 J Clin Aesthet Dermatol. 2022;15(10):52–57.
J Clin Aesthet Dermatol. 2022;15(10):52–57.
by Dèbora Aparecida Oliverira Modena, PhD; Ciro Dantas Soares, PhD; Elaine Cristina Candido, MSc; Felipe Mendonça Chaim, PhD; Everton Cazzo, PhD;
and Elinton Adami Chaim, PhD
Drs. Oliveira Modena, Candido, Chaim, Cazzo, and Chaim are with the Department of Surgery, Medical Sciences Institute at the University of Campinas (UNICAMP) in São Paulo, Brazil. Dr. Soares is with the Oral Pathology Section, Department of Oral Diagnosis, and Piracicaba Dental School at the University of Campinas (UNICAMP) in Piracicaba, Brazil.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Obesity became a worldwide public health problem and its treatment presents a strict relationship with skin flaccidity, for which the development of non-invasive therapies is an emerging field.
Objective. This study aims to evaluate the physiological response of the skin tissue of individuals with obesity with flaccidity to the physiological stimulus of shockwave therapy (ESWT).
Methods. This is a comparative intervention study based on histological and immunohistochemical analyses of a set of samples of skin tissue of women with Grade II obesity who achieved a 10 percent preoperative weight loss before bariatric surgery and complaints of skin flaccidity, subjected to the ESWT treatment. A total of seven sessions were carried out in the abdominal region on the left side, and the collateral side was used as control; the biological material was collected at the moment of the bariatric surgery. Hematoxylin and Eosin, Masson’s trichrome, Picrosirius Red and the markers for immunohistochemical were used for the morphological evaluation.
Results. Fourteen women were included in the research. The results demonstrated that the tissue which underwent the ESWT intervention presented significant increases of fibroblast cells (p<0.0001) and collagen fibers Type I and II (p<0.0002). In the significant expressions of the markers FGF1, FGF2, FGFR1 were identified in the exposed side (p<0.0002, 0.0017, <0.0001, respectively) as well as a significantly higher expression of Ki67 marker of cell proliferation (p<0.0002).
Conclusion. ESWT was associated with a significant increase of cell proliferation and collagen expression in flaccid skin of individuals who achieved weight loss before bariatric surgery.
Keywords. Dermatology, obesity, skin flaccidity, shockwave, quality of life
The skin tissue constantly suffers with biomolecular and structural alterations stemming from chronological aging, photoaging or substantial changes in body dimensions during pregnancy, obesity periods and, particularly, after excessive weight loss. Such alterations generate damage in the collagen and elastin fibers, causing flaccid or saggy skin.1,2
Recently, there has been an increase in the search for treatments for skin flaccidity, including for individuals with flacid skin secondary to rapid weight loss.2,3 Many electrothermal phototherapeutic resources have been researched for skin flaccidity, including radiofrequency, high power laser and ultrasound; however, with the recent technological advancements, new resources have been emerging, such as the extracorporeal shockwave therapy (ESWT).3,4 ESWT is characterized as high intensity mechanical waves which present the ability to generate cavitation resulting in formation of gas bubbles in liquid medium. These waves propagate in the target tissue, stimulating action mechanisms and, consequently, physiological effects. Some studies show that the ESWT has the ability to improve the metabolism and activity of several types of cells, including dermal and epidermal, and thus modulate the repair and tissue regeneration process.5-7
To date, there is no clear evidence that substantiates the real physiological effect of ESWT in the skin tissue, and therefore in this study we aimed to evaluate the efficacy and safety of the ESWT therapy in stimulating the fibroblast cells of the dermis in individuals with obesity aiming to favor neocollagenesis through a histological and immunohistochemical analysis.
This study aims to evaluate the physiological response of the skin tissue of individuals with obesity and skin flaccidity to the physiological stimulus of ESWT.
Methods
Ethical considerations. This is a comparative intervention study that has been performed in accordance to the 1964 Declaration of Helsinki and was approved by the Institutional Ethics Committee of the University of Campinas (Unicamp) under the recommendation number 2.281.487 and registered with ClinicalTrials nº NCT03275259.
As inclusion criteria, women participating in the group of pre-surgical preparation for bariatric surgery at the Hospital de Clinicas da Unicamp, presenting obesity Degrees I and II, who had weight loss of at least 10 percent of the initial weight with indication for bariatric surgery and complaints of abdominal skin flaccidity were selected. As exclusion criteria, women presenting with diabetes mellitus, with skin lesions such as dermatitis and dermatosis, with a history of deep venous thrombosis, smokers, or who had electronic implanted devices such as cardiac pacemakers were ineligible.
Procedures of ESWT. The participants of the treated group received seven therapy sessions by ESWT twice a week, and the last session 20 minutes before the bariatric surgery procedure; the therapy was carried out in an area of 150cm2 on the left side of the abdominal region following the linea alba, with frequency of 15Hz, being 4.000 shots with energy of 180mJ and stainless steel tip of 15mm and 2.000 shots with energy of 100mJ with plastic tip of 15mm, for sliding the tip, the neutral lotion Loção Neutra Thork ® Essencial Cosméticos Indústria LTDA- MED Anvisa nº 25351.419510/2017- 95 was used.
For the therapy, the THORK Shock Wave®- IBRAMED- Indústria Brasileira de Equipamentos Eletromédicos EIRELI approved by Anvisa number 10360310036 equipment was used. The right side of the abdomen of the participants was used as control and did not receive ESWT therapy (control group).
Surgical procedure. First, the anesthetic procedure was carried out. Then, two fragments of skin tissue (average size of 4cm in diameter) were removed during bariatric surgery: (1) the area treated with EWST and (2) the contralateral side without treatment considered as the control.
Histological procedure. The biopsy samples were washed and then fixed in a solution of buffered 10% formaldehyde for 48 hours, histotechnically processed, embedded in paraffin, and cut with a rotating microtome in 3–5μm-thick sections.
For the histological evaluation of the skin tissue, the slides were stained with Hematoxylin and Eosin (HE) and Masson Trichrome (ab150686, Abcam, Cambridge, United Kingdom). For the morphological analyses, the slides were evaluated by light microscopy with a binocular microscope (Nikon YS 100, Japan) adapted with ocular WSCF 10X/18 and objective lenses Nikon 4X/0.10, 10X/0.25, 40X/0.65 and 100X/1.25.
For the differentiation of the types of collagen fibers the coloration the Picrosirius Red (ab150681, Abcam) was used. After the coloration, the slides were analyzed under polarized light with the DMR microscope (Leica) and photographs were taken with magnification of 400x, the collagen fibers were differentiated by means of their coloration. The Type I collagen fibers appears in yellow-orange coloration and Type III in green coloration, for quantification of the analyses, the software ImageJ® (NIH, Bethesda, USA) was used. All analyses were carried out based on previous studies of our group, and followed the standardized protocols of the pathology laboratory of the Hospital de Clinicas da Unicamp.
Immunohistochemical procedures. Three-μm sections were obtained from the blocks and placed over signaled slides (3-aminopropyl-triethoxysilane, Sigma- Aldrich, USA). In all the reactions, positive and negative controls were carried out. The reactions were carried out following the previously published protocols (Meyer et al, 2017; Soares et al, 2019).8,9
To summarize, the tissues were subjected to deparaffinization, rehydration and peroxidase endogenous blocking processes. The antigen retrieval was carried out with ethylenediamine tetra-acetic (EDTA), in an electric pressure cooker for 15 minutes.
For the immunohistochemical analysis, the following antibodies were used: fibroblast growth factor markers (FGFs) and their respective receptors: FGF1 (Antibody sc-55520, Santa Cruz Biotechnology, USA), FGF2 (Antibody sc-365106, Santa Cruz Biotechnology), FGFR1 (Antibody sc-121, Santa Cruz Biotechnology), FGFR2 (Antibody sc-122, Santa Cruz Biotechnology) and to evaluate the cell proliferation the Ki67 (Antibody ab92742, Abcam) marker was applied.
The antigen-antibody reaction was developed using a chromogenic substrate (3.3 diaminobenzidine-DAB, Sigma-Aldrich). The slides were then digitally scanned and analyzed, also according to previously published protocols (Meyer et al, 2017; Soares et al, 2019.8,9
Statistical analysis. First, for all the quantitative variables, two normality tests were used (D’Agostino and Pearson), to evaluate if the data presented a normal distribution. The statistical significance of the results was verified through the parametric analysis of variance of the Student’s t-test. The level of significance adopted was 5 percent (p=0,05).
Results
Twenty women participated in the research, but only fourteen were selected after application of inclusion and exclusion criteria (6 of them did not follow the internal protocol of the pre-surgical program of the bariatric surgery of the Hospital de Clínicas da Unicamp). All the participants included in the research underwent the bariatric surgery procedure, eight of the participants with laparoscopy surgery and six by videolaparoscopy.
The age average was 35.0 ± 8.6 years, initial weight (kg) 101.8±7.9, height (cm) 1.63±0.05, body mass index (BMI) kg/cm2, was 39.0±3.5, considered obesity Degree II. None of the participants presented associated pathologies, such as diabetes and hypertension.
Morphological analysis, increase of number of fibroblast cells. Based on the HE technique, the treated group revealed a significant increase of the number of fibroblast cells, dense collagen fibers in the dermis and inflammatory cells in the epidermis and dermis compared to the non-treated side (p<0.0001), which did not receive the ESWT, as demonstrated in Figure 1.
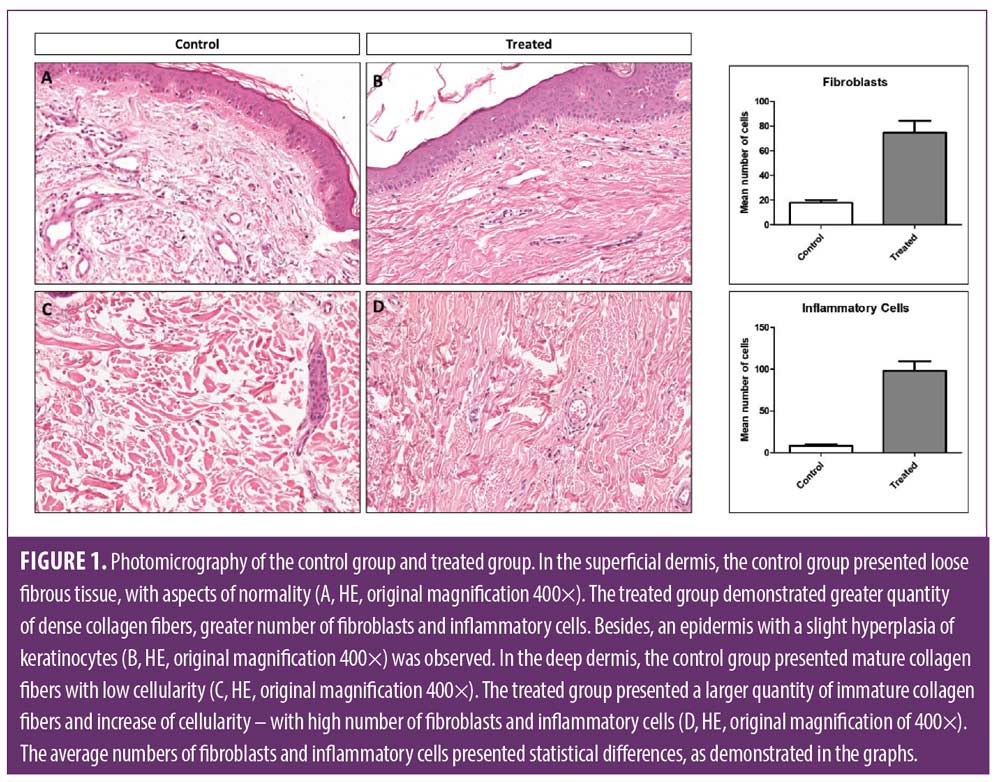
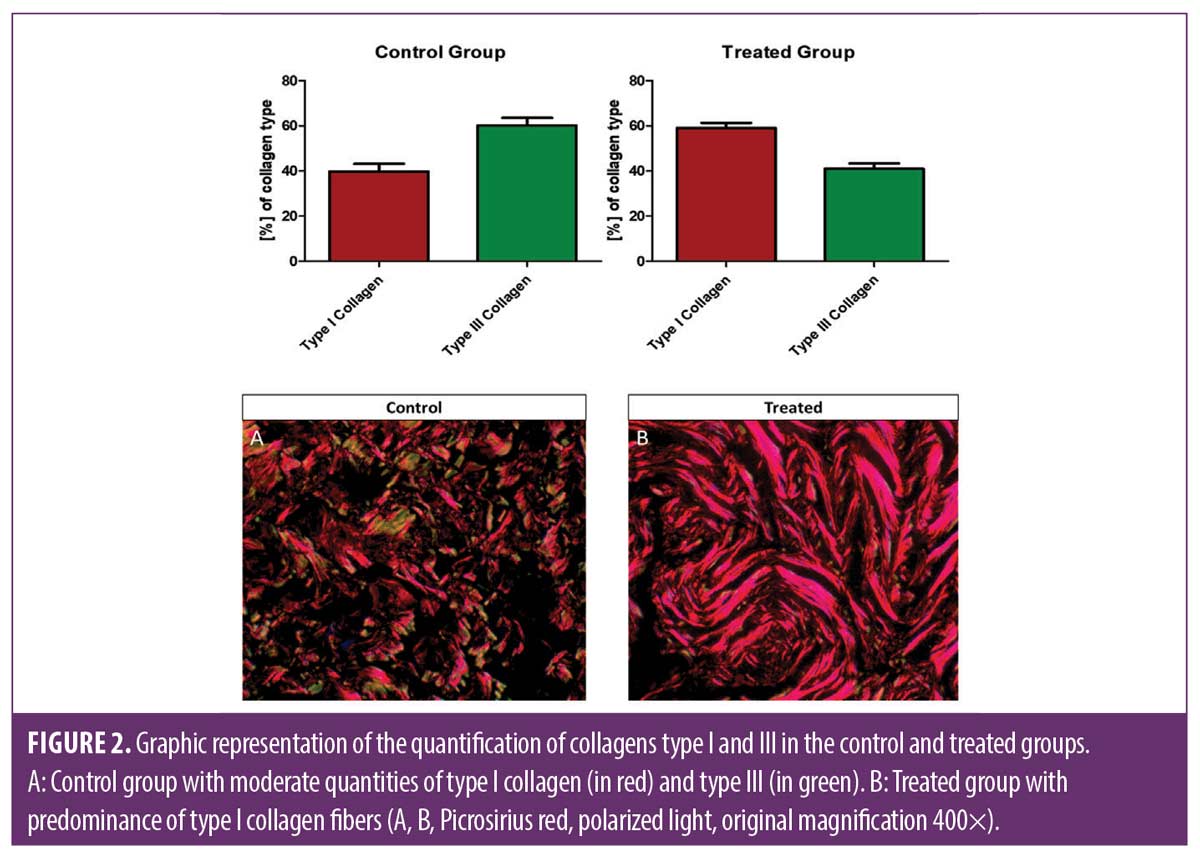
Quantification of Type I- and Type III-collagen. In the analysis with special coloration with Picrosirius red, the side treated by ESWT presented predominance of Type I collagen, and the non-treated side presented predominantly Type III collagen (p< 0.0002). These results indicate a turnover in the neocollagenesis induced by ESWT (Figure 2).
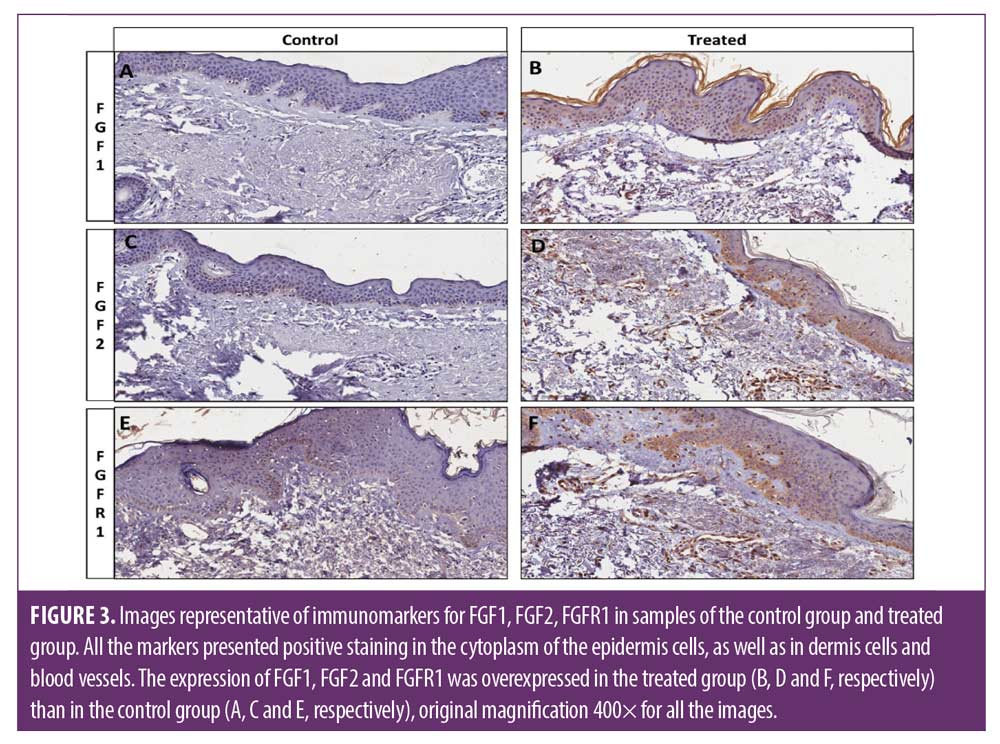
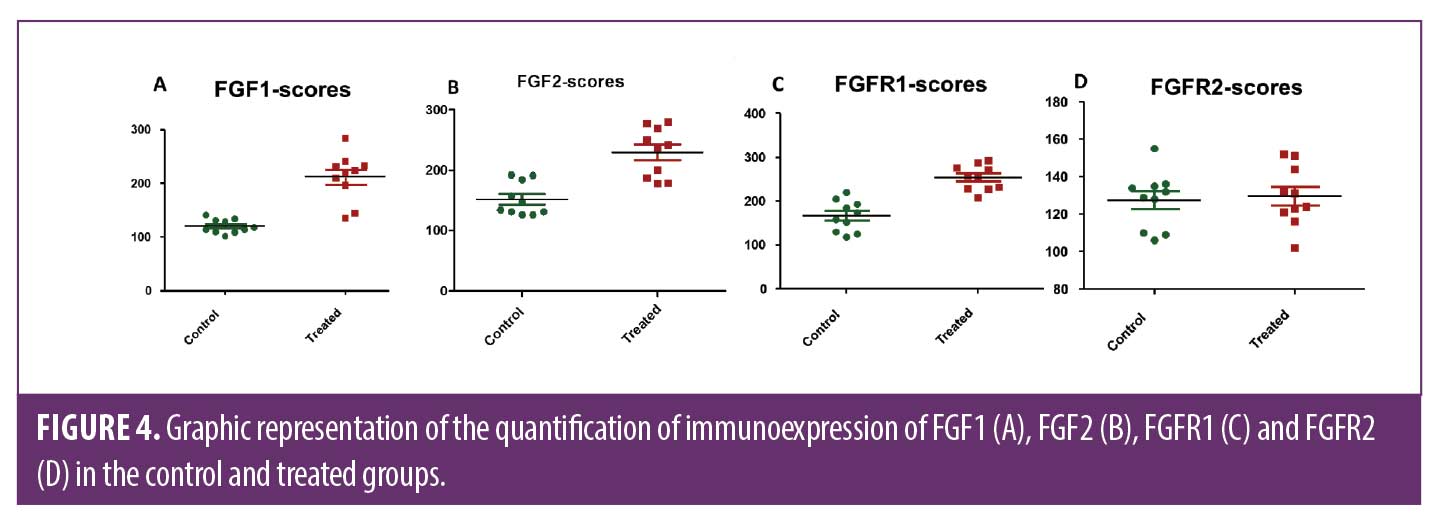
ESWT induces overexpression of fibroblast growth factors and fibroblastic proliferation in the dermis. FGF1, FGF2 and FGFR1 were expressed in the epidermal and dermal cells. In addition, some endothelial and inflammatory cells were also positive for these markers. The treated group showed a significant high expression of FGF1, FGF2, FGFR1 in the epidermis and dermis than control non-treated side, p<0.0002, 0.0017, <0.0001, respectively. The FGFR2 marker, however, did not present significant expression (Figures 3 and 4).
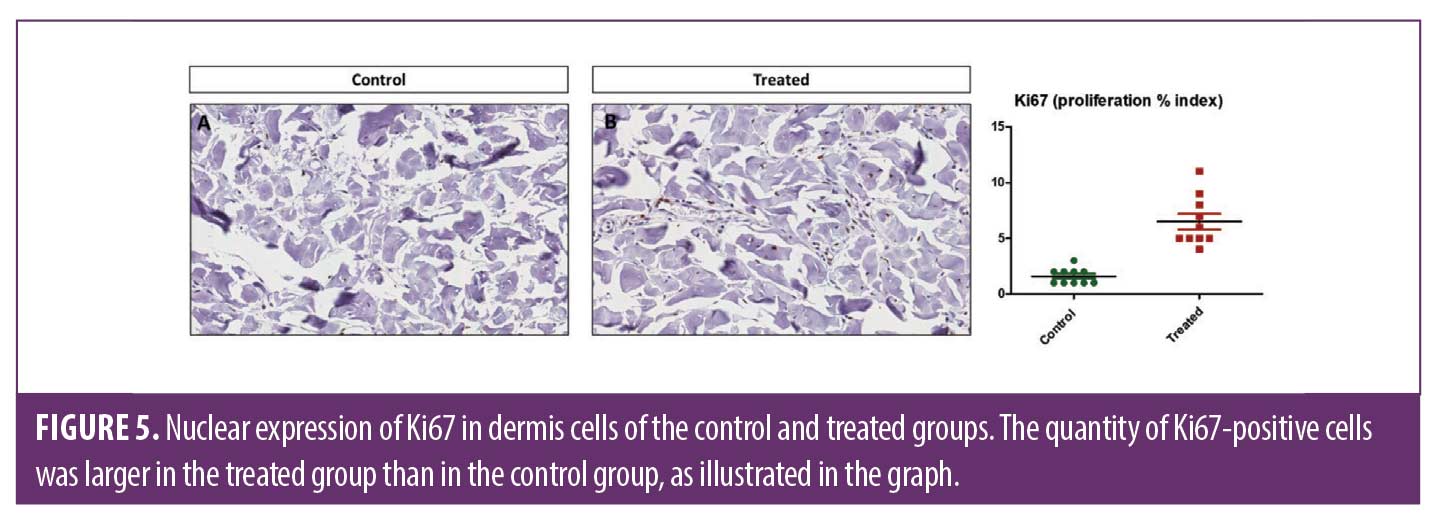
Regarding the Ki67 expression (marker of cellular proliferation), it was observed a higher number of positive cells in the treated group than control non-treated side (p<0.0002). These findings confirm that the ESWT may stimulate cellular proliferation, with particular attention to the dermal fibroblasts that demonstrated a higher cellular proliferative index. In Figure 5, it is possible to determine that in the control non-treated side, most cells were negative. On the other hand, dermal fibroblasts presented nuclear expression of Ki67.
Adverse reactions. As adverse reactions, two of the participants (14.28%) presented erythema in the treated region; however, after approximately 20 minutes the condition had improved, and two other participants (14.28%) presented formation of petechia and edema; however, this was not considered as a contraindication for the surgical treatment.
Discussion
Obesity is a condition which causes alterations in the skin and its annexes. For example, a marked pro-inflammatory state predisposes to infections and cutaneous inflammation; metabolic and hormonal alterations, as increased cortisol, which interferes in the collagen and elastin fibers. These changes, when present for long time, also contributes to poor healing of wounds; and the excess of weight itself, which alters the structure of collagen, given that its fibers are not capable of corresponding to the increase in skin surface, and which leads to the decrease in mechanical resistance and the rupture of both the collagen fibers and elastin fibers.10,12 All of these alterations compromise the skin homeostasis and its ability to self-repair in obese subjects. Understanding these clinical signs and the subjacent systemic disturbances can facilitate the treatment and prevent sequelae. Therefore, this is the first study in patients with obesity which aims to evaluate one of the most important dermatological complications after weight loss, skin flaccidity, and to validate a non-invasive, safe, and efficient which can prevent and treat these complications, since such obesity-related alterations are still currently underestimated.
In our study, we used electromagnetic ESWT therapy, which provides energy propagation coming from the generation of a magnetic field run by electricity displaces a projectile inside the applicator and from that moment on, there is a transfer of energy from the projectile to the tip of the applicator, and this energy is then transformed in mechanical waves, which favor cavitation and the physiological effects in the target tissue.5,13
As we see in our study, ESWT was able to stimulate the proliferation of fibroblast cells in the dermis and inflammatory cells in the epidermis and dermis of obese individuals. Also, the skin treated with EWST demonstrates a greater quantity of collagen fibers, better organization and disposition of epidermal cells, of the dermis-epidermis junction and papillary dermis. A similar result was found by Kuhn et al14, who analyzed the effects of ESWT on the skin of a non-obese woman, 50 years of age, who underwent four applications of ESWT and soon after the last therapy some samples of skin were collected for morphological analysis. They reported that the side which she received ESWT therapy showed better anatomical disposition of the dermis with increase of conjunctive tissue and collagen and elastin fibers, indicating induction of neocollagenesis and neoelastogenesis.14
This ESWT stimulus, according to Aguilera-Sáez et al15, is possible given that the shockwave is able to carry out mechanotransduction, the ability of mechanical energy to alter the potential of the membrane and activate the signaling processes of mediators and finally act in the regulation of migration and cell proliferation of keratocytes and fibroblasts. It can be inferred, therefore, that the behavior of ESWT is stimulating the self-regeneration in the biological tissue.14–17
In the differentiation of the types of collagen present in the skin after the ESWT procedure, there was predominance of Type I, main collagen in the dermis responsible for forming dense fibers in the reticular dermis. On the other hand, in the non-treated control side, there was predominance of Type III collagen, a more inactive collagen fibers. Thus, it is possible to affirm that EWST-treatment was able to stimulate neocollagenesis, and it is in accordance with previous studies.15,17 According to Woodley et al16 the proportion of Type I collagen to Type III collagen is of approximately 4 to 1 in adult skin; however, in adult skin the Type III collagen can increase fibers temporarily in the process of tissue regeneration after lesion, with the formation of neodermis.16
This correlation of the proportion of collagen was maintained in the analysis of the tissue which did not receive the treatment, and became even more evident in the sample of the tissue which received ESWT, where besides the greater quantity of Type I collagen, we observed the realignment and thickening of the fibers. This stimulus to neocollagenesis and neoelastogenesis favors the improvement of skin firmness and elasticity in obese individuals and confirms the findings in some studies.14,19,20 which also complement that the improvement of the skin is progressive, since the effect of ESWT can be maintained for up to six months after the last treatment session.22,23
This prolonged effect of ESWT on the skin can be of extreme importance to individuals with obeesity. Gallo et al18 evaluated the clinical differences of protein composition of the skin of three groups of patients with morbid obesity; individuals with morbid obesity, individuals with excessive weight loss without surgery and individuals who underwent bariatric surgery, to find clinical characteristics of the skin tissue which can favor the development of complications after surgical procedure. The results demonstrated that these individuals presented protein alterations and high expressions of the protein Haptoglobin (HP) marker of inflammation, which indicates that the systemic inflammation condition present in these individuals remains present in the integumentary tissue even after the excessive weight loss and particularly in post-bariatric individuals.18
This finding was extremely relevant, since after the weight loss skin flaccidity is a worrying complication, being a risk factor for the development of skin disease and an important limiting factor for quality of life. For this reason, the researchers in the study alert that it is necessary to develop non-invasive protocols able to address the inflammatory process in the skin of individuals with obesity to reduce the damaging effects of inflammation in the physiology of the integumentary system and, consequently, improve the characteristics of the viscoelastic properties of the skin, optimizing clinical conditions.18
Therefore, ESWT can be beneficial for this type of patient because of the confirmation of its benefits for the improvement in the properties of the integumentary tissue and also for its anti-inflammatory effect when used in low energies, which has been demonstrated in some studies.18–22,24,25
To clearly elucidate the ESWT stimulus in the integumentary tissue and the ability of the tissue in responding by synthetizing their products, immunohistochemical analyses were also carried out with antigen FGFs markers and their receptors. There are 18 proteins in the FGFs family in humans which are naturally stimulated and signaled by cells and interact directly with the extracellular matrix to connect to FGFR receptors, such as tyrosine kinase protein, to regulate the cellular processes mediating metabolic functions of differentiation, cell maturation, tissue repair, and regeneration; this process occurs from the intrinsic stimulus until the product synthetizing and is called signal transduction.26,27,28
We used the markers of FGF1 and FGF2, powerful stimulators of dermal regeneration, for presenting mitogenic effect for the fibroblast cells and keratocytes, and we also employ their receptors FGFR1 and FGFR2; the results evidenced increased expression of FGF1, FGF2, FGFR1 in the epidermis and dermis on the side treated by ESWT. These results correlate with the previous findings of the present study and reaffirm the ability of the therapy in cellular stimulation and the physiological efficacy of the integumentary tissue of individuals with obesity in responding to these stimuli and promoting cellular proliferation of keratocytes and fibroblasts.29,30
We were also able to correlate the pathway of intrinsic physiological stimulus given by the transduction of signal with the pathway of extrinsic stimulus of ESWT by the mechanotransduction of the signal; ability of the resident cells to perceive the extrinsic mechanical stimulus and transmit it over the cells which constitute the properties of the cellular matrix and carry out mechanical homeostasis, that is, ESWT has the ability to stimulate the natural physiological pathway of tissue self-repair in obese individuals.26,30
Lima et al7 evaluated the effect of ESWT in the skin of 45 female rats with histological and immunohistochemical analyses and also proved that the ESWT was able to increase the thickness of the epidermis and dermis, there was suppression of FGF2 and moderate expression of FGF1 and consequent increase in collagen production.7
Many electromedical resources are present or have been developed with objectives similar to those of ESWT; the best known is radiofrequency (RF), which demonstrates safe and efficient physiological effects in the treatment and prevention of skin flaccidity, and there are various experimental studies which confirm the ability of RF to stimulate the increase of FGF2 and consequently the proliferation of fibroblasts and their products, improving the aspect and quality of the skin. However, it is a technology which promotes thermal stimulus in the integumentary tissue and not the mechanical stimulus as ESWT does, and there are no studies which correlate the therapy with individuals with obesity.31,32,33
We also evaluated the marker Ki67, a well-recognized marker of cell proliferation. According to Miller et al34 the levels of Ki67 decrease constantly in inactive cells, and therefore it can be an important marker in cellular activity.34,35 Our results demonstrated a higher expression of Ki67 in dermal fibroblasts and inflammatory cells on the side treated by ESWT, indicating a higher activity in treated than control group. These findings corroborate with the upregulated collagen turnover and increased expression of FGF1, FGF2 and FGFR1.34
However, there are studies which confirm that ESWT induces angiogenesis, stimulates vascular endothelial endothelial growth factor (VEGF), acting in the increase of microvessel density (MVD) and greater endothelial cellular proliferation during tissue repair, besides carrying out the synthesis of the endothelial nitric oxide enzyme (eNOS) which contributes in the regulation of the vascular function.7,19,23
Huang et al36 and Sundaram et al37 confirmed that the ESWT is able to stimulate angiogenesis by means of signaling of the endothelial vascular growth factor (VEGF) and consequently the therapy can be used to accelerate healing and even to improve perfusion of cutaneous flaps.36,37
In summary, we observed that ESWT presents the ability to stimulate biological effects in the skin of individuals obesity. Such effects can contribute to tissue repair and the prevention of flaccidity in individuals who are experiencing the process of weight loss. It is worth pointing out that this was the first research carried out in humans with obesity, aiming to scientifically contribute in the validation of a non-invasive safe, efficient and low-cost resource for this type of dermatological complication, which affects thousands of individuals in this condition.
Conclusion
ESWT was able to stimulate the proliferation of fibroblast and keratocyte cells leading to higher number, density and realignment of collagen fibers Type I, increase of expression of FGF1, FGF2 and FGFR1 indicating the neocollagenesis and neoelastogenesis and the expression of Ki67 for the proliferation of endothelial cells of the skin in individual with obesity. ESWT was associated with a significant increase of cell proliferation and collagen expression in flaccid skin of individuals who achieved weight loss before bariatric surgery, leading to promising results for the repair and prevention treatment of skin flaccidity in patients with obesity.
References
- Dąbrowska AK, Spano F, Derler S, et al. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res Technol. 2018;24(2):165−174.
- Bonté F, Girard D, Archambault JC, Desmoulière A. Skin Changes During Ageing. Sub-cellular biochemistry. 2019; 91: 249–280.
- Greenway FL. Physiological adaptations to weight loss and factor favouring weight regain. International journal of obesity. 2005; 39(8):1188–1196.
- Chooi YC, Ding C, Magkos F. The epidemiology of obesity Metabolism: clinical and experimental. 2019; 92: 6–10.
- Modena D, da Silva CN, Grecco C, et al. Extracorporeal shockwave: mechanisms of action and physiological aspects for cellulite, body shaping, and localized fat Systematic review. Journal of cosmetic and laser therapy. 2017; 19(6): 314–319.
- Knobloch K, Kraemer R. Extracorporeal shock wave therapy (ESWT) for the treatment of cellulite–A current metaanalysis. International journal of surgery. 2015; 24:210–217.
- de Lima Morais TM, Meyer PF, de Vasconcellos LS, et al. Effects of th extracorporeal shock wave therapy on the skin: an experimental study. Lasers Med Sci. 2019; 34(2): 389–396.
- Meyer PF, de Oliveira P, Silva FKBA, da Costa ACS, et al. Radiofrequency treatment inducesfibroblast growth factor 2 expression and subsequently promotes neocollagenesisand neoangiogenesis in the skin tissue. Lasers Med Sci. 2017 32(8):1727–1736.
- Soares CD, Morais TML, Araújo RMFG, et al. Effects of subcutaneous injection of ozone during wound healing in rats. Growth Factors. 2019;37(1-2):95–103.
- Shipman AR, Millington GW. Obesity and the skin. Br J Dermatol. 2011;165(4):743–750.
- Poeggeler B, Schulz C, Pappolla MA, et al. Leptin and the skin: a new frontier. Exp Dermatol. 2010;19(1):12–18.
- Penelope A, David E. Castillo, Gil Yosipovitch, et al. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81(5):1037–1057
- Malliaropoulos N, Crate G, Meke M, et al. Success and Recurrence Rate after Radial Extracorporeal Shock Wave Therapy for Plantar Fasciopathy: A Retrospective Study. Biomed Res Int. 2016;1–8.
- Kuhn C. Impact of extracorporeal shock waves on the human skin with cellulite: A case study of an unique instance. Clinical Interventions in Aging. 2008; 3(1): 201–210.
- Aguilera-Sáez J, Muñoz P, Serracanta J, Monte A, et al. Extracorporeal shock wave therapy role in the treatment of burn patients. A systematic literature review. Burns. 2019; (19)30211–30216.
- Woodley DT. Distinct Fibroblasts in the Papillary and Reticular Dermis: Implications for Wound Healing. Dermatol Clin. 2017; 35(1):95–100.
- Wolf AM, Beisiegel U. The effect of loss of excess weight on the metabolic risk factors after bariatric surgery in morbidly and super-obese patients. Obes Surg. 2007;17(7):910–919.
- Gallo JRB, Maschio-Signorini LB, Cabral CRB, de Campos Zuccari DAP, et al. Skin Protein Profile after Major Weight Loss and Its Role in Body Contouring Surgery. Plast Reconstr Surg. 2019; 19;7(8):e2339.
- Siems W, Grune T, Voss P, et al. Anti-fibrosclerotic effects of shock wave therapy in lipedema and cellulite. Biofactors. 2005; 24(1): 275–282.
- Angehrn F, Kuhn C, Voss A Can cellulite be treated with low-energy extracorporeal shock wave therapy? Clin Interv Aging. 2007; 2(4): 623– 630.
- Christ C, Brenke R, Sattler G, et al. Improvement in Skin Elasticity in the Treatment of Cellulite and Connective Tissue Weakness by Means of Extracorporeal Pulse Activation Therapy. Aesthetic Surg J. 2008; 28(5): 538–544.
- Modena DAO, Silva CN, Delinocente TCP, et al. Shock wave therapy associated with radio frequency in the treatment of abdominal skin flaccidity. J Dermat Cosmetol. 2019;3(3):69–73.
- Modena DAO, Nogueira da Silva C, Delinocente TCP, et al. Effectiveness of the Electromagnetic Shock Wave Therapy in the Treatment of Cellulite. Dermatol Res Pract. 2019;1–6.
- Sukubo NG, Tibalt E, Respizzi S, et al. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int J Surg. 2015;24:124–130.
- de Girolamo L, Stanco D, Galliera E, et al. Soft-focused extracorporeal shock waves increase the expression oftendon-specific markers and the release of anti-inflammatory cytokines in anadherent culture model of primary human tendon cells. Ultrasound Med Biol. 2014;40(6):1204–1215.
- Ornitz DM, Itoh N. [The fibroblast growth factor signaling pathway]. Wiley Interdiscip Rev Dev Biol. 2015;4(3):215–266.
- de Araújo R, Lôbo M, Trindade K, et al. Fibroblast GrowthFactors: A Controlling Mechanism of Skin Aging. Skin Pharmacol Physiol. 2019;32(5):275–282.
- Goetz R, Mohammadi M. [Exploring FGF signaling mechanisms through the lens of structural biology]. Nat Rev Mol Cell Biol. 2013;14(3):166–180.
- Filho D, Antonio Medeiros et al. [Effects of basic fibroblast growth factor and its antifactor on the healing and collagen maturation of the infected skin wound]. Acta Cir Bras. 2007; 22(1): 64
- Humphrey JD, Dufresne ER, Schwartz MA. [Mechanotransduction and extracellular matrix homeostasis]. Nat Rev Mol Cell Biol. 2014; 15 (12): 802–812.
- Alvarez N, Ortiz L, Vicente V, et al. The effects of radiofrequency on skin: experimental study. Lasers Surg Med. 2008; 40(2):76–82.
- Im CN, Kim EH, Park AK, et al. Classification of biological effect of 1,763 MHz radiofrequency radiation based on gene expression profiles. Genomics & Informatics. 2010; 8(1):34–40
- Meyer PF, de Oliveira P, Silva FKBA, et al. Radiofrequency treatment inducesfibroblast growth factor 2 expression and subsequently promotes neocollagenesisand neoangiogenesis in the skin tissue. Lasers Med Sci. 2017; 32(8):1727–1736.
- Miller I, Min M, Yang C, et al. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018; 24(5):1105z-1112.e5.
- Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma. 2018;127(2):175–186.
- Huang T, Sun C, Chen Y, et al. Shock Wave Therapy Enhances Angiogenesis through VEGFR2 Activation and Recycling. Mol Med. 2016;22: 850–862.
- Sundaram S, Sellamuthu K, Nagavelu K, et al. Stimulation of angiogenesis using single-pulse low-pressure shock wave treatment. J Mol Med (Berl). 2018;96(11):1177–1187.

