3a genetic polymorphism in psoriasis are scarce. This study clearly showed a significant association between FOXO3a genetic polymorphism and its serum level and psoriasis. Our study reinforces the findings of Pektas et al, who first reported such an association.13 Our results indicate that FOXO3a rs13217795 gene polymorphism is associated with psoriasis and strongly associated with disease severity.
Phosphatidylinositol-4, 5-bisphosphate 3-kinase (PI3K)/protein kinase B (AKT) signaling cascade contributes markedly to cell proliferation. In this cascade, PI3K and AKT are the upstreaming molecules that connect the ligation of the phosphorylation and activation state of FOXO and mammalian target of Rapamycin complex (mTOR) with growth factor receptor (GFR).27,28
PI3K/AKT signaling enhances cell survival by phosphorylating and inhibiting a FOXO transcription factor. It further motivates cellular proliferation by inactivating cell cycle inhibitors (p27 and p21) while activating the cell cycle proteins (c-Myc and cyclin D1).29-31
AKT phosphorylates FOXO, resulting in FOXO’s confinement in the cytoplasm. Withdrawing survival factors results in dephosphorylation of FOXO, nuclear translocation, and eventually target gene activation. Inside the nucleus, FOXO provokes apoptosis and inhibits proliferation, mainly by inducing the expression of crucial genes for cell death and growth.31 This indicates that the PI3K–AKT–FOXO signaling network is a central intracellular axis regulating cellular proliferation.32 In the epidermis, PI3K/AKT signaling is associated with keratinocyte survival and differentiation and allows the function of the epidermal keratinocyte terminal differentiation program through phosphorylation and inhibition of the FOXO transcription factor.33-35
Psoriasis is a chronic, multifactorial, inflammatory autoimmune disease reflecting hyperproliferative and less differentiated epidermal keratinocytes. Over the years, studies have confirmed that abnormal T-lymphocytes are strongly linked with the pathogenesis of psoriasis. However, the exact immunogenetic etiology behind excessive proliferation besides the defect in keratinocyte apoptosis remains unclear.36
Recent studies have identified the vital pathophysiological role of FOXO3a in various aspects of cell-cycle regulation, apoptosis, and oxidative stress through controlling the expression of target genes.29,37,38 Some research has revealed the important role of oxidative stress and genetic tendency in several dermatological disorders, such as vitiligo and psoriasis.14, 29
Ozel Turkcu et al14 found an association between vitiligo and FOXO3a gene polymorphism and suggested that rs4946936 polymorphism of the FOXO3a gene might be associated with predisposition to vitiligo. Moreover, they found decreased serum FOXO3a levels and catalase activity and increased superoxide dismutase antioxidant enzyme activities in patients with vitiligo. The authors suggested that low serum levels of FOXO3a might be related to oxidative stress and that FOXO3a might regulate superoxide dismutase and catalase antioxidant enzyme activity by removing superoxide anions and H2O2.14
In another study, Pektas et al13 reported a significant association between rs4946936 polymorphism of FOXO3a gene and early-onset psoriasis (EOP) and its severity, suggesting the C allele of rs4946936 polymorphism of the FOXO3a gene is a risk factor for EOP. Their results also showed significantly higher CC frequency in nail retention of EOP.
Our study is in line with the findings of Pektas et al13 by showing a significant association between FOXO3a gene polymorphism and psoriasis. Our results indicate the TT genotype of rs13217795 of the FOXO3a gene and T allele frequency are significantly higher in patients with psoriasis (P≤0.001) (63.2%), compared to healthy controls. Furthermore, our results revealed a significantly higher serum level of TT genotype of rs13217795 of the FOXO3a gene in the psoriasis group, compared to the control group. Interestingly, serum FOXO3a was significantly directly proportional to disease severity (P≤0.005). Several previous studies of polymorphism of FOXO3a have revealed an association between FOXO3a and other inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel diseases, and chronic obstructive pulmonary disease.30-42
Our study findings raise a question about the molecular mechanism behind the association between FOXO3a gene polymorphism and the pathogenesis of psoriasis. Also, our study opens a future investigative window for possible therapeutic options based on the FOXO3a transcription factor and blocking of the PI3K–AKT–FOXO signaling axis.
Conclusion
Our study was the second to investigate the association between FOXO3a gene polymorphism and its serum level and psoriasis. The findings indicate that the rs13217795 polymorphism of the FOXO3a gene is strongly associated with susceptibility to psoriasis. Also, we found significantly higher serum levels of FOXO3a in patients with severe psoriasis than in patients with mild-to-moderate psoriasis. This finding raises questions about the molecular mechanism behind this association and possible target therapy focusing on FOXO3a transcription factor and blocking the PI3K–AKT–FOXO signaling axis. Studies with larger sample sizes are necessary to support our finidngs. Additionally, studies examining serum levels of FOXO3a in patients with early versus late onset psoriasis may be helpful.
Limitations. The small sample size of our study limits our findings. Further studies with a large sample size are required to investigate the molecular mechanism behind the association between FOXO3a gene polymorphism and the pathogenesis of psoriasis and to study other gene polymorphisms.
References
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. Published 2016 Nov 24.
- Christophers E. Psoriasis–epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–946.
- Parisi R, Symmons DP, Griffiths CE, et al. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Gibbs S. Skin disease and socioeconomic conditions in rural Africa: Tanzania. Int J Dermatol. 1996;35(9):633–639.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Danielsen K, Olsen AO, Wilsgaard T, et al. Is the prevalence of psoriasis increasing? A 30-year follow-up of a population-based cohort. Br J Dermatol. 2013;168(6):1303–1310. doi:10.1111/bjd.12230
- Ranza R, Carneiro S, Qureshi AA, et al. prevalence of psoriatic arthritis in a large cohort of Brazilian patients with psoriasis. J Rheumatol. 2015;42(5):829–834.
- Mease PJ, Gladman DD, Papp KA, et al. prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509.
- Nevitt GJ, Hutchinson PE. Psoriasis in the community: Prevalence, severity, and patients’ beliefs and attitudes towards the disease. Br J Dermatol. 1996;135(4): 533–537.
- Henseler T, Christophers E. Psoriasis of early and late onset: Characterization of two types of psoriasis vulgaris. J Am Acad Dermatol. 1985;13(3):450–456.
- Pektas SD, Dogan G, Edgunlu TG, et al. The role of Forkhead Box Class O3A and SIRT1 gene variants in early-onset psoriasis. Indian J Dermatol. 2018;63(3):208–214.
- Ozel Turkcu U, Solak Tekin N, Gokdogan Edgunlu T, et al. The association of FOXO3a gene polymorphisms with serum FOXO3a levels and oxidative stress markers in vitiligo patients. Gene. 2014;536(1):129–134.
- Srivastava RK, Unterman TG, Shankar S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol Cell Biochem. 2010;337(1–2):201–212.
- Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, et al. Redox regulation of FOXO transcription factors. Redox Biol. 2015;6:51–72.
- Lam EW, Brosens JJ, Gomes AR, et al. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13(7):482–495.
- Gao L, Li K, Li F, et al. Polymorphisms in the FOXP3 gene in Han Chinese psoriasis patients. J Dermatol Sci. 2010;57(1):51–56.
- Oda JM, Hirata BK, Guembarovski RL, et al. Genetic polymorphism in FOXP3 gene: Imbalance in regulatory T-cell role and development of human diseases. J Genet. 2013;92(1):163–171.
- Lin YC, Lee JH, Wu AS, et al. Association of single-nucleotide polymorphisms in FOXP3 gene with systemic lupus erythematosus susceptibility: A case-control study. Lupus. 2011;20(2):137–143.
- D’Amico F, Skarmoutsou E, Marchini M, et al. Genetic polymorphisms of FOXP3 in Italian patients with systemic sclerosis. Immunol Lett. 2013;152(2):109–113.
- Donn RP, Plant D, Jury F, et al. Macrophage migration inhibitory factor gene polymorphism is associated with psoriasis. J Invest Dermatol. 2004;123(3):484–487.
- Ramsay B, Lawrence CM. Measurement of involved surface area in patients with psoriasis. Br J Dermatol. 1991;124(6):565–570.
- Long CC, Finlay AY, Averill RW. The rule of hand: 4 hand areas = 2 FTU = 1 g. Arch Dermatol. 1992;128(8):1129–1130.
- Langley RG, Ellis CN. Evaluating psoriasis with Psoriasis Area and Severity Index, Psoriasis Global Assessment, and Lattice System Physician’s Global Assessment. J Am Acad Dermatol. 2004;51(4):563–569.
- Khattab RA, Ahmed HM, Doudar NA. Association study of FOXO3a single-nucleotide polymorphism and bronchial asthma in Egyptian children. Egypt J Immunol. 2020;27(1):1–8.
- Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway–beyond rapalogs. Oncotarget. 2010;1(7):530–543.
- Scott PH, Brunn GJ, Kohn AD, et al. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci U S A. 1998; 95(13):7772–7777.
- Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868.
- Diehl JA, Cheng M, Roussel MF, et al. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–3511.
- Lizcano JM, Alessi DR. The insulin signalling pathway. Curr Biol. 2002;12(7):R236–R238.
- Coffre M, Benhamou D, Rieß D, et al. miRNAs are essential for the regulation of the PI3K/AKT/FOXO pathway and receptor editing during B Cell maturation. Cell Rep. 2016;17(9):2271–2285.
- Calautti E, Li J, Saoncella S, et al. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;280(38):32856–32865.
- O’Shaughnessy RF, Welti JC, Cooke JC, et al. AKT-dependent HspB1 (Hsp27) activity in epidermal differentiation. J Biol Chem. 2007;282(23):17297–17305.
- Peng XD, Xu PZ, Chen ML, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17(11):1352–1365.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29(1):3–9.
- Kedenko L, Lamina C, Kedenko I, et al. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med Genet. 2014;15:112. Published 2014 Oct 2.
- Furukawa-Hibi Y, Kobayashi Y, Chen C, et al. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7(5–6):752–760.
- Emre S, Demirseren DD, Alisik M, et al. Dynamic thiol/disulfide homeostasis and effects of smoking on homeostasis parameters in patients with psoriasis. Cutan Ocul Toxicol. 2017;36(4):393–396.
- Ludikhuize J, de Launay D, Groot D, et al. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum. 2007;56(7):2180–2191.
- Snoeks L, Weber CR, Wasland K, et al. Tumor suppressor FOXO3 participates in the regulation of intestinal inflammation. Lab Invest. 2009;89(9):1053–1062.
- Kalemci S, Edgunlu TG, Kara M, et al. Sirtuin gene polymorphisms are associated with chronic obstructive pulmonary disease in patients in Muğla province. Kardiochir Torakochirurgia Pol. 2014;11(3):306–310.

