 Dear Editor:
Dear Editor:
Dulaglutide is a glucagon-like peptide (GLP-1) receptor agonist, which stimulates insulin release via activation of GLP-1 receptors on pancreatic beta cells. Here, we report the first case of morbilliform drug eruption to dulaglutide.
Case presentation. An 84-year-old male patient with a medical history of Type 2 diabetes mellitus, hyperlipidemia, hypertension, and back pain presented to the emergency department with a rash. The rash appeared on his legs two weeks prior and then spread upwards to his trunk, neck, and upper extremities (Figures 1 and 2). Other symptoms included pruritus, and a burning sensation on the skin and inside the mouth. He reported no recent illnesses or tick bites. The only new medication that had been started was dulaglutide five weeks before the onset of symptoms. The patient reported no arthralgias, myalgias, gastrointestinal symptoms, nausea, vomiting, or fevers.
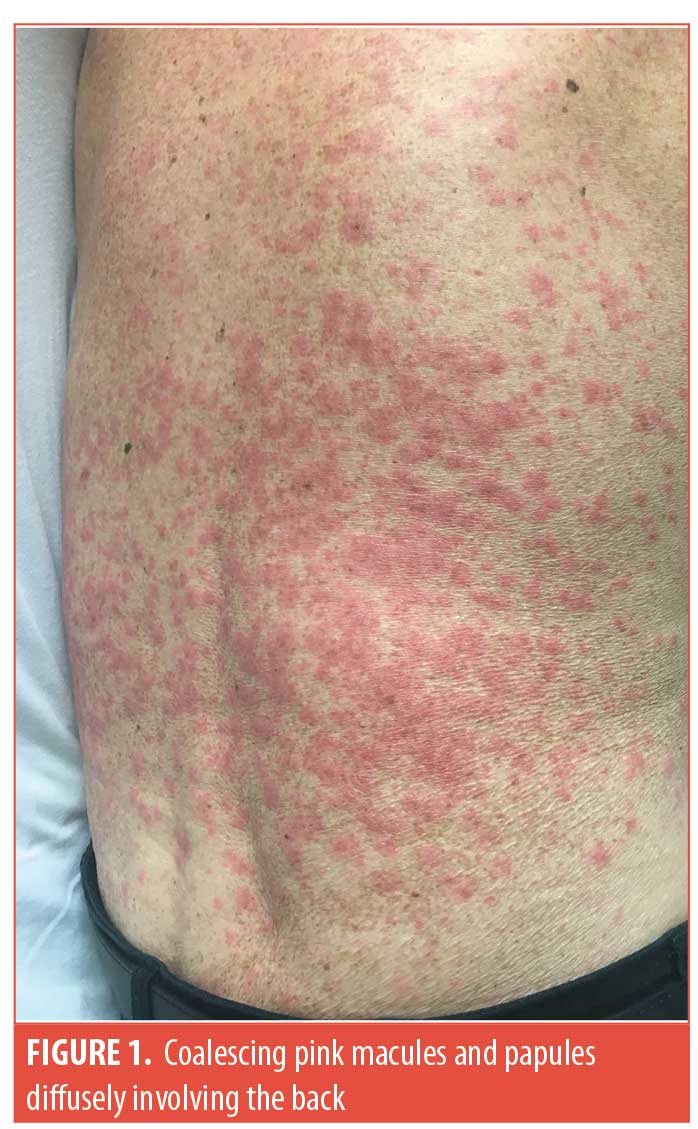
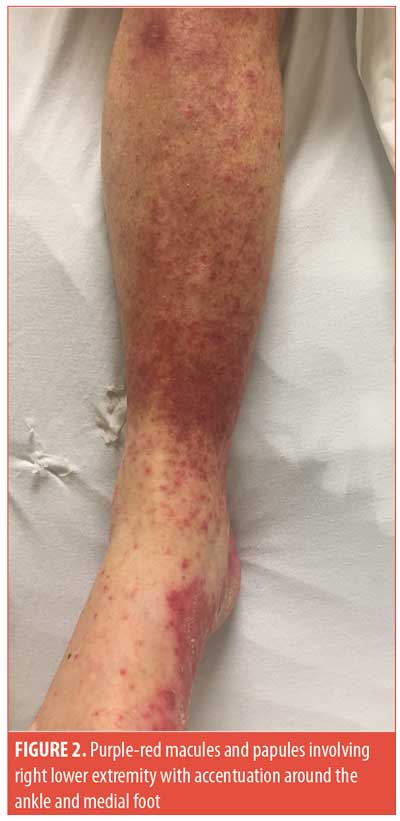
Physical exam demonstrated coalescing pink macules and papules in a morbilliform pattern that affected the patient’s lower extremities, abdomen, neck, upper extremities, and back. He also had purpuric discoloration of dependent lesions on his lower extremities and non-blanching petechiae of his bilateral palms. His buccal mucosa was erythematous, but there were no erosions observed. A punch biopsy was performed and showed interface dermatitis with eosinophils in the dermal inflammatory infiltrate, which suggested a hypersensitivity reaction. Labs revealed complete blood cell count, chemistry, and liver functions tests were within normal limits.
The patient was discharged with triamcinolone 0.1% cream to be applied twice daily to affected areas and a twelve-day prednisone taper starting at 60mg per day and was instructed to discontinue dulaglutide and follow up with his primary care physician for close glucose monitoring. His rash quickly improved following the treatments and the cessation of dulaglutide. The rash did not return, and the patient declined a retrial of dulaglutide.
Discussion. Dulaglutide is administered subcutaneously once a week. Common adverse effects of dulaglutide include nausea (13%), diarrhea (9%), and abdominal distension (8%).1 While gastrointestinal adverse events are most common, they are usually mild to moderate in severity and tend to diminish over time.2 Additional reported adverse events include injection-site reactions, cholelithiasis, and pancreatitis-related events.3
To our knowledge, morbilliform drug eruptions to dulaglutide have not been reported. Utilizing the Naranjo criteria of adverse drug eruptions, a score of 5 (the adverse event appeared after the suspected drug was administered, the adverse reaction improved when the drug was discontinued, there are no alternate causes that could on their own have caused the reaction) corresponding to probable chance was obtained. Therefore, it is likely that dulaglutide can contribute to morbilliform drug eruptions and providers should be aware of this possible reaction.
With regard,
Polina V. Rzepka, MD and Jessica A. Kaffenberger, MD
Affiliations. Drs. Rzepka and Kaffenberger are with the Division of Dermatology at the Ohio State University Department of Internal Medicine in Columbus, Ohio.
Funding. No funding was provided for this study.
Disclosures. The authors have no conflicts of interest relevant to the content of this article.
Correspondence. Jessica A. Kaffenberger, MD; Email: Jessica.Kaffenberger@osumc.edu
References
- Madsbad S, Kielgast U, Asmar M, et al. An overview of once-weekly glucagon-like peptide-1 receptor agonists—available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13(5):394–407.
- Gentilella R, Pechtner V, Corcos A, Consoli A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev. 2019;35(1):e3070.
- Goldenberg RM, Steen O. Semaglutide: review and place in therapy for adults with Type 2 diabetes. Can J Diabetes. 2019;43(2):136–145.

