J Clin Aesthet Dermatol. 2020;13(2):61–66
 by Mukta Sachdev, MD; Keerthi Velugotla, DDVL; Sapna Revanker, MD; and Geetha Somasekhar, DO, DNB
by Mukta Sachdev, MD; Keerthi Velugotla, DDVL; Sapna Revanker, MD; and Geetha Somasekhar, DO, DNB
All authors are with MS Clinical Research in Bangalore, India. Drs. Sachdev and Velugotla are also with MS Skin Clinic in Bangalore, India.
FUNDING: This study was funded by an educational grant from SkinGen International Inc.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. We sought to evaluate the efficacy of a polygrowth factor serum for increasing length, luster, thickness, and volume of eyelashes in a group of healthy Indian women.
Design. This was a 90-day, open-label, single-center safety and efficacy study. Thirty Indian female participants, aged 15 to 45 years old, were enrolled in the study; 29 of these subjects completed the study. There were four assessment visits: at baseline (Day 0) and on Days 30, 60, and 90. Subjects were instructed on the application of the test product uniformly to both eyes on the upper and lower eyelid margins. Subjects applied the product once nightly for 90 days.
Measurements. At each visit, subjects underwent ophthalmological and dermatological assessments and digital image photographs using Visia CR imaging system. Improvement in eyelash length, density/volume, luster, and curl were evaluated using imaging and software technologies.
Results. Improvement in test parameters was observed 30 days after initiation of product usage. Among the 29 subjects who completed the study, improvements in length (10.52%), volume (9.3%), luster (11.43%), thickness (35%), and curl (50.83%), compared to baseline, were recorded.
Conclusion. The study demonstrated efficacy in improving eyelash length, luster, thickness, and curl. The results were observed as early as 30 days of product usage and persisted until the last visit following 90 days of product usage. There were no adverse events associated with the product. We concluded that the polygrowth factor serum was well tolerated and effectively improved eye lash length, luster, thickness, and volume in our patient sample. Additional randomized, controlled studies with larger samples are needed to confirm our findings.
KEYWORDS: Polygrowth factor serum, eyelash growth
Eyelash growth enhancement to obtain thicker, fuller, and longer lashes is one of the most sought-after aesthetic desires.1 Mascaras, artificial eyelashes, eyelash reconstruction, eyelash growth stimulating serums and solutions, eyelash transplantation are the various modalities currently available.2
The function of eyelashes is to protect the eyeball from debris and foreign material and act as triggers for the blink reflex.3 The eyelash growth cycle has three phases: anagen (i.e., growth phase), catagen (i.e., transition phase) and telogen (i.e., resting phase).4,5
The growth cycle of eyelashes is postulated to last 5 to 12 months.6,7 Factors such as signaling molecules, growth factors, cytokines, and hormones influence the growth cycle. The effect of growth factors and cytokines on the hair follicle has been studied in vitro as well as in vivo.8–10 Various growth factor families including epidermal growth factor(EGF)-related ligands, fibroblast growth factors (FGF), transforming growth factor-beta (TGF-beta), insulin-like growth factor (IGF), hepatocyte growth factor/scatter factor (HGF/SF), and platelet-derived growth factor (PDGF) have been shown to be involved in the regulation of the hair cycle and hair growth, in addition to hormones, cytokines, and growth peptide molecules.11,12 However, research is lacking regarding which growth factors are involved in eyelash growth.
Eyelash growth enhancement has been reported to be a side effect of prostaglandin analogues used in the treatment of glaucoma and other ocular diseases.13As a result, many eyelash growth enhancing serums have been developed using prostaglandin analogues as key ingredients. The efficacy of the various prostaglandin analogues and bimatoprost 0.03% in enhancing eyelash growth has been extensively studied.3,14,15 However adverse effects, including conjunctival hyperemia, cataracts, ocular dryness and pruritus, and periocular skin pigmentation limit their use and underscore the need for safer alternatives.12,14,15
Eyelash hypertrichosis has also been observed in association with malignancy, drugs (especially anticancer medication and in patients with inflammatory eyelid diseases), and connective tissue disorders, suggesting the high levels of growth factors and peptides characteristic of these conditions might promote excessive eyelash growth.16–21
Based on this observation, an eyelash polygrowth factor serum was developed using advanced growth factor technology that combined growth factors into a solution at an optimal concentration. We conducted a human volunteer study to determine the efficacy of eyelash polygrowth factor serum in a group of healthy Indian female participants.
Materials and Methods
Study design. This was an open-label, single-center, single-arm efficacy study that included 30 healthy Indian female subjects. Included participants were women aged 18 to 45 years with an expressed desire for eyelash improvement.
Key exclusion criteria included the following:
- A history or present condition of allergic response to any cosmetic products
- Presence of active skin diseases (e.g., moderate-to-severe acne vulgaris or nodulocystic acne, psoriasis, active atopic dermatitis, melasma, lichen planus pigmentosus/ashy dermatosis, pigmented contact dermatitis, or other cutaneous manifestations) which might interfere with the test readings
- Use of oral medications (e.g., steroids, antioxidant, antihistamine) that might compromise study results
- A history of high fever lasting for more than 48 hours within seven days prior to start of the study
- Use of topical medications, such as ointments and creams
- Systemic treatments (e.g., retinoid therapy) within 30 days of study
- History of intense sun exposure and/or photo toxicity/allergy
- Presence of wounds, sunburn, scars, tattoos, or piercings at the skin test site
- Pregnancy or lactation
- Presence of any underlying, uncontrolled medical illness, including diabetes mellitus, hypertension, liver disease or history of alcoholism, HIV, hepatitis, or any other serious medical illness.
The primary objective of the study was to evaluate the efficacy of the once-daily application of a polygrowth factor serum to the eyelid margins for 90 days for the purpose of improving eyelash length, luster, thickness, volume, color, and curl compared to baseline. The secondary objective was to evaluate the eyelash quality in terms of appearance, color, and volume through dermatological evaluation compared to baseline and to evaluate the ophthalmologic and dermatological safety of the test product.
Thirty subjects were enrolled in the study and were treated for 90 days. Quantitative and qualitative measurements of the eyelashes were recorded to ensure that evidence could be provided for the safety and efficacy of the test serum. The key response evaluation tools included high-resolution digital images taken at baseline, and 30 days, 60 days, and 90 days after treatment initiation. In addition, changes in eyelash length, volume, luster, thickness, and curl were measured using advanced measurement tools, including Visia CR imaging, Caslite Nova software, and Dinolite microscopic camera. Patients completed questionnaires to assess subjective improvement and satisfaction.
The study was conducted after approval from an independent ethics committee and in accordance with the principles stated in the Declaration of Helsinki and its subsequent amendments and the good clinical practice (GCP) guidelines. Subjects who satisfied the study inclusion criteria were enrolled after obtaining voluntary informed consent.
Subject assessments and visits Each subject participated for approximately three months, and attended a total of four visits— at baseline (Day 0) and on Days 30, 60, and 90. During their baseline assessment, the subjects were instructed to wash their face and undergo acclimatization for about 10 minutes. After this, initial dermatological and ophthalmological examinations and imaging were completed without application of the test product. After baseline assessments, the test products were applied on the eyelashes at the site. The subjects were instructed on the application to ensure that the test product was applied along the cutaneous margins of the eyelids to both the eyes uniformly and in a similar fashion. After product application, the subjects underwent product tolerance assessment by the ophthalmologist and dermatologist, who evaluated the participants for any localized ocular and skin irritation. Subjects were given the eyelash serum to apply once nightly at home for three months. Subjects were instructed to revisit the center after 30, 60, and 90 days (Visits 2, 3, and 4, respectively). During each visit, the subjects underwent ophthalmologic and dermatologic assessments for safety. Additionally, images were captured by a professional photographer under standardized, controlled conditions to determine the improvement of eyelashes, using an imaging system (VISIA®-CR, Canfield Scientific; Parsippany, New Jersey) .
Results
Safety. Twenty-nine out of thirty subjects completed the study. The one subject who did not complete the study was lost to follow-up. Two subjects experienced a mild stinging sensation upon application of the product, which lasted 2 to 5 seconds. No other adverse events were reported.
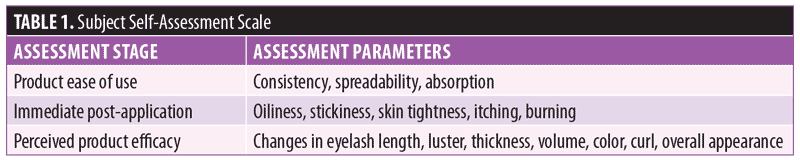
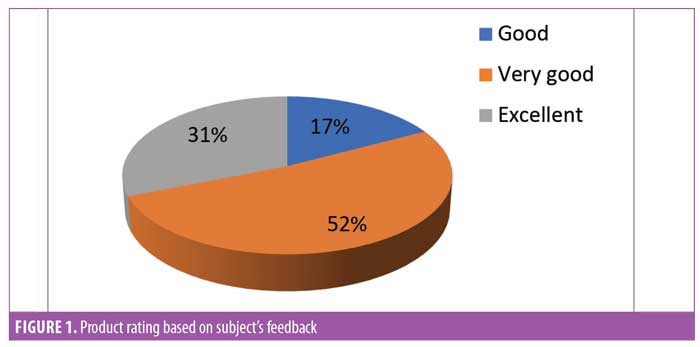
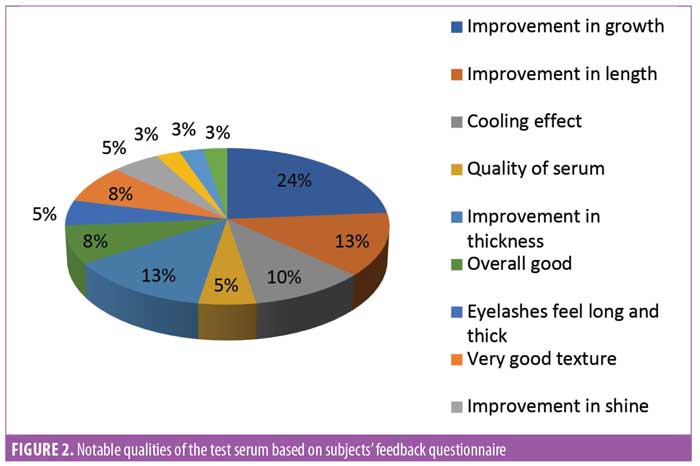
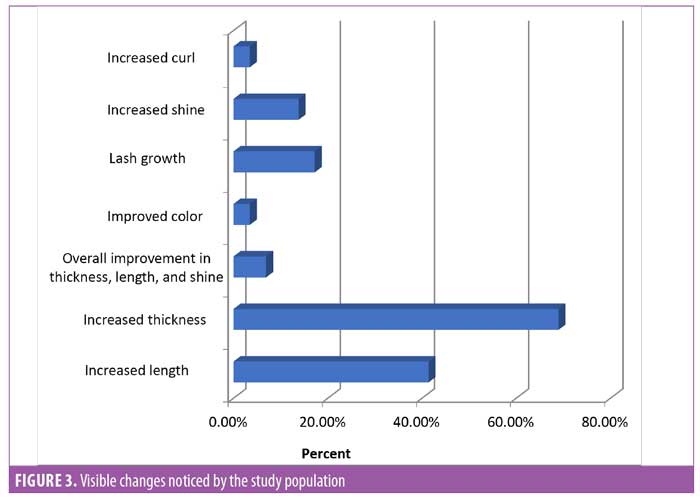
Subject feedback (Table 1). Eighty-three percent of subjects rated the product as “very good” or “excellent.” Out of the parameters evaluated, the subjects perceived the most noticeable improvements in thickness and length of eyelashes (Figures 1, 2, and 3).
Dermatologist’s assessment. The dermatologist assessment was performed using image comparison and showed a significant improvement in quality, color, and volume of the eyelashes over the three-month period. Statistical analyses were done by paired two sample t-test; the dermatological assessment for improvement in quality, color, and volume of lashes was statistically significant with a p-value of <0.0001.
Ophthalmologist’s assessment. The ophthalmologist assessed the subjects for irritation using the Draize Scale for Erythema, Dryness, Wrinkles, and Edema (Table 2). The ophthalmologist also graded the eyes for ocular reaction (conjunctivae, cornea, and iris). Evaluations were performed at baseline, and Visits 2,3, and 4.
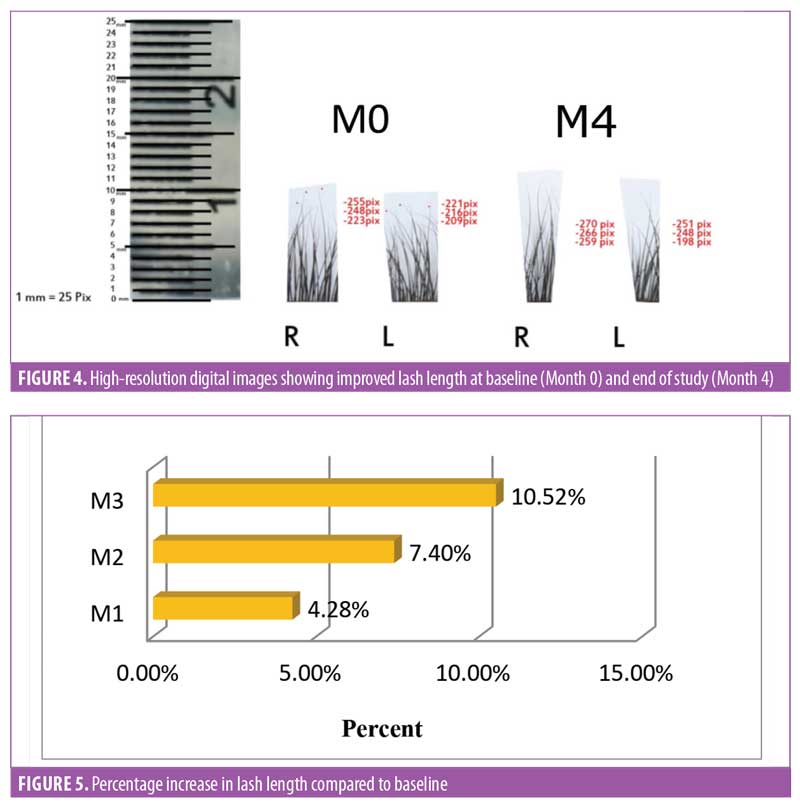
Lash length. There was an improvement in eyelash growth as measured using the advanced digital photography (Figure 4). The increase in eyelash length was significant (p<0.0001) after one month of product usage. Lash length continued to increase until Day 90. After three months of regular use, a 10.52-percent increase in the eyelash length was observed (Figure 5), and increases in eyelash length was observed in over 95 percent of the treated subjects (n=28).
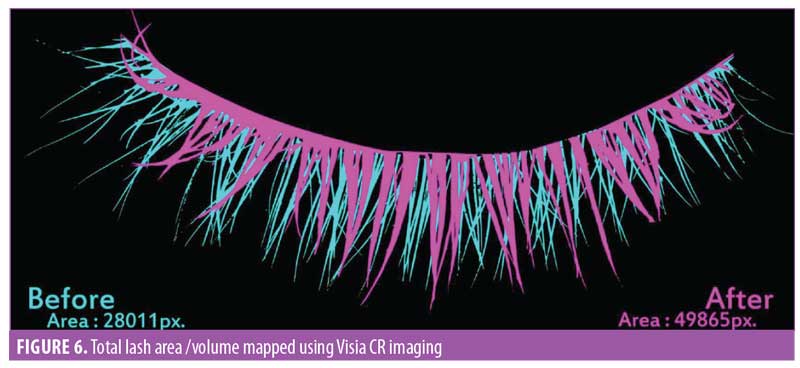
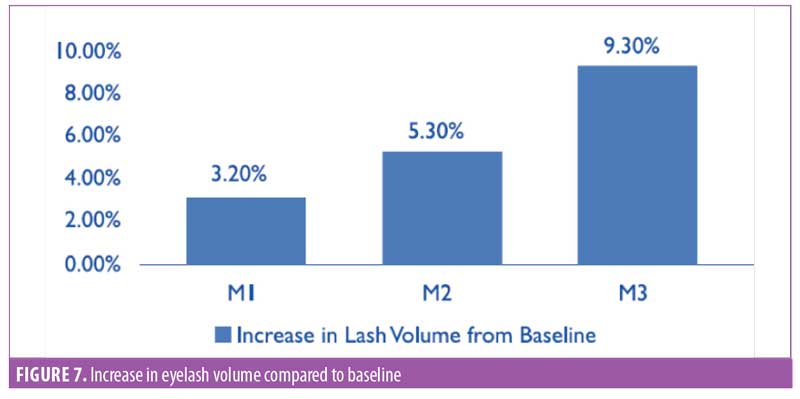
Eyelash volume. A software-based assessment for eyelash volume was performed by mapping the total eyelash area using the imaging software (Figure 6). The area was noted by pixels, then converted to centimeters squared. The improvement in eyelash volume was statistically significant (p<0.0001) after one month of product use. A visual comparison showed an improvement over the period of the trial. A 9.3-percent increase in the average volume was noted by the end of the study (Figure 7). The improvement was noted in 95 percent of participants (n=28).
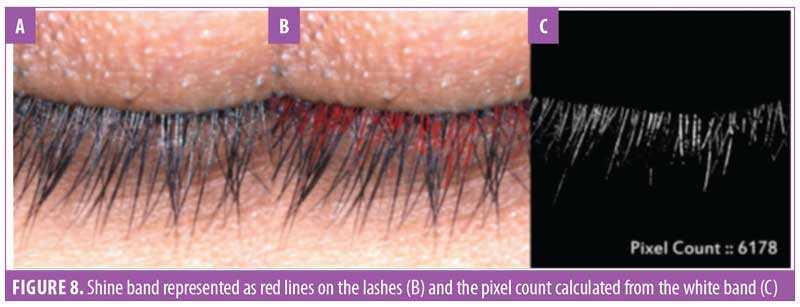
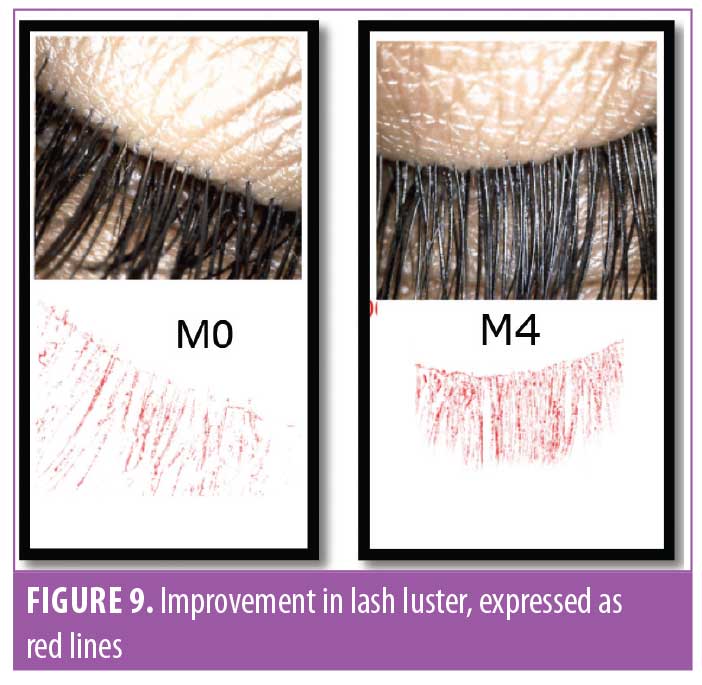
Eyelash luster. Hair luster is the shine profile identified by the interaction of light with the hair fiber.22 When light reflects off of the hair surface, it can be visualized as a white band, also called the shine band (Figure 8). The luster is a result of reflectance, refraction, and diffusion of background light. In simpler terms, it is directly proportional to the shine band; the longer the shine band, more lustrous the hair appears.22 The shine band area and the total picture area are counted in pixels and the luster index is calculated. The improvement in lash luster was statistically significant (p<0.0001) after one month of instructed use and was progressive until the last visit at Day 90 (Figure 8). The entire study population who completed the study (N=29, 100%) showed an improvement in lash luster. The VISIA®-CR imaging system was used to capture the shine band, which is expressed as red lines in Figure 9. The percent area with shine band was calculated to perform the statistical analysis (Luster Area Index=Px [shine band area]/ Total Px [area of the image]x100). An 11.43-percent improvement in lash luster index was recorded.
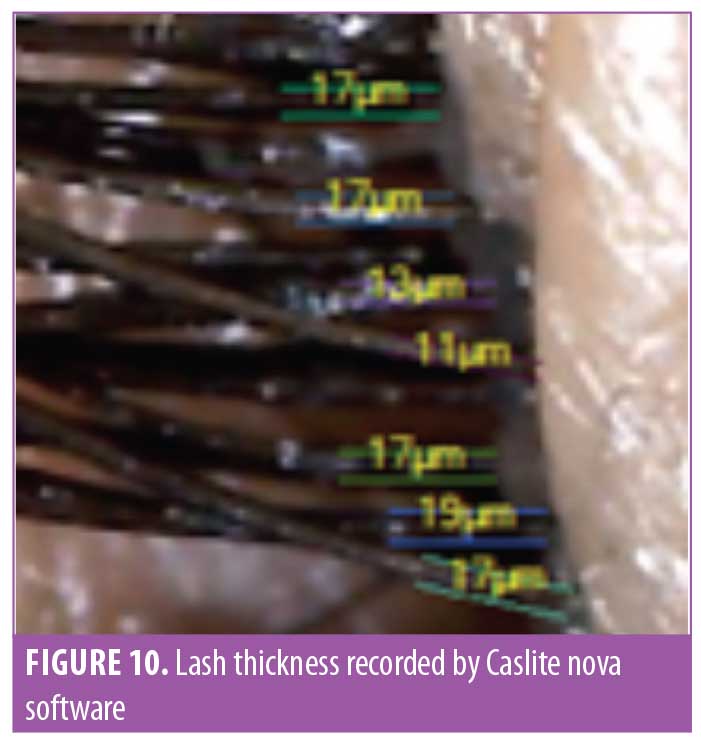
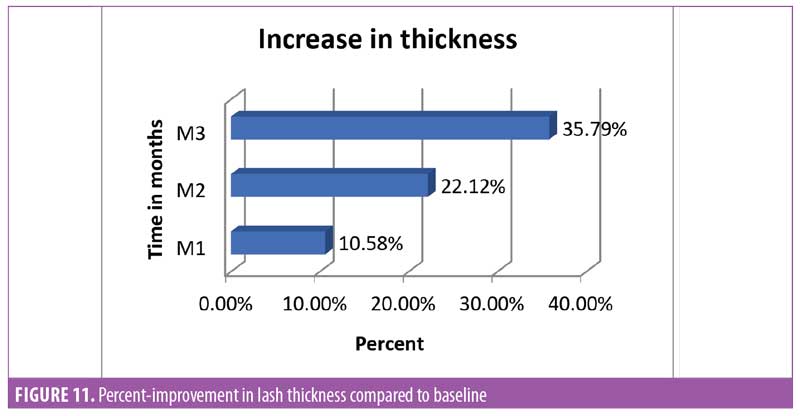
Lash thickness. Using the Dinolite microscopic camera (AnMo Electronics Corporation, Taiwan) and Caslite Nova software (Catseye Systems and Solutions Private Limited, Mumbai, India) the thickness of individual eyelashes from each participant were measured across the cross-section. The measurement was performed for each eyelash hair fiber with visibly clear edges (Figure 10). The average value reported by the software was considered for statistical analysis. The improvement was statistically significant (p<0.0001) after 30 days of product use. The improvement was progressive until the last observation was made at Day 90. The entire study population (N=29, 100%) showed improvement in eyelash hair thickness (Figure 11).
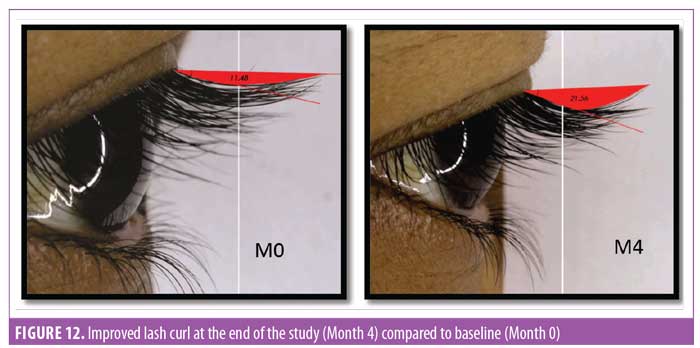
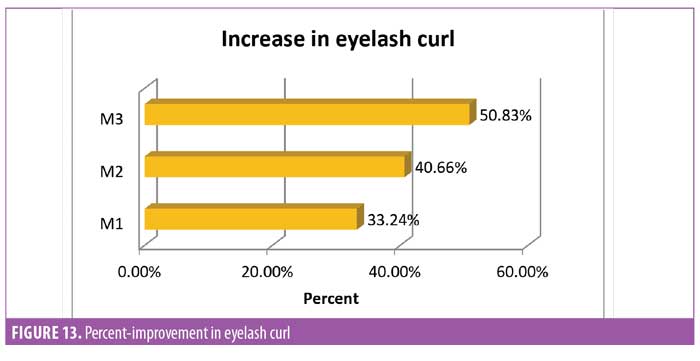
Eyelash curl. Curled eyelashes have a higher aesthetic visual appeal.23 Measurement of improved curl was considered one of the key parameters in this study. To measure curl, the angle between the base of the hair and tip of the hair was measured (Figure 12). It was noted that after 30 days of regular use, the product appeared to yield statistically significant (p<0.0001) improvement in the curl angle. By the end of the study at Day 90, the improvement was about 50 percent compared to baseline and improvement was noted in all subjects (N=29) (Figure 13).
Discussion
Our study evaluated the efficacy of an eyelash polygrowth factor serum in improving length, luster, thickness, volume, and curl compared to baseline and established the safety of this product in the test population.
Growth factors are the key active ingredients in the test serum. Growth factors are giant molecules with high molecular weight (nearly 2kDa), making it impossible for them to permeate through the epidermis.24,25 Studies suggest that the probable route of penetration of these molecules is through hair follicles, sweat glands, and in conditions with disrupted barrier functions.26,27 The growth factors in the serum are manufactured by recombinant technology using Escherichia coli (E.coli). They are nano-encapsulated to enhance penetration and ensure their delivery to the hair follicle through the intact epidermis. The product is formulated with these three key aspects in mind to enhance eyelash growth—the actives in the serum have been formulated to 1) stimulate eyelash growth; 2) minimize the body’s natural inhibition to eyelash growth; 3) nourish the eyelash follicles to ensure the results last.
The growth factors in the test serum are keratinocyte growth factor (KGF), fibroblast growth factor (FGF), insulin-like growth factor-2 (IGF-2), and vascular endothelial growth factor (VEGF), which target at various levels of hair growth cycle.1,4 Growth factors are theorized to stimulate bulge stem cells in the dermal papilla of hair to proliferate and modulate growth-phase transitions; the same theory posits that stem cells play a role in regulating the duration of the anagen phase.28 The growth factors in the serum formulation are thought to act on these stem cells and promote eyelash growth by prolonging the anagen phase.
The Salvia officinalis oil in the serum is thought to enhance eyelash growth by inhibiting the androgen-mediated shedding of lashes and providing nourishment to the eyelash follicles.29 Other key ingredients include the two peptide proteins, myristoyl pentapeptide-17 and myristoyl hexapeptide-16, which have demonstrated the potential to stimulate eyelash growth.30 These peptides act by stimulating keratin production, which is a key structural protein of hair.31,32 In addition, myristoyl pentapeptide-17 promotes the delivery of key ingredients in the serum, such as the growth factors, Salvia officinalis oil, and lysophosphatidic acid.33
Conclusion
Efficacy was observed after 30 days of product usage, and the results were progressive until the last observation made at the 90-day assessment. The improvement in eyelash curl was a unique measurement recorded by the center, as there are not many studies done on the eyelash improvement in the literature that has included this feature. The ability to increase and enhance lash curl, length, thickness, and volume with minimal adverse reactions makes the product unique compared to other products in the market.
Limitations. Small sample size, limited study duration, lack of control, and lack of investigator blinding are the limitations of our study.
With the exception of minor, transient irritation experienced by two subjects, the product was well tolerated by all participants, and according to the subject questionnaire, yielded high patient satisfaction. Overall, our study suggests that this polygrowth factor serum is safe and effective for the enhancement of eyelashes. Additional larger, randomized, controlled studies are needed to confirm our results.
Acknowledgments
We acknowledge Rachana Shilpakar, MD, Mukesh Ramnane, MD, and Ms. Ritambhara for their contributions to this study.
References
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424–430.
- Jones D. Enhanced eyelashes: prescription and over-the-counter options. Aesthetic Plast Surg. 2011;35:116–121.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010; 3: 39–48.
- Aumond S, Bitton E. The eyelash follicle features and anomalies: a review. J Optom. 2018;11(4):211–222.
- Kloepper JA, Sugawara K, Al-Nuaimi Y, et al. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol. 2010;19:305–312.
- Elder MJ. Anatomy and physiology of eyelash follicles: relevance to lash ablation procedures. Ophthalmic Plast Reconstr Surg. 1997; 13(1):21–25.
- Na JI, Kwon OS, Kim BK, et al. Ethnic characteristics of eyelashes: a comparative analysis in Asian and Caucasian females. Br J Dermatol. 2006;155(6):1170–1176.
- Tsuboi R. Growth factors and hair growth. Korean Journal of Investigative Dermatology. 1997; 4(2):103–108.
- Philpott MP, Kealey T. Effects of EGF on the morphology and patterns of DNA synthesis in isolated human hair follicles. J Invest Dermatol. 1994;102: 186–191.
- Philpott MP, Kealey T. Rat hair follicle growth in-vitro. Br J Dermatol. 1992;27:600–607.
- Peus D, Pittelkow MR. Growth factors in hair organ development and the hair growth cycle. Dermatol Clin. 1996;14(4):559–572.
- Rosenquist TA, Martin GR. Fibroblast growth factor signaling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn. 1996;205(4): 379–386.
- Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53(Suppl 1):93–105.
- Giannico AT, Lima L, Russ HH, et al. Eyelash growth included by topical prostaglandin analogues, bimatoprost, travoprost, travoprost and latanoprost in rabbits. J Ocular Pharmacol Ther. 2013;29(9): 817–820.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010; 36(5):638–649.
- Vélez A, Kindelán JM, García-Herola A, et al. Acquired trichomegaly and hypertrichosis in metastatic adenocarcinoma. Clin Exp Dermatol. 1995; 20:237–239.
- F Braiteh, R Kurzrock, FM Johnson. Trichomegaly of the eyelashes after lung cancer treatment with the epidermal growth factor receptor inhibitor erlotinib. J Clin Oncol. 2008;26:3460– 3462.
- Bouché O, Brixi-Benmansour H, Bertin A, et al. Trichomegaly of the eyelashes following treatment with cetuximab. Ann Oncol. 2005;16:1711–1712.
- Pucci N, Novembre E, Lombardi E, et al. Long eyelashes in a case series of 93 children with vernal keratoconjunctivitis. Pediatrics. 2005;115: 1542–1555.
- Sharma RC, Mahajan VK, Sharma NL, Sharma A. Trichomegaly of the eyelashes in dermatomyositis. Dermatology. 2002;205:305.
- Santiago M, Travassos AC, Rocha MC, Souza S. Hypertrichosis in systemic lupus erythematosus (SLE). Clin Rheumatol. 2000;19:245–246.
- Wortmann F ,Schulze zur Wiesche E, Bourceau B. Analyzing the laser-light reflection from human hair fibers. II. Deriving a measure of hair luster. J Cosmet Sci. 2004;55:81–93.
- Kwak TJ, Lee SM, Cho WG. The character of eyelashes and the choice of mascara in Korean women. Skin Res Technol. 2002 Aug;8(3):155–163.
- Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000; 9(3):165–169. 21.
- Andrews SN, Jeong E, Prausnitz MR. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm Res. 2013;30(4): 1099–1109.
- Schaefer H, Lademann J. The role of follicular penetration. A differential view. Skin Pharmacol Appl Skin Physiol. 2001;14 (Suppl)1:23–27. 23.
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525
- Hoover E, Alhajj M, Flores JL. Physiology, Hair. Updated 2019 Aug 10. In: StatPearls. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm. nih.gov/books/NBK499948/.
- Rastegar H, Ashtiani HA, Aghaei M, et al. Herbal extracts induce dermal papilla cell proliferation of human hair follicles. Ann Dermatol. 2015;27(6): 667–675.
- Kemper FH, Luepke NP, Umbach W. Blue List: Cosmetic Ingredients. Aulendorf: Editio Cantor Verlag; 2000. Er. 568
- Zangl A. The evolution of peptides. Dermascope site. https://www.dermascope.com/ingredients/ the-evolution-of-peptides. Accessed Jan 21 2020.
- Myristoyl Hexapeptide-16. INCI Decoder site. https://incidecoder.com/ingredients/myristoylhexapeptide-16. Accessed Jan 21 2020.
- Myristoyl Pentapeptide-17. Creative Peptides site. https://www.creative-peptides.com/product/ myristoyl-pentapeptide-item-cpc1655-164.html. Accessed Jan 21 2020.

