J Clin Aesthet Dermatol. 2020;13(4):14–21
by Leon Kircik, MD; James Q Del Rosso, DO; Jonathan S Weiss, MD; Vassilis Stakias, PharmD; Anat London, PhD; Rita Keynan; Yohan Hazot; Russell Elliott, PhD; and Iain Stuart, PhD
Dr. Kircik is with the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Del Rosso is with JDR Dermatology Research/Thomas Dermatology in Las Vegas, Nevada. Dr. Weiss is with Georgia Dermatology Partners in Snellville, Georgia. Drs. Stakias, Elliott, and Stuart are with Foamix Pharmaceuticals Inc. in Bridgewater, New Jersey. Dr. London, Ms. Keynan, and Mr. Hazot are with Foamix Pharmaceuticals Ltd in Rehovot, Israel.
FUNDING: This research was funded by Foamix Pharmaceuticals.
DISCLOSURES: Leon Kircik is an investigator and consultant for Foamix Pharmaceuticals Inc. James Q. Del Rosso is a consultant for Aclaris, Almirall, Athenex, Cutanea, Dermira, Ferndale, Galderma, Genentech, LEO Pharma, Menlo, Novan, Ortho, Pfizer, Promius, Sanofi/Regeneron, SkinFix, and SunPharma; he has received research support from Aclaris, Almirall, Athenex, Botanix, Celgene, Cutanea, Dermira, Galderma, Genentech, LEO Pharma, Menlo, Novan, Ortho, Promius, Regeneron, SunPharma, and Thync; he receives honoraria from Aclaris, Celgene, Galderma, Genentech, LEO Pharma, Novartis, Ortho, Pfizer, Promius, Sanofi/Regeneron, and SunPharma; and he participates in speakers bureaus for honoraria from Aclaris, Celgene, Galderma, Genentech, LEO Pharma, Novartis, Ortho, Pfizer, Promius, Sanofi/Regeneron, and SunPharma. Jonathan S. Weiss is a principal investigator for AbbVie, Aclaris, Endo, Foamix, Galderma, LEO Pharma, Moberg, Promius, and Valeant; he is a speaker for AbbVie, Almirall, Dermira, and Ortho Dermatologics and a consultant for Aclaris and LEO Pharma, as well as an advisor for Foamix, Galderma, and Valeant. Vassilis Stakias, Anat London, Rita Keynan, Yohan Hazot, Russell Elliott, and Iain Stuart are employees of Foamix.
ABSTRACT: FMX101 4% minocycline is a hydrophobic, topical foam formulation of minocycline recently approved by the United States Food and Drug Administration (FDA) for the treatment of non-nodular inflammatory lesions in moderate-to-severe acne vulgaris. It was developed to harness the anti-inflammatory and antibiotic activity of minocycline while minimizing potentially serious systemic adverse events associated with oral delivery. The composition and profile of this novel treatment have yet to be described. This article discusses the components of the foam-based product and the rationale for their selection. It reviews microbiologic data for FMX101 4% and presents previously unpublished data regarding sebum penetration, minocycline permeation, and disposition into skin structures. The effects of FMX101 4% were compared with those of several commercially available acne preparations to determine how the FMX101 4% formulation affects the physical properties of model human sebum in vitro. The hydrophobic formulation of FMX101 4% was found to lower the melting temperature of model human sebum below that of normal skin temperature, decreasing its viscosity. FMX101 4% achieved high concentrations of minocycline in the sebaceous appendage, while minimizing permeation beyond the dermal layer. Finally, this article summarizes efficacy and safety data for FMX101 4% from three Phase III studies (FX2014-04, FX2014-05, and FX2017-22). FMX101 4% appeared to be safe, effective, and well tolerated for the treatment of non-nodular inflammatory lesions in moderate-to-severe acne vulgaris. In conclusion, the topical formulation of minocycline in FMX101 4% represents a unique treatment for acne vulgaris and a viable alternative to oral administration.
KEYWORDS: Micronized minocycline, moderate-to-severe acne vulgaris, inflammatory lesions, hydrophobic foam formulation, topical preparation
Acne vulgaris (AV) is a common chronic inflammatory skin disorder involving pilosebaceous follicles.1–3 It typically affects adolescents and young adults; however, acne can occur in any age group and persist into adulthood.4–6 It most commonly affects the face and trunk and is characterized by papules, pustules, and comedones, and is frequently associated with scarring.1,5–7 AV pathogenesis is believed to be multifactorial, involving overproduction of sebum, colonization by Cutibacterium acnes (C. acnes) within pilosebaceous follicles, follicular hyperkeratosis, and inflammation.1,5–8 Factors secreted by C. acnes, including lipases, chemotactic factors, metalloproteases, and porphyrins, interact with molecular oxygen to generate reactive oxygen species, which can damage keratinocytes and induce a proinflammatory state.9 Furthermore, AV is associated with significant physical and psychological comorbidity, such as pain, scarring, depression, anxiety, and low self-esteem.6,10,11
Compounding the challenges associated with AV, acne medication adherence rates are reported to be especially low, although adherence is crucial for any acne treatment to be effective. Adherence rates to acne medications are generally reported to be about 50 percent but vary significantly depending on the methods used.12 A systematic review examined subjective adherence rates from 14 clinical trials and objective adherence rates from a large pharmacy database.12 The overall adherence rate to oral acne medications in the analysis of clinical trials was 76.3 percent, similar to the overall adherence rate for topical acne medications of 75.8 percent (p=0.927). However, adherence rates based on pharmacy prescription records and other objective means found that adherence to oral acne therapies (10.3%) was better than that for topical therapies (3.2%, p<.05).12 Reasons for suboptimal adherence to acne medications include early discontinuation due to unwanted side effects, lack of understanding of AV or AV-related treatment(s), and overall dissatisfaction with treatment.2,12 The presence of two commonly used excipients, ethanol and propylene glycol, in topical preparations has been associated with skin irritation, dryness, burning, pruritus, redness, or allergic contact dermatitis.13,14 A relatively slow onset of action for many acne therapies also makes these treatments innately discouraging for patients. Specifically, there is often a latency period of at least 6 to 8 weeks until the appearance of a definite clinical improvement, which often progresses slowly.12
Oral antibiotics of the tetracycline class (doxycycline and minocycline) are considered first-line therapy for the treatment of moderate-to-severe AV and a mainstay in acne treatment as recommended by the American Academy of Dermatology.5,6,10 Minocycline is a semi-synthetic, second-generation tetracycline with broad-spectrum bacteriostatic and anti-inflammatory activity, and is one of the most commonly prescribed oral antibiotics for AV.10,15 As a tetracycline, in-vitro studies with minocycline have shown it to have a bacteriostatic effect on C. acnes by binding to 16s rRNA and overlapping the anticodon stem loop of the aminoacyl-site tRNA in the 30S subunit. This action blocks the accommodation of the tRNA into the A-site tRNA in a reversible manner, preventing protein synthesis and microbial replication.16 Recent findings suggest that tetracyclines, specifically minocycline, have other biological functions outside of their antimicrobial properties that include anti-inflammatory properties, with a specific modulatory effect on epidermal cytokines.17,18
A significant risk and concern of using oral antibiotics to treat dermatologic conditions is the potential for development of antibiotic resistance, including antibiotic-resistant C. acnes.5,8,15 Topical erythromycin and clindamycin have been used for many years for the treatment of moderate-to-severe AV; however, their widespread use has resulted in the development of drug resistance as well as C. acnes strains with cross-resistance to different antibiotics.1,6,10,19 Thus, the emergence of these resistant strains of C. acnes has been associated with therapeutic failure of topical erythromycin for inflammatory and noninflammatory lesions, leading to a decrease in the use of topical erythromycin and clindamycin to treat acne.20,21 By comparison, the tetracycline class of antibiotics are less susceptible to resistance, with minocycline having the lowest rates of antibiotic resistance and low cross-reactivity.1,10 A topical preparation of minocycline should be able to harness the antimicrobial effects of the active ingredient while minimizing systemic exposure, but attempts to produce a topical preparation of minocycline have been previously unsuccessful, until the recent approval of the first topical minocycline, FMX101 4% foam, for the treatment of inflammatory lesions of non-nodular moderate-to-severe AV in patients nine years of age or older.
Administration of oral antibiotics has also been associated with systemic side effects.6,10,15 Oral minocycline has been linked to tinnitus, dizziness, and hyperpigmentation of skin, mucous membranes, and teeth.6 In rare instances, serious side effects, including autoimmune disorders (e.g., systemic lupus erythematosus, drug rash with eosinophilia), systemic symptoms, and pseudotumor cerebri, have been attributed to oral minocycline.6,10 Systemic side effects might be circumvented by topical acne treatments.
FMX101 4% was developed with the goal of harnessing the anti-inflammatory and antibiotic activity of minocycline, while minimizing potentially serious systemic adverse events through topical delivery.4,10 In the treatment of acne with a topical medication, delivery of the active ingredient into the pilosebaceous unit (PSU) is believed to be critical to achieve efficacy.22 In-vitro studies have demonstrated that FMX101 4% was able to deliver high amounts of minocycline to the epidermis and PSU, including the hair follicle and the sebaceous gland, while minimizing transdermal drug absorption.23 In four Phase I dermal safety studies, FMX101 4% did not show any evidence of phototoxicity, photosensitivity, or skin sensitization, and no treatment-emergent adverse events (TEAEs) were related to treatment. A pharmacokinetic study also demonstrated minimal systemic absorption and accumulation of minocycline following maximal-use dosing of FMX101 4% for 21 days compared with oral minocycline.4 Systemic exposure to minocycline with daily topical application of a 4g dose of FMX101 4% was 730 to 765 times lower than that following a single dose (~1mg/kg) of oral minocycline.4 A pharmacokinetic study conducted in patients aged 9 to 17 years (FX2016-21) showed similarly low levels of systemic exposure following maximal-use dosing of FMX101 4% for seven days.
In three Phase III studies (FX2014-04, FX2014-05, and FX2017-22), the novel foam formulation of FMX101 4% appeared effective, safe, and well tolerated, leading to its approval by the United States (US) Food and Drug Administration (FDA) for the treatment of non-nodular inflammatory lesions in moderate-to-severe AV.10,24 In Phase III clinical studies for AV, FMX101 4% significantly reduced inflammatory (Figure 1) and noninflammatory lesions and led to significant improvement in Investigator’s Global Assessment (IGA) treatment success (clear or almost clear) in studies FMX2014-05 and FMX2017-22. Clinical improvements in the reduction of inflammatory lesions were observed as early as Week 3 and were maintained through 52 weeks in open-label extension studies. Across the three studies, FMX101 4% appeared to be safe and well tolerated, with most TEAEs considered to be mild to moderate and the frequency of TEAEs similar between treatment groups. During the open-label extension study, there were no serious treatment-related TEAEs leading to subject discontinuation, and efficacy continued to improve until the end of the study. At the conclusion of the 52-week treatment, FMX101 4% achieved high levels of patient satisfaction and good tolerability as a treatment for moderate-to-severe acne.
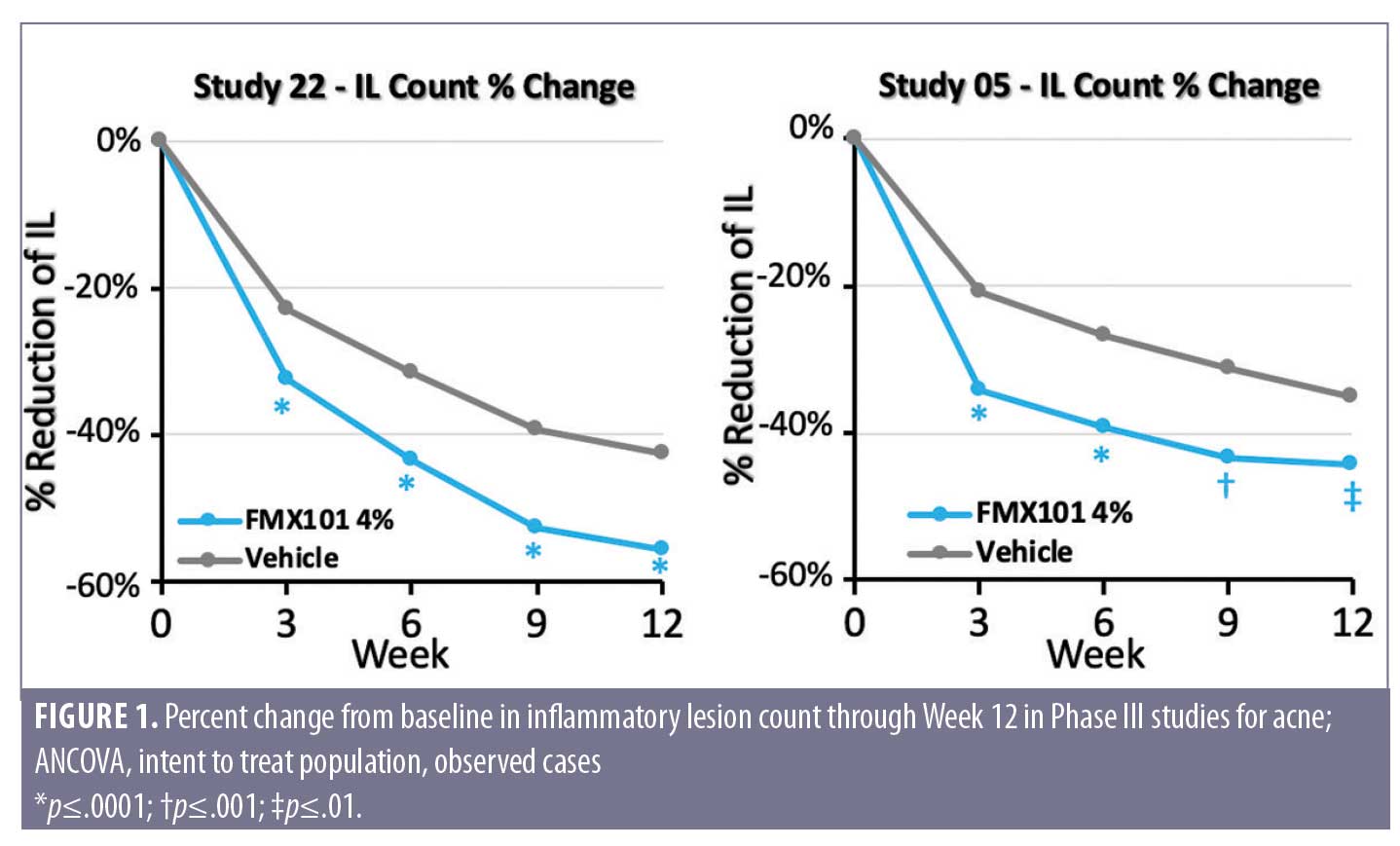
This review will discuss the composition and characteristics of FMX101 4%, a novel hydrophobic foam formulation of minocycline being developed for the topical treatment of AV. In addition, this manuscript will review microbiologic data, summarize efficacy and safety data for FMX101 4%, and present previously unpublished data regarding sebum penetration, minocycline permeation, and disposition into skin structures.
Rational Design of FMX101 4%
Foam technology. Although minocycline is stable in the solid state, it degrades extensively in the presence of water or protic solvents, complicating its ability to be formulated in an aqueous vehicle. In addition, it is sensitive to both light and oxidation, making it very unstable.10,23 However, the hydrophobic nature of minocycline relative to other tetracycline antibiotics offers an opportunity to improve skin penetration and delivery of antibiotic to the follicle.23 The challenges in developing a topical preparation, therefore, involve maintaining the chemical stability of minocycline during manufacturing and shelf life, while delivering high concentrations of the antibiotic to the PSU.
In order to determine the optimal vehicle for FMX101 4% with respect to minocycline solubility and stability, a carrier screening study evaluated three vehicle classes. Additionally, for each carrier, the solubility of minocycline was assessed using high-pressure liquid chromatography. Results indicated that minocycline undergoes significant degradation in water and polar organic solvents, while remaining more stable in non-polar carriers. For carriers in which minocycline solubility was low, minocycline exhibited greater stability upon exposure to elevated temperatures. Based on these results, a suspension of minocycline HCl in a non-aqueous hydrophobic matrix was determined to provide the greatest stability for minocycline.
A combination of soybean oil, coconut oil, light mineral oil, and cyclomethicone was selected as suitable carriers for the formulation of FMX101 4%. Plant oils are known to have several benefits to the skin, including antimicrobial effects and occlusive effects, which allow the skin to retain moisture. Coconut oil, for example, has been found to be an emollient and has demonstrated the ability to increase skin capacitance, reduce trans-epidermal water loss (TEWL), and reduce SCORing Atopic Dermatitis (SCORAD) index values in patients with mild-to-moderate AD.25 In addition to improving barrier function (as demonstrated by decreased TEWL and increased skin capacitance), coconut oil contains a monoglyceride known as monolaurin that displays antimicrobial activity by disintegrating lipid membranes of bacteria.26 Similarly, soybean oil containing soy phytosterols has been shown to positively influence skin barrier recovery and to decrease TEWL on forearm skin. Mineral oil has also been shown to act as an emollient and improve barrier function by suppressing TEWL.27 The beneficial effect of coconut oil, soybean oil, and mineral oil on barrier function, along with their moisturizing and antibacterial properties, make them ideal choices as carriers for minocycline delivery.26
Following recent trends in topical drug delivery, FMX101 4% uses a foam-based vehicle.28,29 Foam preparations are generally preferred by patients over conventional gels, creams, and ointments due to their spreadability, pleasant skin “feel,” and ease of use.30,31 The FMX101 4% minocycline foam is stabilized by adsorption of fatty acid or fatty alcohol crystals around air bubbles, preventing bubble coalescence at the oil-air interface. Oil foams are also stabilized by the non-absorbed fatty acid or fatty alcohol crystals, which increases oil viscosity to form an oleogel, thus reducing the possibility of oil drainage and subsequent foam breakdown. Pressurized aerosol packaging in canisters with hydrophobic propellants protects the composition from air and moisture. This further reduces the risk of minocycline degradation, and improves its long-term stability.
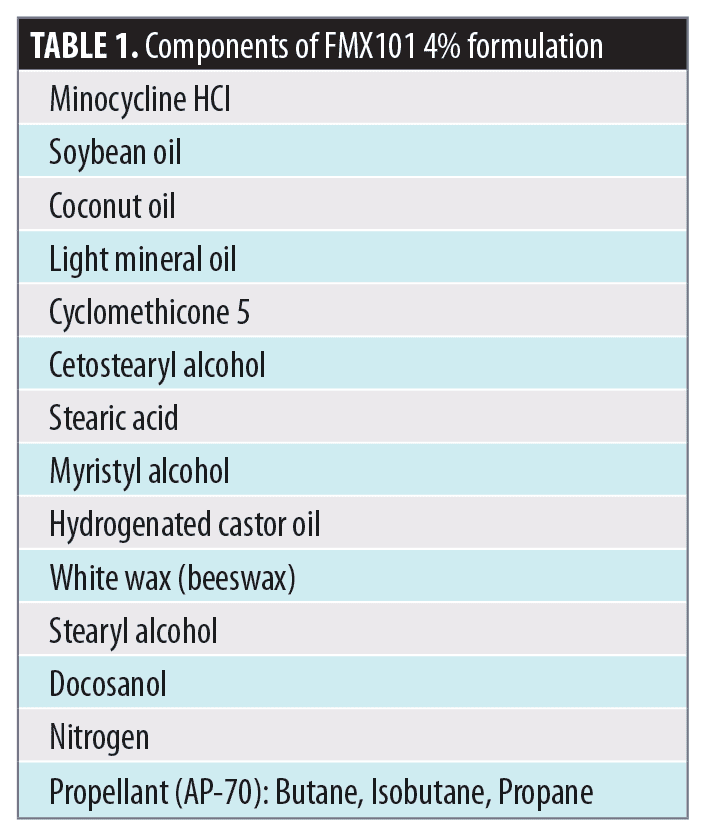
The foam formulation of FMX101 4% has micronized minocycline hydrochloride (HCl) crystals suspended and dispersed throughout the product (Figure 2). Particle size plays an important role in effective topical acne treatment, because smaller particles can better penetrate the PSU, delivering the active ingredient to the target site without resulting in an increase in the transdermal penetration. In addition to improving the ability of a drug to penetrate into the skin, micronization helps protect the stability of drugs, making them more photostable.32 Suspension of micronized minocycline in the non-aqueous vehicle limits its interaction with other components of the composition (Table 1). Micronization of minocycline in FMX101 4% also prevents a grainy sensation on the skin, ensures consistency of the product, and facilitates application by preventing clogging of the actuator valve.

In-Vitro Effects on Sebum
In order to effectively treat AV with a topical formulation, it is critical to deliver the active ingredient into the PSU. To achieve successful intrafollicular delivery, the active molecule must access the sebum-laden target site. Sebum has a high content of saturated components, including triglycerides, fatty acids, wax esters, and cholesterol, which increase its viscosity and raise its melting temperature above normal skin temperature. A topical formulation capable of lowering the melting temperature of sebum should reduce its overall viscosity, making the sebum more permeable to the active ingredient. Decreasing sebum viscosity should also minimize clogging of pilosebaceous ducts, which putatively impacts comedonal formation and the anaerobic environment in which C. acnes flourishes.
In order to evaluate the effects of FMX101 4% on sebum, artificial human sebum was used to mimic the composition and characteristics of human sebum. A study was conducted to compare the effect of FMX101 4% (Table 1) and an oil-in-water emulsion (OIWE) on the physical properties of model human sebum (Table 2). The mixtures were tested using three methods: differential scanning calorimetry (DSC) to measure melting temperatures, rheometry to measure viscosity, and light microscopy to determine miscibility of test formulations with model human sebum. An additional study compared the miscibility of five commercially available topical acne treatments with FMX101 4% using polarized light microscopy.

Differential scanning calorimetry (DSC). DSC was used to evaluate the effects of FMX101 4% and OIWE on the melting temperature of model sebum. The test samples were model human sebum alone, a 50:50 (w/w) mixture of OIWE: model sebum, and a 50:50 (w/w) mixture of FMX101 4%: model sebum. Samples were cooled to 0°C (32°F) at a rate of 5°C/min (9°F/min) and held isothermally at 0°C (32°F) for five minutes; then they were heated to 60°C (140°F) at a heating rate of 5°C/min. The peak melting temperature of model sebum was approximately 37°C (98.6°F). When model sebum was combined with FMX101 4%, the peak melting temperature decreased to approximately 33.5°C (92.3°F), which was about 3.5°C lower than that of pure sebum and 1.5°C lower than normal skin temperature of 35°C (95°F). Conversely, the mixture of sebum and OIWE had a melting temperature of 39.6°C (103.3°F), which was more than 2.5°C (36.5°F) higher than that of pure sebum (Figure 3) and more than 4.5°C higher than normal skin temperature of 35°C (95°F). These data demonstrate that FMX101 4% lowered the melting temperature of model sebum below that of normal skin temperature. Conversely, the OIWE, which approximates creams used in acne treatments, raised the melting temperature well above normal skin temperature.

Rheometry. Rheometry was performed to measure any effects of the test formulations on viscosity. A Discovery HR-2 rheometer was used to conduct rheological measurements on model human sebum alone, and the 50:50 mixtures of model sebum with FMX101 4% vehicle and OIWE. Temperature-dependent flow behavior of the samples was assessed, which found that the inflection point of the viscosity curve for sebum alone was 37.7°C (99.9°F), whereas the inflection point for the 50:50 (w/w) mixture of model sebum with FMX101 4% vehicle was 2°C lower: 35.7°C (96.3°F). The 50:50 mixture of model sebum with OIWE demonstrated an inflection point of approximately 40°C (104°F), more than 2°C higher than model sebum alone. These results align with the observations in the DSC experiments, and demonstrate that FMX101 4% decreases the viscosity of model sebum at skin temperature. The liquification of sebum at skin temperature would be expected to increase the diffusion coefficient for minocycline and provide better penetration through sebum into the pilosebaceous duct.
Light microscopy. Light microscopy was used to visually assess the miscibility of the test formulations with model human sebum. A small amount of each test formulation was placed in contact (but not mixing) with a small amount of model sebum on a microscope slide in order to visually examine miscibility. The slide was mounted on a hot stage, and the temperature was raised from 25°C (77°F) to 35°C (95°F) at 2°C/min (3.6°F/min) and then held isothermally. A video of the observed changes was recorded, and photomicrographs were taken using polarized light. Test formulations included FMX101 vehicle, FMX101 4%, OIWE, and five commercially available acne treatments (dapsone 7.5% gel, tretinoin 0.08% gel microspheres, tazarotene 0.1% foam, adapalene 0.3%/benzoyl peroxide 2.5% gel, or clindamycin phosphate 1.2%/benzoyl peroxide 3.75% gel).
Model human sebum and the test formulation were initially separated with clearly visible borders when held at 25°C (77°F) before heating (Figure 4, images on left). Upon heating to 35°C (95°F), components of model sebum and FMX101 4% (having lower melting points) liquified and began to intermix (Figure 4, top row, middle image). This observation became more pronounced as the sample was held isothermally at skin temperature 35°C (95°F) for one minute (Figure 4, top row, right image). Conversely, the border between model sebum and each of the test formulations other than FMX101 4% remained intact even after heating to 35°C (95°F) and maintaining at this temperature (Figure 4, lower rows, middle and right images). These observations visually confirmed the results seen with DSC and rheology for FMX101 4%, and indicated enhanced miscibility at 35°C (95°F). Similar to the behavior seen with OIWE, each of the five commercially available acne treatments showed a relatively intact interface between model human sebum and the test formulation when heated and held at 35°C (95°F), indicating poor miscibility.

Based on these observations, it was concluded that the hydrophobic formulation of FMX101 4% impacts the physical properties of sebum to a higher degree than other tested formulations. By reducing the melting temperature of sebum, and consequently decreasing its viscosity, sebum became more fluid at normal skin temperature. These effects should increase the permeability of sebum to minocycline, have a softening effect on sebum, and potentially reduce clogging of pilosebaceous ducts.
In-Vitro Permeation and Penetration of Minocycline into Skin
Although oral minocycline is used primarily to treat skin conditions such as acne and rosacea, it has a high molecular weight of 457 daltons and might not penetrate efficiently into or through human skin when applied topically. Understanding the permeation and penetration of the active agent into skin structures, particularly the epidermis, dermis, and sebaceous appendage, is critical to characterizing topical formulations of minocycline. Thus, an ex-vivo permeation and penetration study was performed using two prototype foam formulations of minocycline, FMX101 4% and FMX103 1.5%, each differing only in their antibiotic concentrations.
A flow-through diffusion cell (MedFlux-HT™) was used to assess drug delivery between donor and receptor compartments across dermatomed human skin. Foam samples were collapsed and approximately 10mg of foam was dosed onto a total of 10 skin samples. The flow was set at 10µL/min and the formulation was allowed to penetrate the skin for 12 hours. After 12 hours of penetration, five skin samples were cleaned and heated at 60°C (140°F) for two minutes in order to manually separate the dermis from the epidermis. The other five skin samples were cleaned and then treated with SurgiSeal® to separate the sebaceous appendage. The minocycline in the sebaceous appendage, dermis, and remaining epidermis was measured using an LC-MS/MS analytical method, which provided sensitive quantification of minocycline from receptor solution and skin tissue homogenates. The ex-vivo skin permeation and penetration experiments consisted of two active (FMX101 4% and FMX103 1.5%) and two placebo formulations. Minocycline penetration was examined by extracting drug from epidermis (n=5), dermis (n=5), and the sebaceous appendage (n=5).
The amount of minocycline that permeated into the receptor solution 12 hours after skin application ranged from 24–417ng/cm2 for FMX101 4% and 55–129ng/cm2 for FMX103 1.5%. There were no statistically significant differences between the two formulations. Penetration of minocycline into the sebaceous appendage 12 hours following application was 3539ng (0.67% of applied dose) for FMX101 4% and 909ng (0.52%) for FMX103 1.5% (p<.001). Approximately half of the minocycline delivered to the epidermis was recovered from the sebaceous appendages for both formulations. After the sebaceous appendage was separated, delivery of similar percentages of the applied minocycline dose to the epidermis was observed for FMX101 4% (0.28%) and FMX103 1.5% (0.29%). Similar percentages of the applied minocycline dose were also observed in the dermis for FMX101 4% (0.45%) and FMX103 (0.39%). When the sebaceous appendages were intact, FMX101 4% delivered 1.02% of the applied dose (4818ng) to the epidermis, while FMX103 1.5% delivered 1.54% (2838ng). Minocycline delivery to the dermis layer was 1680–2407ng for FMX101 4% and 676–1419ng for FMX103 1.5% (p<.05).
Observed amounts of minocycline were converted to minocycline concentrations (µg/mL) for each layer, under the assumptions of average weights and density of skin, average volume of sebaceous appendages, and cellular volume corrections. Based on these assumptions, delivery of minocycline to the epidermis was 560ug/mL for FMX101 4% and 330ug/mL for FMX103 1.5%, with the sebaceous appendage intact. Minocycline delivery to the dermis was 17ug/mL with FMX101 4% and 15ug/mL with FMX103 1.5%, with the sebaceous appendage intact. After removal of the sebaceous appendages, epidermal delivery of minocycline for FMX101 4% and FMX103 1.5% was 166ug/mL and 59ug/mL, respectively, and dermis delivery was 25ug/mL and 7ug/mL, respectively. Both FMX101 4% and FMX103 1.5% were shown to deliver similar, high concentrations of minocycline to the epidermis and sebaceous appendage, much lower concentrations to the dermis skin layer, and minimal concentrations permeated through the skin. These data, obtained using human skin from a single donor, confirm similar observations conducted in porcine skin samples.23
Microbiology Profile of FMX101 4%
C. acnes (previously called Propionibacterium acnes) and inflammatory mechanisms play a key role in the development of AV.6, 33 Oral minocycline inhibits clinical isolates of C. acnes and is recommended for the treatment of moderate-to-severe AV. Studies were conducted to investigate microbiologic susceptibility and potential for resistance to FMX101 4% topical minocycline foam and comparator antibiotics using a phenotypically diverse set of clinical isolates of C. acnes primarily originating from the US.34 In these studies, FMX101 4% had a minimum inhibitory concentration for 90% of organisms (MIC90) of 0.25µg/mL and was four-fold more potent than bacitracin and tetracycline, eight-fold more potent than clindamycin, and greater than or equal to 32-fold more potent than neomycin, erythromycin, fusidic acid, and mupirocin. Spontaneous resistance to FMX101 4% occurred at low frequencies ranging from less than or equal to 5×10-9 to less than 1×10-8 in C. acnes, with no mutants exhibiting a minocycline minimum inhibitory concentration (MIC) above 0.5ug/mL. Mutations were identified in rpsJ, a gene encoding 30S ribosomal protein S10; however, no cross-resistance was observed outside of the tetracycline family. Furthermore, no second-step mutation in previously isolated mutants or strains containing rpsJ±16S rRNA mutations was detected following minocycline challenge. Minocycline retained potent antibacterial activity against C. acnes over 15 serial passages; thus, no selective growth advantage for minocycline-resistant mutants occurred under these experimental conditions. These studies demonstrated that FMX101 4% has the potential to retain the favorable resistance profile of oral minocycline in diverse C. acnes isolates while offering the potential of minimizing serious systemic side effects in the treatment of AV. Importantly, the concentration of minocycline in the epidermis and sebaceous appendage following FMX101 4% administration, as measured above in the ex-vivo study, is well above the MIC90 for minocycline, which suggests low potential for development of antimicrobial resistance.
Conclusion
AV is frequently associated with scarring, leading to low self-esteem, anxiety, and depression. Oral minocycline is widely used as first-line therapy for moderate-to-severe AV. A broad-spectrum antibiotic of the tetracycline class, minocycline is currently associated with the lowest rates of antibiotic resistance. Oral antibiotics, including minocycline, are effective in treating dermatological conditions, but are limited by systemic adverse effects. A topical preparation of minocycline should be able to harness the antimicrobial effects of the active ingredient while minimizing systemic exposure, but attempts to produce a topical preparation of minocycline have previously been unsuccessful, until the recent approval by the FDA of the first topical minocycline foam formulation.
FMX101 4% and FMX103 1.5% are novel, topical formulations of micronized minocycline being developed to treat dermal conditions, including acne and rosacea. These unique products result from a novel foam vehicle rationally designed to stabilize and deliver minocycline in a topical preparation for the first time. While the hydrophobic carrier, composed primarily of natural plant oils, was selected for its ability to protect minocycline from degradation, coconut and soybean oils have also been shown to have beneficial moisturizing effects on skin. The hydrophobic nature of the formulation helps to improve miscibility with model human sebum by lowering its melting temperature and decreasing its viscosity. Ex-vivo studies demonstrated that this hydrophobic formulation is capable of delivering high concentrations of minocycline to the epidermis and sebaceous appendage without correspondingly high systemic exposure. Importantly, the delivery of minocycline to the epidermis and sebaceous appendage is well above the MIC90 for minocycline, which suggests low potential for development of antimicrobial resistance.
In summary, FMX101 4% minocycline represents a novel approach to treating AV with an effective antibiotic formulated for the first time as a topical preparation. The hydrophobic foam formulation maintains minocycline in stable conditions and delivers high concentrations of the active agent to the epidermis and the sebaceous appendage, where C. acnes resides. These concentrations of minocycline are well above the MIC for C. acnes. Despite delivering high minocycline concentrations to the skin, the topical preparation appears to be well-tolerated and minimizes systemic exposure to minocycline.
References
- Biswal I, Gaind R, Kumar N, et al. In vitro antimicrobial susceptibility patterns of Propionibacterium acnes isolated from patients with acne vulgaris. J Infect Dev Ctries. 2016;10(10):1140–1145.
- Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448–456.
- Pena S, Hill D, Feldman SR. Use of topical retinoids by dermatologists and non-dermatologists in the management of acne vulgaris. J Am Acad Dermatol. 2016;74(6): 1252–1254.
- Jones TM, Ellman H, deVries T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16(10):1022–1028.
- Mendoza N, Hernandez PO, Tyring SK, et al. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013;52(6):688–692.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5): 945–973. e33.
- Thiboutot DM, Dreno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2 suppl 1):S1–S23. e1.
- Odsbu I, Selmer R, Stalsby Lundborg C, Blix HS. Increased prescribing of systemic tetracyclines and isotretinoin for treatment of acne. J Antimicrob Chemother. 2017;72(5):1510–1515.
- Beylot C, Auffret N, Poli F, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. J Eur Acad Dermatol Venereol. 2014;28(3):271–278.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80(1):168–177.
- Kraft J, Freiman A. Management of acne. CMAJ. 2011;183(7):E430–E435.
- Snyder S, Crandell I, Davis SA, Feldman SR. Medical adherence to acne therapy: a systematic review. Am J Clin Dermatol. 2014;15(2):87–94.
- Lopedota A, Cutrignelli A, Denora N, et al. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-beta-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev Ind Pharm. 2015;41(5):728–736.
- Lachenmeier DW. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J Occup Med Toxicol. 2008;3:26.
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77(3):456–463. e4.
- Chukwudi CU. rRNA binding sites and the molecular mechanism of action of the tetracyclines. Antimicrob Agents Chemother. 2016;60(8):4433–4441.
- Garrido-Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–352.
- Ochsendorf F. Minocycline in acne vulgaris: benefits and risks. Am J Clin Dermatol. 2010;11(5):327–341.
- Ross JI, Snelling AM, Carnegie E, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148(3):467–478.
- Eady EA, Cove JH, Holland KT, Cunliffe WJ. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121(1):51–57.
- Walsh TR, Efthimiou J, Dreno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16(3):e23–33.
- Rhein L, Zatz JL, Motwani MR. Targeted delivery of actives from topical treatment products to the pilosebaceous unit. 2007:223–252.
- Hazot Y, Malinov T, Gazal E, et al. Topical oleaginous minocycline foam: efficacious delivery into skin layers. J Anal Pharm Res. 2017;4(5):1–5.
- Raoof TJ, Hooper D, Moore A, et al. Efficacy and safety of a novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: a phase 3 study. J Am Acad Dermatol. 2020;82(4):832–837.
- Evangelista MT, Abad-Casintahan F, Lopez-Villafuerte L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double-blind, clinical trial. Int J Dermatol. 2014;53(1):100–108.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19(1).
- Rawlings AV, Lombard KJ. A review on the extensive skin benefits of mineral oil. Int J Cosmet Sci. 2012;34(6):511–518.
- Zhao Y, Jones SA, Brown MB. Dynamic foams in topical drug delivery. J Pharm Pharmacol. 2010;62(6):678–684.
- Tamarkin D. Foam: a unique delivery vehicle for topically applied formulations. In: Handbook of Formulating Dermal Applications: A Definitive Practical Guide. 2016:233–260.
- Cash KQ, M.O. The vehicle found in ketoconazole foam 2% is preferred by patients with mild to severe seborrheic dermatitis over other vehicles, regardless of gender, age, or ethnicity. J Am Acad Dermatol. 2008;58(suppl 2):AB92.
- Kahanek N, Gelbard C, Hebert A. Desonide: a review of formulations, efficacy and safety. Expert Opin Investig Drugs. 2008;17(7): 1097–1104.
- Vyas A, Kumar Sonker A, Gidwani B. Carrier-based drug delivery system for treatment of acne. ScientificWorldJournal. 2014;2014:276260.
- Dreno B, Bagatin E, Blume-Peytavi U, et al. Female type of adult acne: physiological and psychological considerations and management. Journal der Deutschen Dermatologischen Gesellschaft / Journal of the German Society of Dermatology: JDDG. 2018;16(10):1185–1194.
- Sutcliffe J, McLaughlin R, Webster G, et al. Susceptibility of Cutibacterium acnes to topical minocycline foam [published online ahead of print January 28, 2020]. Anaerobe. doi: 10.1016/j.anaerobe.2020.102169.

