 J Clin Aesthet Dermatol. 2022;15(10):58–61.
J Clin Aesthet Dermatol. 2022;15(10):58–61.
Dr. Salah is an Assistant Professor of Dermatology, Venereology and Andrology at Zagazig University in Zagazig, Egypt
by Eman Salah, PhD
FUNDING: No funding was provided for this article.
DISCLOSURES: The author reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Systemic isotretinoin is the most effective treatment for acne vulgaris (AV). However, numerous side effects are associated with isotretinoin. Oral zinc has a better safety profile and has been used to treat AV with variable results.
Objective. We sought to evaluate the safety and efficacy of combining oral zinc to low-dose systemic isotretinoin in AV patients.
Methods. Sixty AV patients were divided into two groups. Group A received oral zinc sulfate plus low-dose isotretinoin and Group B received the standard isotretinoin dosage. At each visit acne severity, photos, side effects, and patient-reported satisfaction were recorded.
Results. In the two groups, no significant difference in reduction of lesion count and Global Acne Grading System scores. The frequency of treatment-related side effects was (20%) in Group A and (76.7%) in Group B. Furthermore, there was no difference regarding the relapse rates between both groups (p>0.05). Finally, the patients’ satisfaction rates did not differ between the two groups.
Conclusion. Oral zinc plus low-dose isotretinoin resulted in satisfactory improvement in AV patients with fewer side effects. Further studies are recommended to compare the efficacy of other zinc preparations if combined with systemic isotretinoin at different concentrations.
Keywords. Acne vulgaris, zinc, isotretinoin, sebaceous glands.
Zinc is an essential trace element that performs catalytic, structural, and regulatory functions in different cells including keratinocytes. Zinc abnormalities have been incriminated in acne vulgaris (AV) due to its anti-inflammatory, anti-bacterial and anti-androgenic effects.1 Therefore, different zinc preparations have been used successfully to treat AV. Systemic isotretinoin, a vitamin A analog, is the most effective treatment for AV but comes with numerous side effects1–4 that may mimic zinc deficiency.5 Interestingly, zinc can affect the absorption, transportation, and utilization of vitamin A.6
Here, we compared the efficacy and safety of oral zinc and low-dose isotretinoin combination versus standard-dose isotretinoin monotherapy in moderate to severe AV.
Methods
Ethical approvals. The Institutional Review Board approved this work in accordance with the Declaration of Helsinki. Before enrollment, all subjects provided a well-informed and written consent.
Patients. This study included 60 patients with moderate-to-severe AV according to the global acne grading system (GAGS).7 Two blinded independent investigators evaluated the patients for enrollment and follow-up. Serum zinc was measured to exclude zinc deficiency. Before initiating isotretinoin, regular laboratory workup was performed, and lesions were counted with photo-documentation.
A software program was used to randomly assign a patient in 1 of 2 groups. Group A, the “experimental group”; who received low-dose isotretinoin (0.25mg/kg/d) and oral zinc sulfate (each 110mg zinc sulfate per capsule yields 25mg of elemental zinc) at a dose of (1mg/kg/d) while Group B, the “control group”; was maintained on standard-dose of (0.5mg/kg/d) isotretinoin. Included patients were males and females at aged at least 15 years and with moderate to severe AV, absent contraindications for isotretinoin or zinc sulfate and a two-week washout period for topical or four weeks for systemic acne therapies. Exclusion criteria were pregnancy, lactation, low serum zinc, and patients who reported interruptions of therapy or did not follow the scheduled monitoring visits were excluded. All patients were evaluated at baseline and monthly for five months evaluating lesions count, GAGS, and possible side effects. At the fifth follow-up visit patients rated their satisfaction level with the used treatment. Finally, all patients were observed for six months after completion of the treatment course to report possible early relapses. Furthermore, the patients were scheduled for a long-term follow up with a maximum of two years to detect any future late relapses.
Statistical analysis. Quantitative data were expressed as the mean ± SD and median (range), and qualitative data were expressed as absolute frequencies (number) and relative frequencies (percentage). Continuous data were checked for normality by using the Shapiro Walk test. The Mann Whitney U test was used to compare two groups of non-normally distributed data. Friedman’s test was used to compare between more than two dependent groups of non-normally distributed data. Wilcoxon signed rank test was used to compare between two dependent groups of non-normally distributed data. Categorical data were compared using the Chi-square test or Fisher’s exact test when appropriate. All tests were two-sided. P-value <0.05 was considered statistically significant. All data were collected, tabulated, and statistically analyzed using SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, USA), and MedCalc 13 for windows (MedCalc Software bvba, Ostend, Belgium).
Results
The experimental group involved 15 males and 15 females with a median age of 23.5 years while the control group included 15 males and 15 females with a median age of 25 years. The demographic data of all patients who completed the study are illustrated in Table 1.
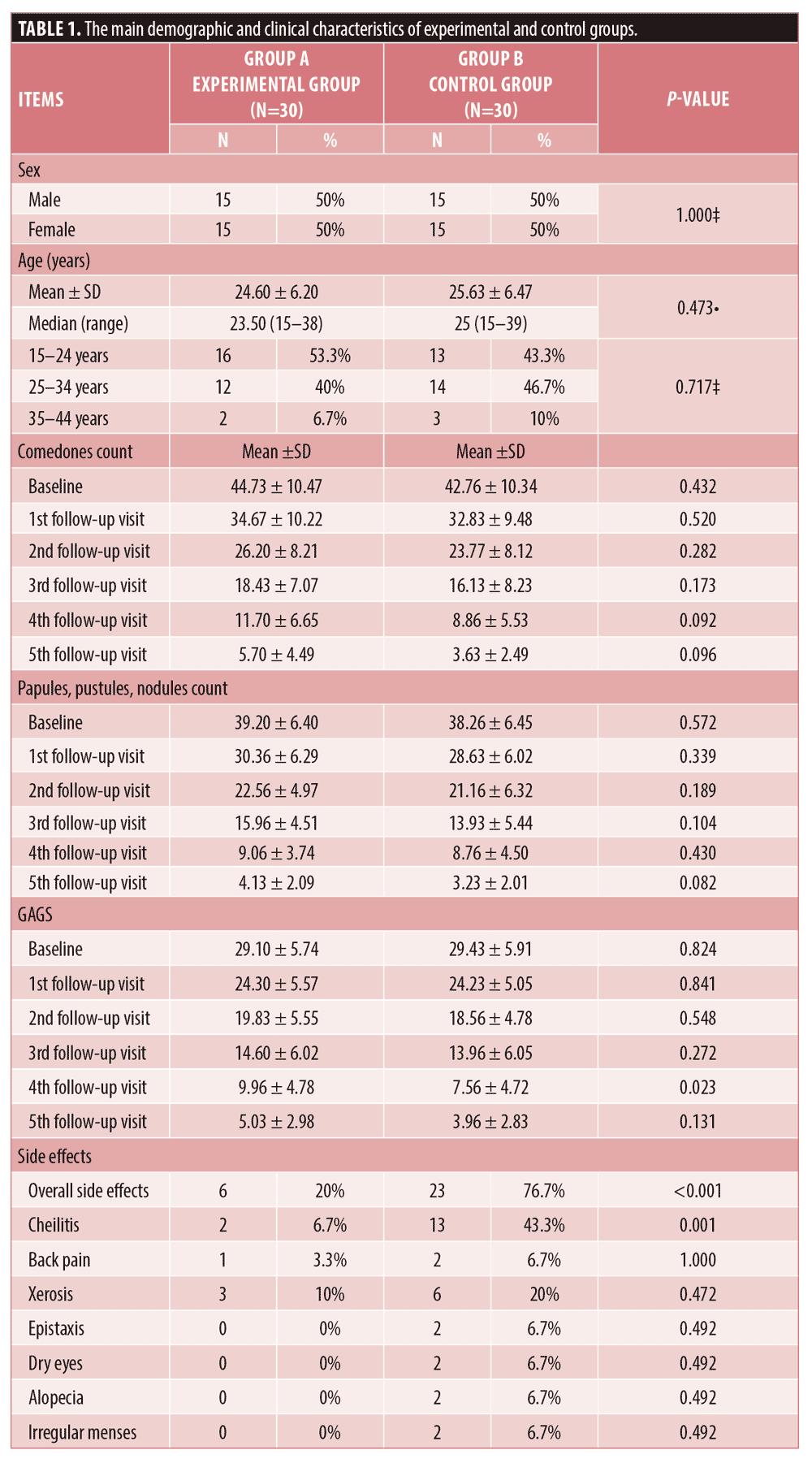
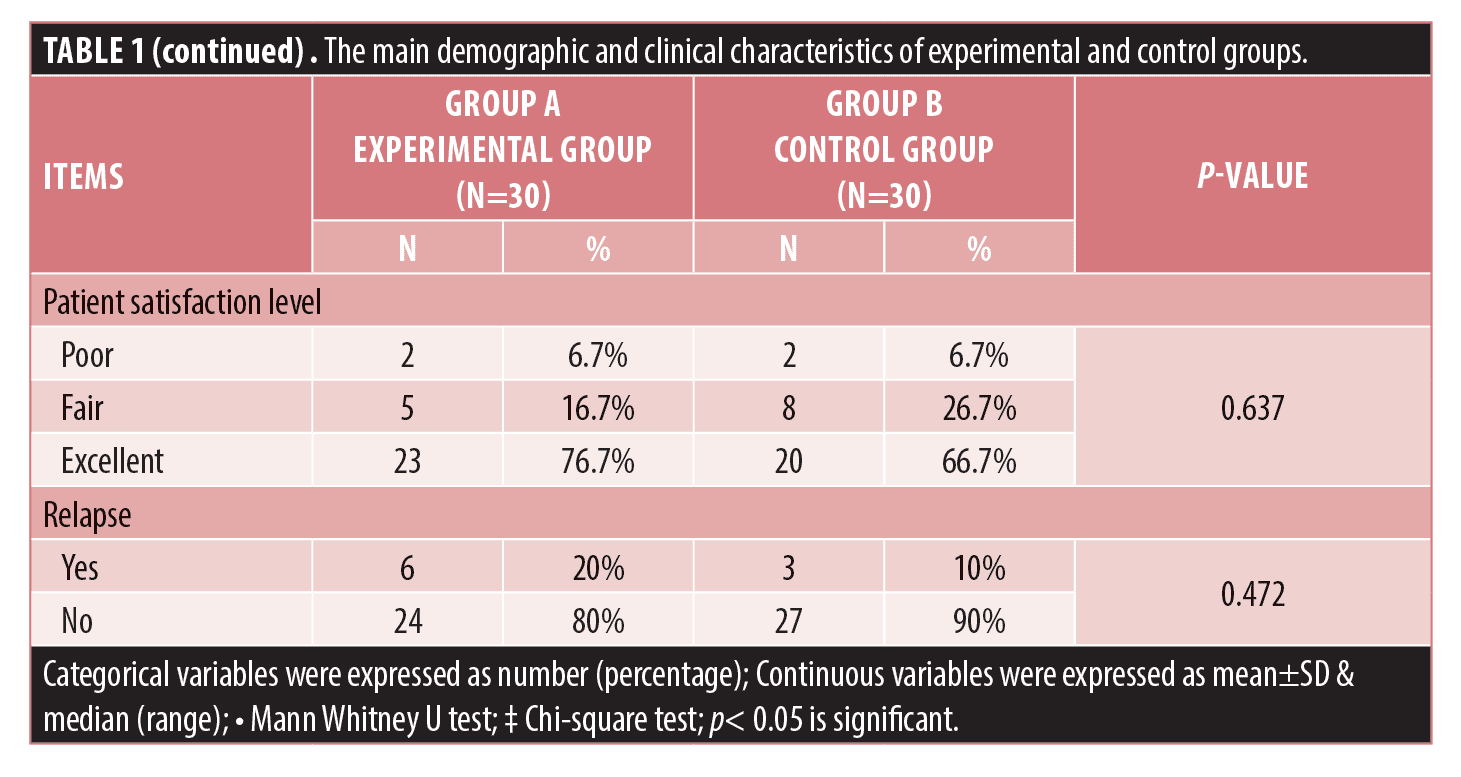
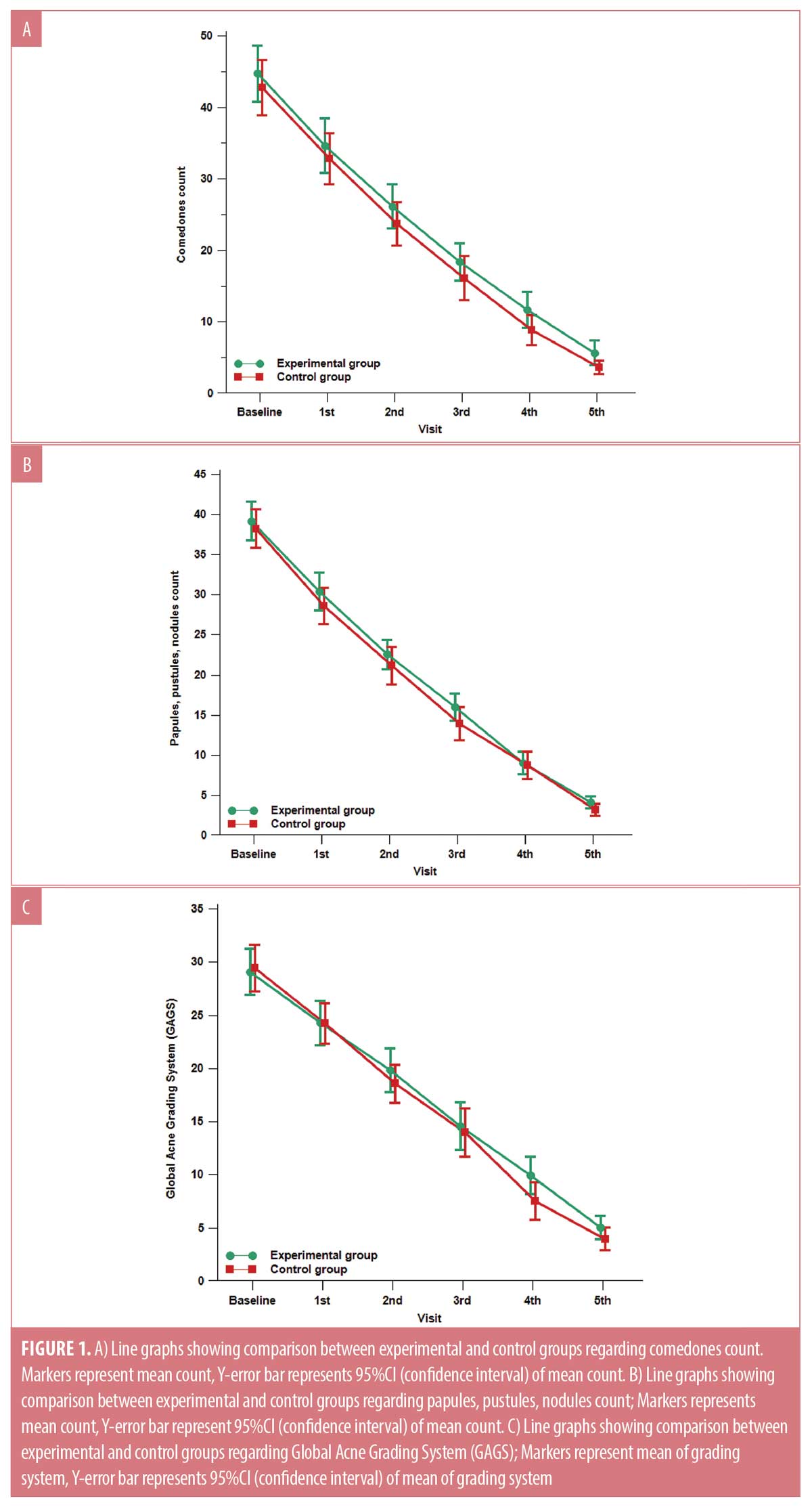
There was no statistically significant difference between the two studied groups regarding the reduction of comedonal and non-comedonal lesions count as well as GAGS assessment at both bassline baseline and monthly follow-up visits except at the fourth visit as demonstrated in Table 1 and Figure 1. At the fourth follow-up visit the control group showed a more significant improvement in GAGS than the experimental group, however, the difference became insignificant by the fifth visit. (Table 1, Figure 1C) Furthermore, the frequency of treatment-related side effects was more reported in the control (76.7%) group than the experimental group (20%) as shown in Table 1. Furthermore, there was no statistically significant difference regarding the relapse rates between both groups (p>0.05). Finally, the levels of patients’ satisfaction did not show a statistically significant difference between the two studied groups.
Discussion
To the best of our knowledge, the present study is the first to evaluate the efficacy and safety of a combination of oral zinc and systemic low-dose isotretinoin therapy in acne vulgaris AV patients. We hypothesized that the role of combining zinc to isotretinoin in AV is not always to correct a quantitative deficiency rather than a qualitative or functional imbalance. So, we have excluded patients with low serum zinc levels for testing this theory. Interestingly, patients who used oral zinc and low-dose systemic isotretinoin combination therapy showed similar improvement levels in comparison with patients who were given a monotherapy of higher isotretinoin dosage. Moreover, the experimental group did not experience higher rates of treatment-related side effects in contrast to the control group with similar relapse rates. Also, the lower cost of the used combination for Group A was an extra benefit. The patients in both groups reported a comparable level of satisfaction about the used treatment.
It was Michaelsson who first noted an improvement of acne vulgaris lesions after zinc administration to treat patients with acrodermatitis enteropathica.8 Subsequently, different zinc preparations have been extensively used both topically and systemically for the management of AV.9 Bilgiç et al1 did not find relationships among zinc levels, AV and isotretinoin use, which supports our hypothesis. Additionally, numerous studies relating the association between zinc status and AV severity are contradicting. Some studies reported a correlation between lower zinc levels and AV severity, but others did not detect such relationship.1
The beneficial effects of combining vitamin A and zinc supplementation were used in the treatment of some non-dermatological conditions such as acute diarrhea in children10 and pulmonary tuberculosis.11 Several mechanisms have been proposed to explain zinc and vitamin A interactions. Zinc is essential for the synthesis and release of retinol-binding protein, which transfers vitamin A from the liver to target tissues. Furthermore, zinc can facilitate vitamin A absorption6,12 and retinoic acid-mediated gene expression.13 On the other hand, isotretinoin could lead to increasing zinc utilization in human cells, because the drug enhances DNA transcription, in which zinc plays a critical role. Moreover, most of the side effects observed during isotretinoin use are similar to the symptoms of zinc deficiency.1
The exact mechanisms by which zinc exerts its effects to improve acne vulgaris is not fully understood. However, existing knowledge suggests multiple mechanisms including regulation of protein, lipid, and nucleic acid metabolism as an essential cofactor in many metalloenzymes and regulation of gene transcription via zinc-finger motif-containing proteins. Also, wound repair activity, maintenance of immunologic response by preserving macrophage and neutrophil function and stimulating natural killer cell and complement activity, anti-inflammatory by inhibition of IL-6, TNF-α, nitric oxide production and integrin and toll-like receptor expression by keratinocytes, direct inhibition of Propionibacterium acnes proliferation and finally inhibition of 5α-reductase with suppression of sebaceous gland activity,14 have been all proposed.
The safety margin for oral zinc supplementations is very wide with a lethal dose of 27 g/d of oral zinc in humans. Several reports have shown that the toxic side effects of oral zinc are associated with extremely high doses or accidental intoxication. These toxic side effects may include lethargy, nausea, vomiting, and copper deficiency.15 Subsequently, the used doses in this study were not expected to exhibit any zinc-induced toxic manifestations.
In this study, oral zinc combined with low-dose isotretinoin resulted in satisfactory improvement in acne vulgaris patients with fewer side effects. Another benefit of this combination would lower the treatment cost as systemic isotretinoin products are known to be expensive in comparison to oral over the counter zinc supplementations.
Conclusion
Oral zinc is an inexpensive, adjuvant, and sparing therapy to systemic isotretinoin in AV. Additionally, oral zinc minimizes isotretinoin adverse effects however, the rate of treatment relapse is like higher dose isotretinoin monotherapy. Further studies are recommended to assess using other zinc preparations and higher zinc doses with different isotretinoin regimens to outline the best combination. It would be compelling to investigate if oral zinc is able to diminish the various known side effects encountered with higher isotretinoin doses, which can secure more patients’ adherence and satisfaction as well as a lower relapse rate.
References
- Bilgiç Ö, Altınyazar HC, Sivrikaya A, et al. The relationship between erythrocyte zinc levels and isotretinoin use in acne vulgaris patients. Cutan Ocul Toxicol. 2015;34(4):303–306.
- Barbieri JS, Spaccarelli N, Margolis DJ et al. Approaches to limit systemic antibiotic use in acne: Systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019 Feb;80(2):538–549.
- Goodfield MJ, Cox NH, Bowser A et al. Advice on the safe introduction and continued use of isotretinoin in acne in the U.K. 2010. Br J Dermatol. 2010 Jun;162(6):1172–1179.
- Zaenglein AL, Pathy AL, Schlosser BJ et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016 May;74(5):945–973.e33.
- Alarco´n OM, Burguera JL, Burguera M. Effect of acute hypervitaminosis A on serum concentrations of Na, K, Mg, Fe, Zn and Cu in rats. Arch Latinoam Nutr. 1987; 37: 305–311.
- Christian P, West Jr KP. Interactions between zinc and vitamin A: an update. Am J Clin Nutr. 1998; 68:435–441.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol.1997 Jun; 36(6):416–418.
- Michaelsson G. Zinc therapy in acrodermatitis enteropathica. Acta Derm Venereol. 1974;54(5):377–381.
- Sardana K, Garg VK. An observational study of methionine bound zinc with antioxidants for mild to moderate acne vulgaris. Dermatol Ther. 2010 Jul; 23(4):411–418.
- Strand TA, Chandyo RK, Bahl R, et al. Effectiveness and efficacy of zinc for the treatment of acute diarrheain young children. Pediatrics. 2002 May;109(5):898–903.
- Karyadi E, West CE, Schultink W, et al. A double-blind, placebo-controlled study of vitamin A and zincsupplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr. 2002 Apr;75(4):720–727.
- Kim ES, Noh SK, Koo SI. Marginal zinc deficiency lowers the lymphatic absorption of alpha-tocopherol in rats. J Nutr. 1998;128: 265–270.
- Morris DR, Levenson CW. Zinc regulation of transcriptional activity during retinoic acid-induced neuronal differentiation. J Nutr Biochem. 2013;24:1940–1944.
- Cervantes J, Eber AE, Perper M et al. The role of zinc in the treatment of acne: A review of the literature. Dermatol Ther. 2018 Jan; 31(1).
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010 Apr;7(4):1342–1365.

