 J Clin Aesthet Dermatol. 2022;15(8):16–21.
J Clin Aesthet Dermatol. 2022;15(8):16–21.
by Natthachat Jurairattanaporn, MD, MSc; Poonkiat Suchonwanit, MD; Teerapong Rattananukrom, MD, MSc; and Vasanop Vachiramon, MD, MSc
All authors are with the Division of Dermatology, the Faculty of Medicine Ramathibodi Hospital at Mahidol University in Bangkok, Thailand. Dr. Jurairattanaporn is also with the Department of Dermatology, Faculty of Medicine at Srinakharinwirot University in Bangkok, Thailand.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Postinflammatory hyperpigmentation (PIH) is a common problem, especially in patients with darker skin tones. It can occur on any area of the body following external injuries or intense inflammatory conditions. However, there is limited evidence regarding the differences in dermatoscopic patterns between facial acne-related PIH and nonfacial acne-related PIH.
Objective. We sought to determine the dermatoscopic features of acne-related PIH in facial and nonfacial areas in an Asian population.
Methods. Patients with acne-related PIH in both facial and nonfacial areas were enrolled. Baseline demographic data, location, and duration of PIH were recorded. Dermatoscopic and clinical pictures of each patient were taken from the darkest PIH lesions of both areas. Differences in dermatoscopic patterns were analyzed.
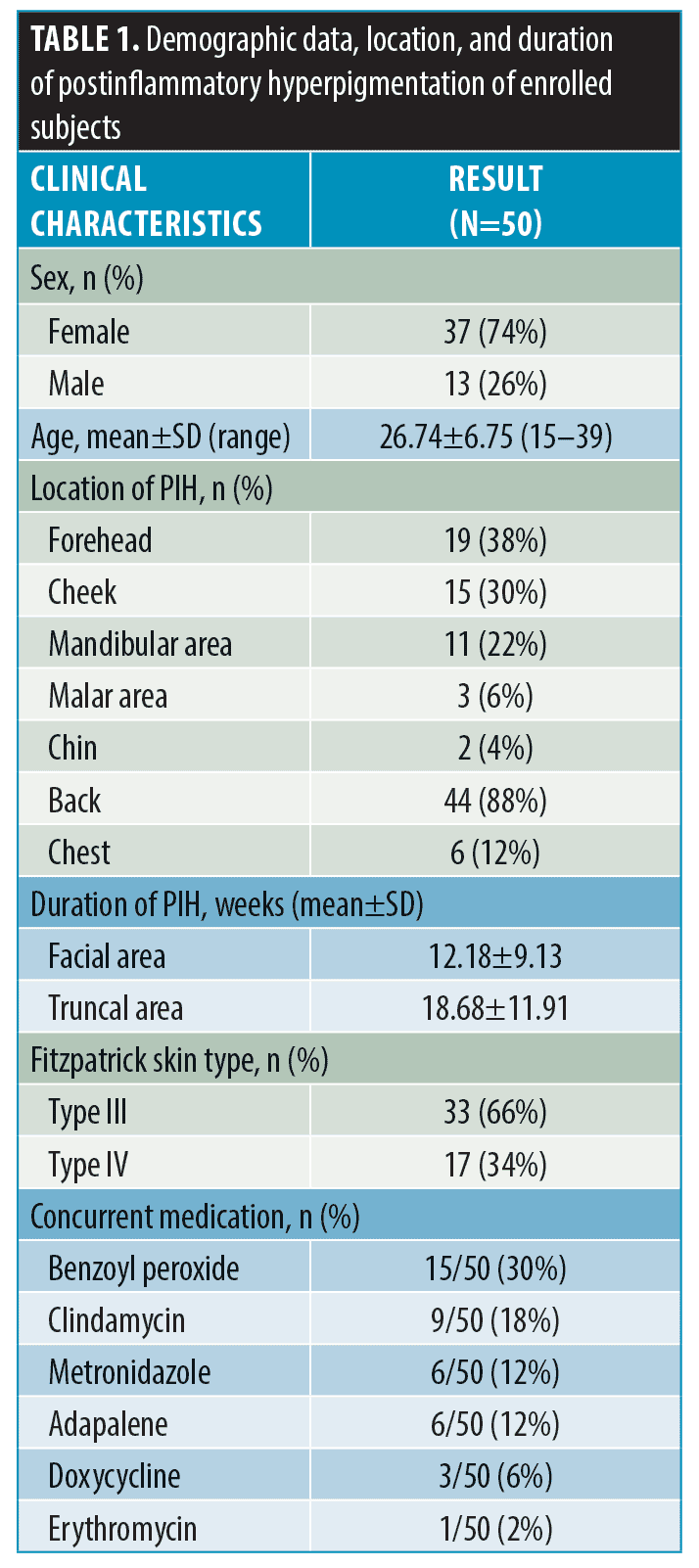
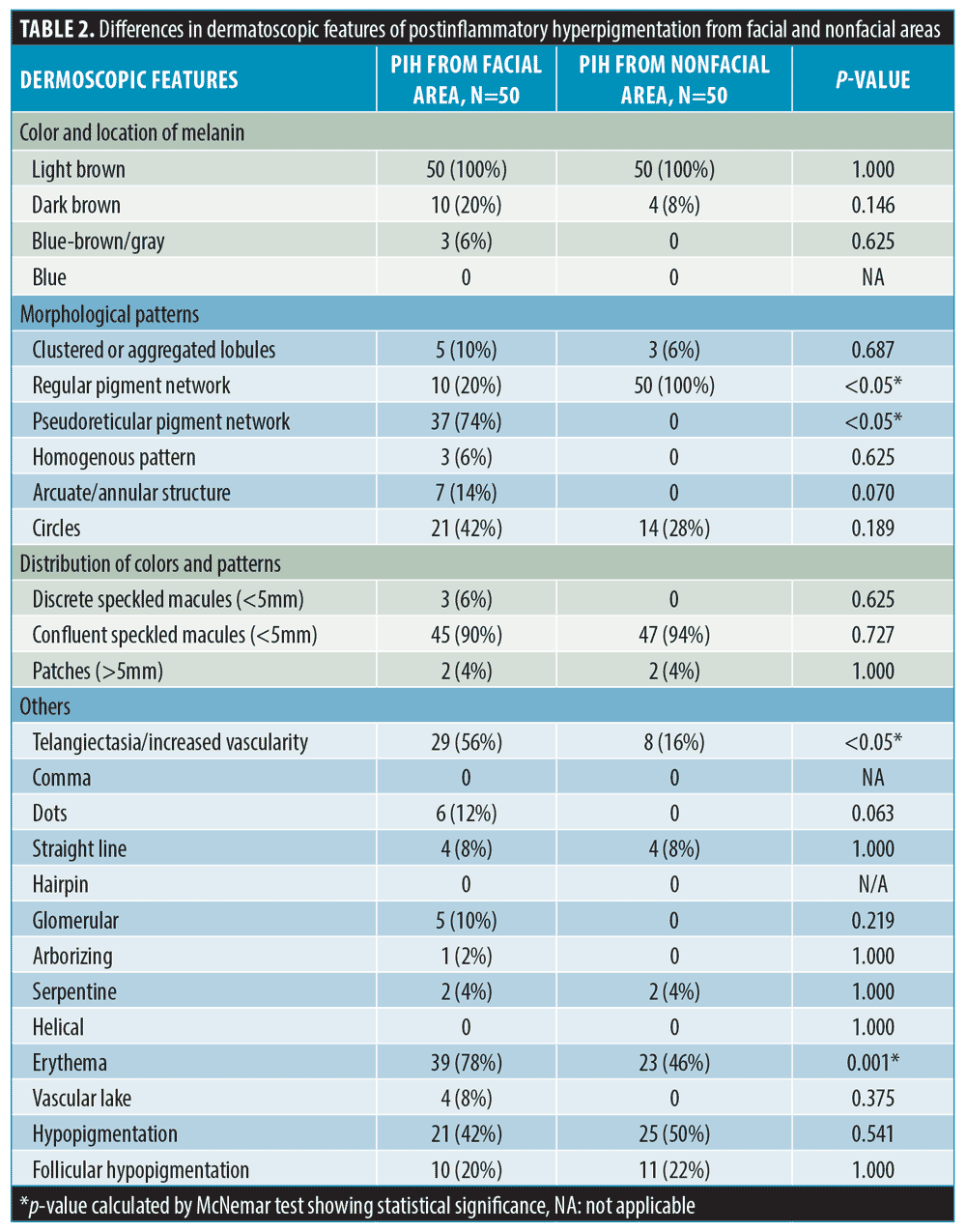
Results. Fifty patients were enrolled. The mean age was 26.74 (+ 6.75) years, and the Fitzpatrick Skin Types were III (66%) and IV (34%). In terms of morphological patterns of melanin, nonfacial PIH showed a significantly more regular pigment network than facial PIH (100% vs. 20%, p<0.05), while facial PIH exhibited a more pseudoreticular pigment network than nonfacial PIH (70% vs. 0%, p<0.05). In terms of vascularity, facial PIH demonstrated more telangiectasia and an increased vascular component compared to nonfacial PIH (56% vs. 16%, p<0.05). Moreover, hypopigmentation within the PIH lesion was demonstrated in both facial and nonfacial lesions (42% vs. 50%, p=0.541).
Conclusion. Acne-related PIH in facial and nonfacial areas showed different morphological pigment patterns and degrees of vascularity. Dermatoscopic examination should be performed before treatment initiation.
Keywords: Acne vulgaris, dark spot, erythema, pigmentation disorder, scar
Postinflammatory hyperpigmentation (PIH) is a frequently encountered problem, especially in patients with darker skin tones. It lasts several months to years and causes both physical and psychological burdens to affected patients. PIH can occur on any area of the body after internal cutaneous conditions or external injuries to the skin.1-3 PIH can occur after endogenous factors such as intense inflammatory dermatosis or cutaneous infection. Inflammatory conditions such as acne, atopic dermatitis, lichen planus, discoid lupus, and vesiculobullous conditions can cause significant dyspigmentation after the healing process. Moreover, infectious conditions such as bacterial, fungal, or viral infection can result in dyspigmentation.4,5 Exogenous PIH can be caused by physical trauma to the skin, such as manual excoriation, trauma, or burns. Aggressive dermatological procedures, such as lasers, chemical peels, or cryotherapy can cause PIH, especially in dark-complexioned skin.6 PIH results from the deposition of excessive melanin that is produced after inflammation of the skin. Injury that does not cause damage to the dermoepidermal junction results in stimulation of epidermal melanin, which results in brown macules or patches. Meanwhile, significant damage to the dermoepidermal junction results in the dropping of melanin to the upper dermis and the formation of melanophage. This results in the formation of dermal PIH that has a gray to blue color upon examination. According to a previous study, dermal PIH has more lymphocytic perivascular infiltration than epidermal PIH.7
Isedah et al8 compared the differences between PIH occurring after acne and that following application of trichloroacetic acid (TCA). Clinical examination of PIH from both groups peaked at 28 days, and no clinical difference was found at 42 and 56 days afterward. Skin biopsy revealed more melanophages in the acne-PIH group than in the TCA group, but the difference was not statistically significant. However, this study did not compare dermatoscopic differences.8 Regarding dermatoscopic features of PIH, epidermal PIH usually shows unpatterned brown pigment, while dermal PIH shows gray dot or blotch.9
Currently, there is no study comparing dermatoscopic patterns between facial and nonfacial PIH. This study aims to compare differences in dermatoscopic patterns between facial and nonfacial PIHs by focusing on acne-induced PIH. This study will provide information regarding the color distribution pattern and vascularity pattern that may help guide and plan proper treatment for each type of acne-related PIH.
Methods
Study design. A case-control study was conducted at a university-based hospital (Ramathibodi Hospital, Mahidol University, Bangkok, Thailand). The study protocol and patient consent to both clinical and dermatoscopic photography received approval from the Mahidol Institutional Review Board for Human Subject Research (Protocol Number MURA2019/913). The study protocol conformed to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from enrolled subjects before participation.
Study subjects. Patients with acne-related PIH were enrolled in this study. Inclusion criteria included patients who had both facial and nonfacial acne-related PIHs in the same person and who were willing to participate in the study. Exclusion criteria included patients with pigmentary conditions other than PIH, currently using contraceptive methods in any form (oral, injection, or patch), and prior application of topical whitening or treatment with lasers or energy-based devices in the study areas. The enrolled subjects signed the informed consent form and were interviewed for basic demographic data such as age, sex, Fitzpatrick Skin Type, and history of concurrent medication.
Clinical and dermatoscopic photography. The darkest PIH lesions were selected from both facial and nonfacial areas. The location and duration of selected PIHs were recorded in each subject. Dermatoscopy (DermLite DL3N, handheld dermatoscope, San Juan Capistrano, California) was used to examine dermatoscopic features of both facial and nonfacial PIHs. Clinical and dermatoscopic photography of PIH lesions were taken by a high-resolution camera (Canon EOS 80D, Canon Inc., Tokyo, Japan) connected to the dermatoscope by a universal connector.
Evaluation. Dermatoscopic evaluations include the determination of color and location of melanin, morphological patterns, distribution of colors and patterns, and other abnormalities such as hypopigmentation or vascular components. Regarding the color and location of melanin, different shades of color were graded and represented different levels of melanin deposition, such as black (spinous layer), light or dark brown (dermoepidermal junction), gray-bluish (papillary dermis) and blue (reticular dermis). The morphological pattern of melanin was recorded, which could be clustered, reticulated lines, streaks, dots, or structureless areas. The symmetry of the distribution of colors was also evaluated. Finally, hypopigmentation and vascular patterns were evaluated. Dermatoscopic features were evaluated by one blinded dermatoscopy-experienced dermatologist.
Statistical analysis. Statistical analyses were performed using the IBM statistical package for the social sciences version 19.0. Descriptive statistics were used to describe the clinical demographic data of the enrolled subjects. Categorical data are expressed in percentages. Continuous data are expressed as the mean ± standard deviation. Pearson’s chi-square and McNemar tests were used to evaluate the differences in dermatoscopic features between facial and nonfacial acne-related PIHs.
Results
Subject demographics. Fifty subjects with both facial and nonfacial acne-related PIH were recruited (Table 1). Thirty-seven subjects were female, and 13 subjects were male. The mean age was 26.74 (+ 6.75) years. The Fitzpatrick Skin Types were type III (66%) and Type IV (34%). Regarding the location of the facial PIH, the forehead was the most common area (38%), followed by the cheek (30%), mandibular (22%), malar (6%), and chin (4%) areas. The back was the most common location for nonfacial PIH (88%). Regarding the duration of PIH, the nonfacial area had a significantly longer mean duration than the facial area (18.68 ± 11.91 vs. 12.18 ± 9.13 weeks, p<0.05). Recruited subjects used topical acne medications such as benzoyl peroxide, clindamycin, adapalene, metronidazole, and erythromycin. Doxycycline was the only recorded systemic medication.
Dermatoscopic features. Analysis of dermatoscopic features between facial and nonfacial areas was compared and is shown in Table 2.
Regarding patterns of pigment deposition (Figure 1), all PIH lesions from facial and nonfacial areas exhibited a light brown color. A dark brown color was demonstrated in facial PIH more than in nonfacial PIH (20% vs. 8%, p=0.146). Blue to brown or gray color was found in facial PIH (6%) but not in nonfacial PIH. There was no blue color pattern in either facial or nonfacial PIH.
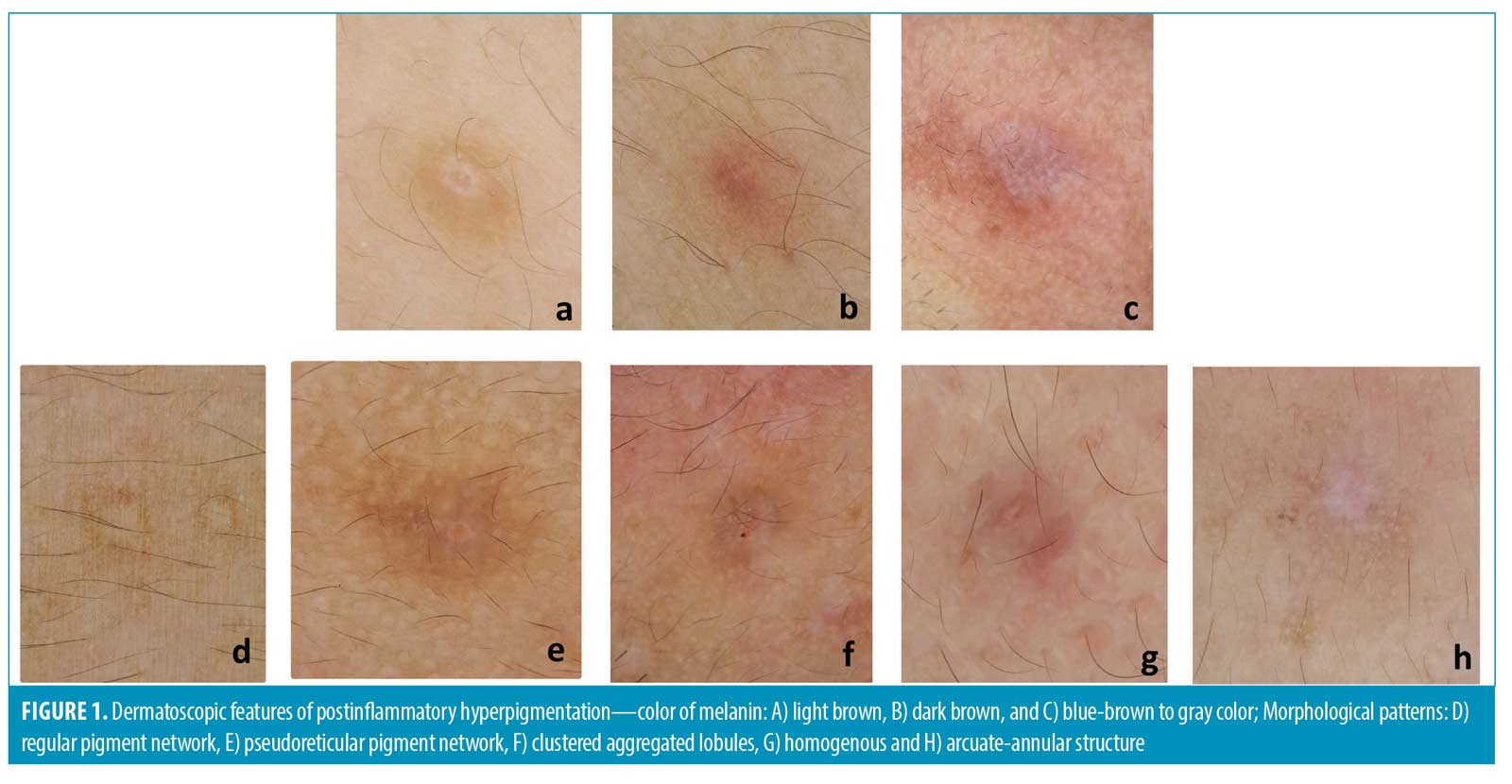
Concerning morphological patterns of pigment (Figure 1), facial PIH showed a significantly more pseudoreticular pigment network than nonfacial PIH (74% vs. 0%, p<0.05). Nonfacial PIH showed a more regular pigment network than facial PIH (100% vs. 20%, p<0.05). Circles and aggregated lobules were seen in both areas but showed no significant difference. Homogenous and annular patterns were detected only in PIH from the facial area.
The pigment distribution of PIH from both facial and nonfacial areas was mostly arranged into confluent speckled macules (size less than 5mm) (90% vs. 94%, p=0.727). Only 6 percent of facial PIH showed discrete speckled macules. Patches (size more than 5mm) were found equally in both areas (4%).
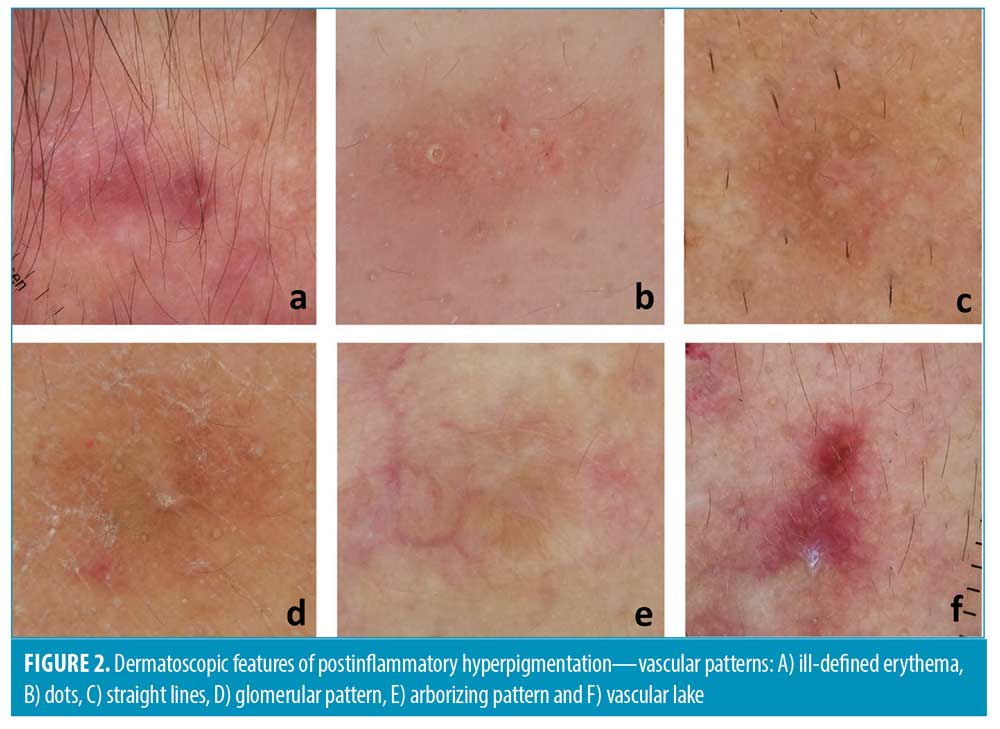
In addition to the pattern of hyperpigmentation, other dermatoscopic features, such as vascular components and hypopigmentation, were also observed. Interestingly, facial PIH significantly exhibited more telangiectasia and vascularity than nonfacial PIH (56% vs. 16%, p<0.05) (Figure 2). The majority of facial PIH showed vascular patterns in dots followed by glomerular, straight line, serpentine, and arborizing patterns. Meanwhile, nonfacial PIH demonstrated vascular patterns in a straight line, serpentine, and glomerular patterns. Ill-defined erythema was seen in facial PIH more than nonfacial PIH (78% vs. 46%, p=0.001). The vascular lake was seen slightly more often in facial PIH (8% vs. 2%, p=0.375).
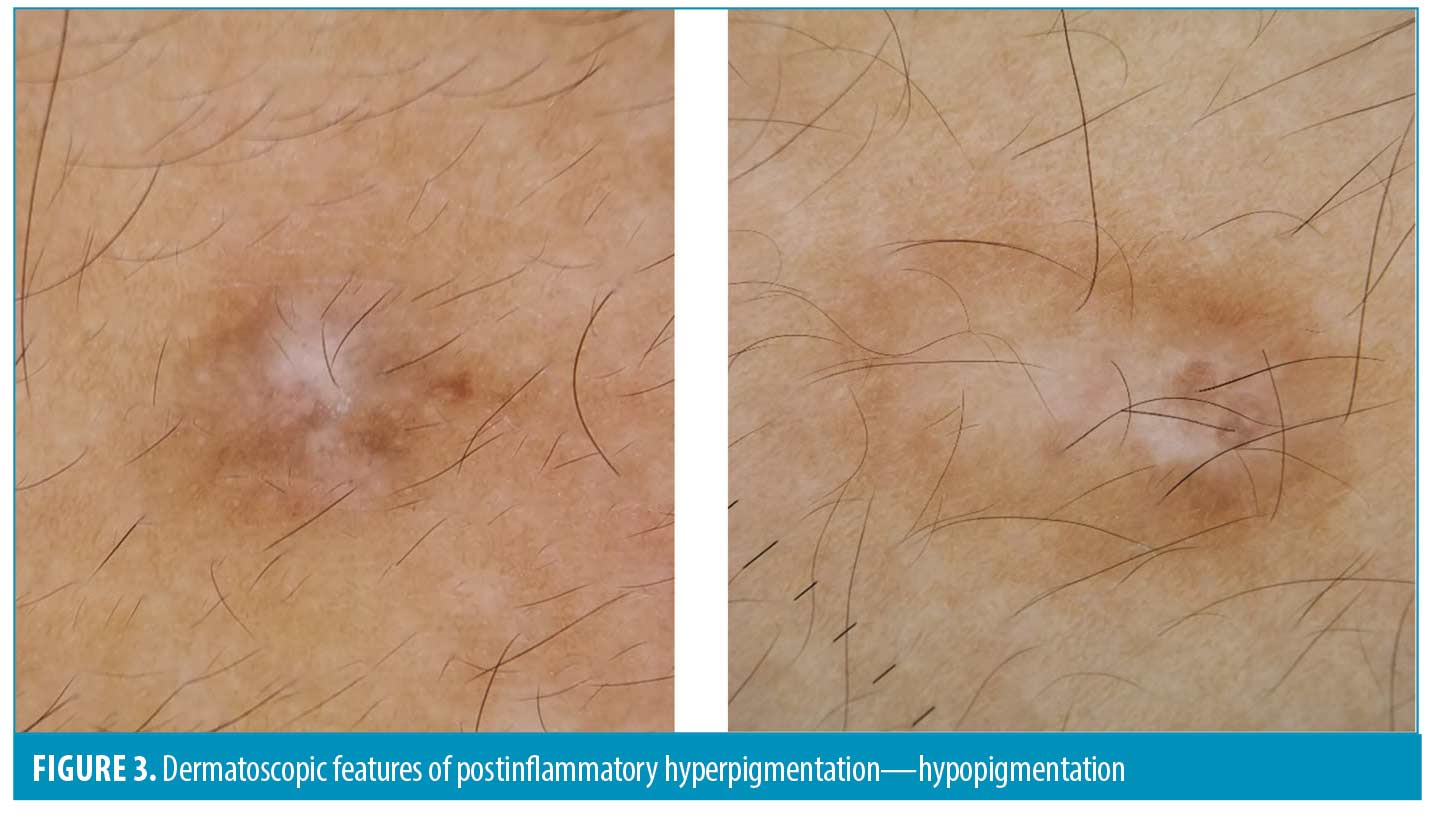
Hypopigmentation was seen equally in facial and nonfacial areas (42% vs. 50%, p=0.541). Some hypopigmentations were localized around the follicular area (follicular hypopigmentation). Follicular hypopigmentation was detected in both nonfacial PIH (22%) and facial PIHs (20%) (Figure 3).
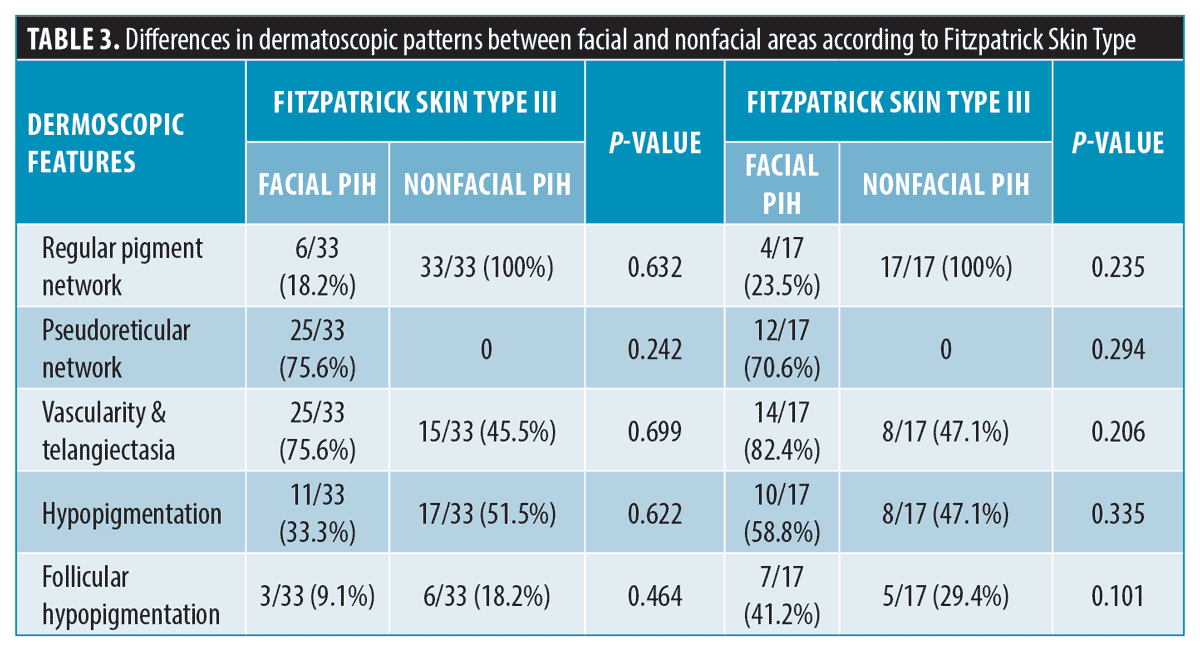
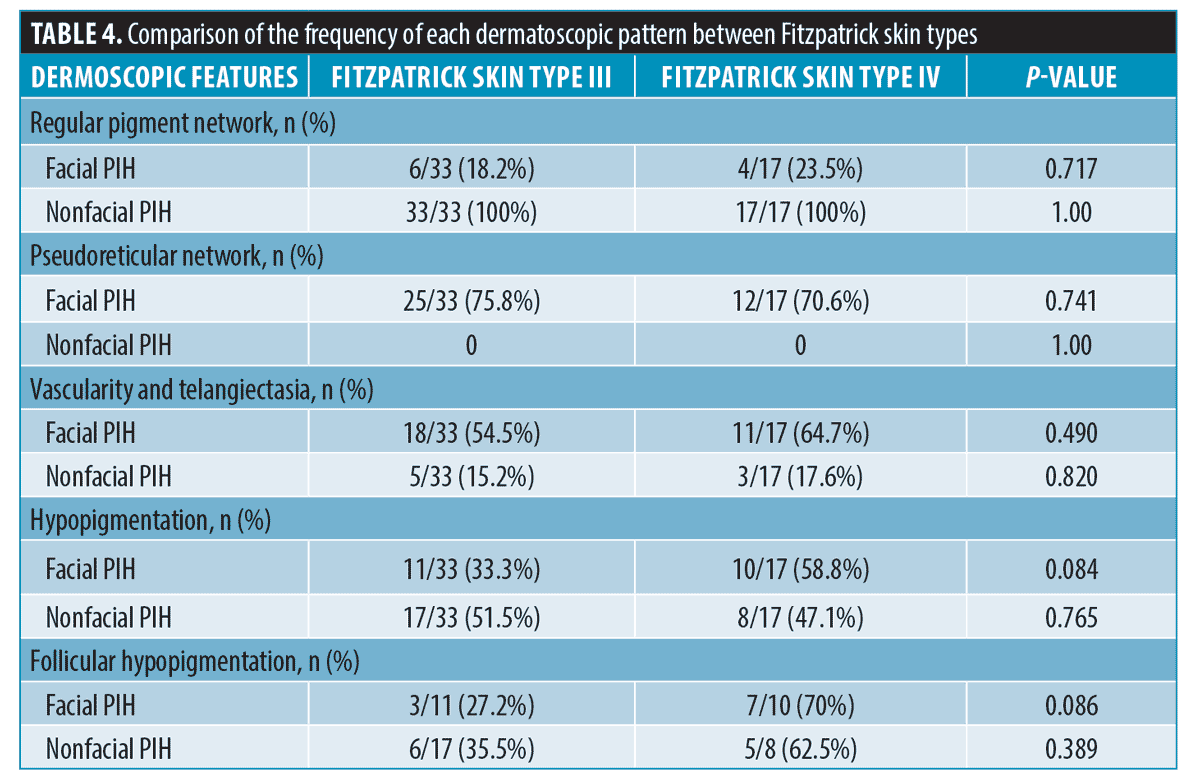
Further evaluation was performed to determine a specific pattern according to Fitzpatrick Skin Type; there was no significant difference in terms of pigment network and patterns of vascularity and hypopigmentation (Tables 3 and 4).
Discussion
PIH is a regularly encountered problem in patients with dark-complexioned skin. The degree of pigmentation is directly related to the severity of previous inflammation and the extent of dermoepidermal injury.10 Our study recruited acne-related PIH patients with Fitzpatrick Skin Types III and IV. Each patient was significantly affected by PIH at various time intervals. The majority of patients noticed that PIH located in the nonfacial area takes a longer time to heal than facial PIH.
Concerning the color of pigments, our study found that facial PIH has both brown and gray color patterns in facial PIH, while nonfacial PIH exhibits only light to dark brown. Normally, the light to dark brown color found in dermatoscopy represents pigment deposition at the epidermal layer. Meanwhile, the blue to gray color that was found only in PIH from the facial area represents a deeper deposition of melanophages in the dermis. This phenomenon could be explained by facial acne being easier to reach for picking than nonfacial acne. Intense physical trauma that causes dermoepidermal junction disruption may cause pigmentary incontinence in the upper dermis.10 Moreover, facial PIH is more prone to be affected by sun exposure than nonfacial PIH. These reasons could be the explanations of blue-gray to brown color found exclusively in facial PIH. However, the true pathogenesis behind this phenomenon still needs to be elucidated.
Regarding pigmentary patterns found in dermatoscopy, we found a pseudoreticular network among PIH from the facial area significantly more than among PIH from the nonfacial area. The pseudoreticular network is described when annular pigmentation is seen at follicular openings. Facial skin has a flatter rete ridge pattern and denser adnexal openings than nonfacial skin. These anatomical differences could explain the predominance of a pigmentary pattern of pseudoreticular networks in facial areas.11,12 We also found a regular pigment network in acne-induced PIH from the nonfacial area more than the facial area. The regular pigment network is formed by aggregations of melanin or melanocytes in basal keratinocytes. The denser pigment line represents the deepest part of pigment in rete ridges. The central paler area depicts the pigment at the tip of dermal papillae. This pigmentary pattern can be found more commonly in the nonfacial area because the skin has a longer rete ridge than the facial area.11 The regular pigment network can also be seen in other pigmented lesions, such as junctional nevus, compound nevus, or atypical nevus.13 However, we did not find any differences in either pigmentary pattern among different Fitzpatrick Skin Types.
Interestingly, we found that PIH that looked clinically brown somehow dermatoscopically demonstrated different vascular components. Facial PIH exhibited significantly more vascular components than nonfacial PIH, with an ill-defined area of erythema being the most common vascular pattern found in both facial and nonfacial PIH. Other vascular patterns were also described slightly more often in PIH from facial areas than in nonfacial areas, such as dots, straight lines, glomerular areas, arborizing areas, serpentine vessels, and large vascular lakes. These patterns have been previously reported in various skin conditions, such as dots in inflammatory skin diseases or flat melanocytic lesions, glomerular vessels in Bowen disease, arborizing vessels in basal cell carcinoma (BCC), or serpentine vessels in BCC or melanoma, etc.12 However, the morphological differences of these dermatoscopic vascular patterns between both areas did not reach a statistically significant difference. According to a study by Abd El All et al14, inflammatory acne vulgaris demonstrated immunoreactivity to interleukin-8 (IL-8) compared to normal skin. High IL-8 expression correlated with more dermal angiogenesis and inflammatory response in acne vulgaris. Moreover, Baran et al15 studied the evolution of acne lesions using optical coherence tomography (OCT)-based microangiography. They found that blood vessels in the acne area are coarser and less organized than those in the surrounding area. The vascular density increased at the early stage of acne inflammation and gradually decreased after a one-month period after inflammation.Another OCT imaging study focusing on different blood vessel morphologies in different body locations found that facial blood vessels show broader net and larger vessels than other body regions.16 These studies could explain the positive finding in our study regarding more vascular patterns in the facial area. Our study demonstrated that previously mentioned dermatoscopic patterns of blood vessels detected in other skin diseases can also be seen from PIH, especially from the facial area.
Based on the results of our study, increased vascularity may contribute to the manifestation of PIH. This finding was supported by a previous clinical study using long-pulsed dye lasers (LPDL, 595nm) for the treatment of acne-induced PIH. LPDL with the compression method was reported to decrease the overall intensity of PIH by targeting the vascular component underneath the PIH lesion and the pigment in the overlying epidermis.5, 17
In addition to hyperpigmentation, the counterpart, postinflammatory hypopigmentation, can also occur after intense inflammation in dark-skinned patients. Our findings demonstrated that hypopigmentation can be found in PIH lesions from both facial and nonfacial areas. Moreover, some of the hypopigmentations that occurred around follicular ostia can be found in both areas. We assume that the inflammatory process after acne usually intensifies around the pilosebaceous follicle, which explains the occurrence of hypopigmentation along the follicular area. Therefore, it is important to perform the dermatoscopic examination of PIH lesions as some treatment modalities for PIH (e.g., bleaching agents, pigment-specific lasers, etc.) may contribute to the further development of hypopigmentation.
The limitation of this study was the difference in the amount of previous sun exposure in each subject that could affect the intensity or pattern of dermatoscopic features of PIH. Regarding the duration of PIH, some individuals might fail to precisely recognize the exact duration of PIH, which could lead to inevitable recall bias. In addition, there might be a confounding effect of adapalene. According to a study by Jacyk WK, a beneficial effect of adapalene in acne-associated hyperpigmentation in African patients has been demonstrated.18 However, all subjects in our study had Fitzpatrick Skin Types III to IV. Hence, the effect of adapalene in terms of altering the course of PIH in this population is still questionable.
The value of this study is to broaden the horizon of unprecedented descriptions of dermatoscopic features of acne-associated PIH from facial and nonfacial areas. Knowing the detailed structure and composition of PIH may help physicians give proper advice and suitable treatments for individual patients. PIH with more vascular components seen from dermatoscopy may benefit from treatment with vascular-specific lasers. PIH with dermatoscopic features that represent deeper deposition of pigments may require proper selection of pigment-specific lasers and appropriate parameter settings. Hence, dermatoscopic examination is preferably recommended before treatment planning and initiation. Future studies may focus on frequent monitoring of inflammatory acne lesions to detect and treat subclinical PIH early and to fully elucidate the relationship between dermatoscopic features and the duration of PIH.
Conclusion
Our study is the first to focus on dermatoscopic patterns of acne-induced postinflammatory hyperpigmentation by comparing differences between facial and nonfacial areas. We found that PIH from the facial area exhibited a more pseudoreticular pigment network, vascularity, and telangiectasia. Dermatoscopic examination of acne-induced PIH should be performed.
References
- Callender VD, St Surin-Lord S, Davis EC, Maclin M, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12(2):87–99.
- Darji K, Varade R, West D, et al. Psychosocial Impact of Postinflammatory Hyperpigmentation in Patients with Acne Vulgaris. J Clin Aesthet Dermatol. 2017;10(5):18–23.
- Lacz NL, Vafaie J, Kihiczak NI, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43(5):362–365.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3(7):20–31.
- Eimpunth S, Wanitphadeedecha R, Manuskiatti W. A focused review on acne-induced and aesthetic procedure-related postinflammatory hyperpigmentation in Asians. J Eur Acad Dermatol Venereol. 2013;27 Suppl 1:7–18.
- Lamel SA, Rahvar M, Maibach HI. Postinflammatory hyperpigmentation secondary to external insult: an overview of the quantitative analysis of pigmentation. Cutan Ocul Toxicol. 2013;32(1):67–71.
- Park JY, Park JH, Kim SJ, et al. Two histopathological patterns of postinflammatory hyperpigmentation: epidermal and dermal. J Cutan Pathol. 2017;44(2):118–124.
- Isedeh P, Kohli I, Al-Jamal M, et al. An in vivo model for postinflammatory hyperpigmentation: an analysis of histological, spectroscopic, colorimetric and clinical traits. Br J Dermatol. 2016;174(4):862–868.
- Chatterjee M, Neema S. Dermoscopy of Pigmentary Disorders in Brown Skin. Dermatol Clin. 2018;36(4):473–485.
- Silpa-Archa N, Kohli I, Chaowattanapanit S, et al. Postinflammatory hyperpigmentation: A comprehensive overview: Epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J Am Acad Dermatol. 2017;77(4):591–605.
- Adya KA, Inamadar AC, Palit A. Dermoscopic Pigment Network: Characteristics in Non-melanocytic Disorders. Indian Dermatol Online J. 2020;11(2):146–153.
- Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74(6):1093–1106.
- Braun RP, Rabinovitz HS, Oliviero M, et al. Dermoscopy of pigmented skin lesions. J Am Acad Dermatol. 2005;52(1):109–121.
- Abd El All HS, Shoukry NS, El Maged RA, et al. Immunohistochemical expression of interleukin 8 in skin biopsies from patients with inflammatory acne vulgaris. Diagn Pathol. 2007;2:4.
- Baran U, Li Y, Choi WJ, et al. High resolution imaging of acne lesion development and scarring in human facial skin using OCT-based microangiography. Lasers Surg Med. 2015;47(3):231–238.
- Schuh S, Holmes J, Ulrich M, et al. Imaging Blood Vessel Morphology in Skin: Dynamic Optical Coherence Tomography as a Novel Potential Diagnostic Tool in Dermatology. Dermatol Ther (Heidelb). 2017;7(2):187–202.
- Togawa Y. Review of vasculature visualized on dermoscopy. J Dermatol. 2017;44(5):525–532.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15 Suppl 3:37–42.7.

