J Clin Aesthet Dermatol. 2019;12(12):39–43

by James P. Foshee, MD; Oleksandr Trofymenko, MD, MEd; and Nathalie C. Zeitouni, MDCM, FRCPC
Dr. Foshee is with the Division of Dermatology, University of Arizona College of Medicine in Tucson, Arizona. Dr. Trofymenko is with the University of Arizona College of Medicine in Tucson, Arizona. Dr. Zeitouni is with the Department of Dermatology, University of Arizona College of Medicine, Phoenix, Arizona.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Dermatofibrosarcoma protuberans (DFSP) is a locally aggressive tumor, uncommonly occurring on the head and neck where these deeply infiltrating tumors might violate underlying neurovascular structures. Treatment is typically surgical, whether by Mohs micrographic surgery (MMS) or wide local excision (WLE). However, there is a paucity of literature describing functional neurologic outcomes following surgical extirpation of facial DFSP. Thus, we sought to examine the functional neurologic outcomes in patients undergoing either MMS or WLE for facial DFSP.
Methods. Two patients with DFSP involving facial nerve danger zones treated by the multidisciplinary team with MMS and subsequent reconstruction were studied. Additionally, a comprehensive literature review of facial DFSP with regard to neurologic functional status was performed.
Results. From our research, only 10 of 46 patients with facial DFSP had neurologic functional status reported, with four of these cases having notable facial nerve deficits. Of our cases, both patients experienced transient neurologic deficits and neither had evidence of recurrence.
Conclusion. The proper assessment and reporting of postoperative functional recovery should be undertaken following facial DFSP resection and consideration should be given to a multidisciplinary treatment approach.
KEYWORDS: Dermatofibrosarcoma protuberans, facial nerve, Mohs micrographic surgery, nerve injury
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally aggressive, low-grade sarcoma with a tendency to spread along fascial planes, with deep infiltration into underlying subcutaneous tissues, muscle, and bone.1,2 Surgical resection is curative, whether by Mohs micrographic surgery (MMS) or wide-local excision (WLE), although recurrences are not uncommon, with reported rates ranging from 1 to 8 percent versus 7.3 to 30.8 percent, respectively.3–10 While these lesions typically occur on the trunk or extremities, they might uncommonly occur on the face.10,11 Given the complex neurovascular anatomy of the head and neck, compounded with the propensity for deep tissue invasion, neurologic deficits, either from direct tumor invasion into neural tissue or damage to nerves during surgical treatment, are a reasonably expected complication. However, despite the wealth of information published on DFSP surgical therapy and outcomes, there exists a paucity of data exploring functional outcomes after surgical therapy. We aim to examine neurological functional outcomes following surgery for facial DFSP, both within the published literature and in our own experience.
Materials and Methods
Two patients with DFSP involving facial nerve danger zones treated by the multidisciplinary team with MMS and subsequent reconstruction were studied. In addition to our own experience, a comprehensive review of the published literature from 1977 to 2015, using the keywords “dermatofibrosarcoma protuberans,” “dermatofibrosarcoma,” and “DFSP” was performed, with attention paid to cases detailing facial DFSP.
Patient 1. A 36-year-old Caucasian male patient with a biopsy-proven DFSP of the right temple was referred for MMS. Given the locally aggressive nature of the tumor, and presumed proximity to the temporal branch of the facial nerve, a multidisciplinary surgical approach (intraoperative Mohs micrographic surgery [iMMS]) was planned with both Mohs and otolaryngology—head and neck surgeons. The head and neck surgeon dissected out the temporal nerve to preserve function and allow complete tumor extirpation from the surrounding tissue. The tumor, measuring 2.0 × 2.5cm in the greatest diameter, was cleared with three stages of Mohs, with negative margins and a resultant defect measuring 3.7 × 4.8cm. The soft-tissue defect was repaired by our head and neck surgery colleagues using a radial forearm free flap. Postoperatively, the patient was noted to have right eyebrow ptosis, which resolved slowly over an approximately six-month period. He was followed for six years with no evidence of recurrence or permanent neurologic deficits.
Patient 2. A 51-year-old Native American female patient with a biopsy-proven DFSP of the right cheek was referred for Mohs. During her initial consultation, firm induration was noted extending from the malar cheek superolaterally, to the upper cutaneous lip inferomedially. A preoperative magnetic resonance imaging scan revealed a deeply infiltrating soft tissue mass, without evidence of bony involvement. Given the large size and deep involvement of the tumor, we opted for MMS with subsequent reconstruction by otolaryngology—head and neck surgery. The tumor was excised with three stages of Mohs, with negative margins and a resultant defect measuring 6.0 × 3.2cm, with exposure of the buccal branch of the facial nerve. Head and neck surgery performed a partial closure with a cervicofacial advancement flap and left a residual tissue defect near the right temple to heal by secondary intention. Postoperatively, she was noted to have an inability to raise the right corner of her mouth, with mild oral incompetence, as well as paresthesia of the right upper lip. These neurologic deficits were noted to be slowly improving at the time the patient was lost to follow-up approximately 13 months after surgery.
Results
In addition to our own experience, a comprehensive review of the published literature was performed, searching for surgical outcomes in DFSP localized to the face. Our review subsequently yielded 44 such cases of facial DFSP reported from 1977 to 2015 (Table 1). Including our two cases (n=46), the mean patient age at time of surgical resection was 32 years old, with 65.2 percent (n=30) being male. Sixteen (34.8%) underwent MMS, while the remainder of patients received surgical excision (n=30). Nine patients received adjuvant radiation therapy. Of the identified cases, 84.7 percent (n=39) had documented follow-up, with a mean follow-up duration of 34.7 months. There were four recurrences in the cohort, ranging from three to 78 months postoperatively, all of whom received WLE (13.3% recurrence rate with WLE) and one of whom who received adjunct radiation therapy in addition to WLE.21–23 Comparatively, there were no recurrences among the patients treated with MMS (0% recurrence rate with MMS; p=0.13). Surface area of the resultant surgical defect after tumor extirpation was reported in 34 patients, and eight of the 10 patients with neurologic outcomes, although there was no statistically significant difference between the surface area of those with and without neurologic deficits (69.0cm2 vs. 39.3cm2, respectively; p=0.13) or between those treated with MMS and WLE.
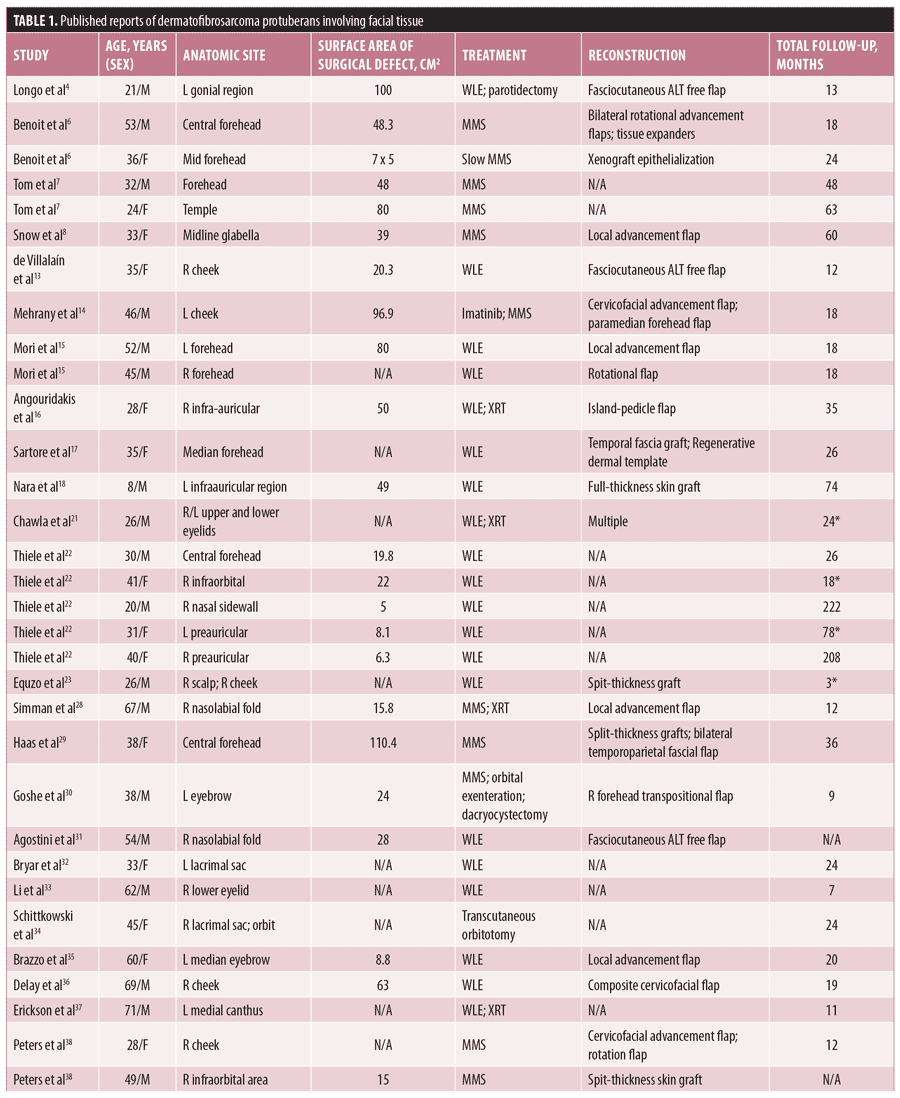
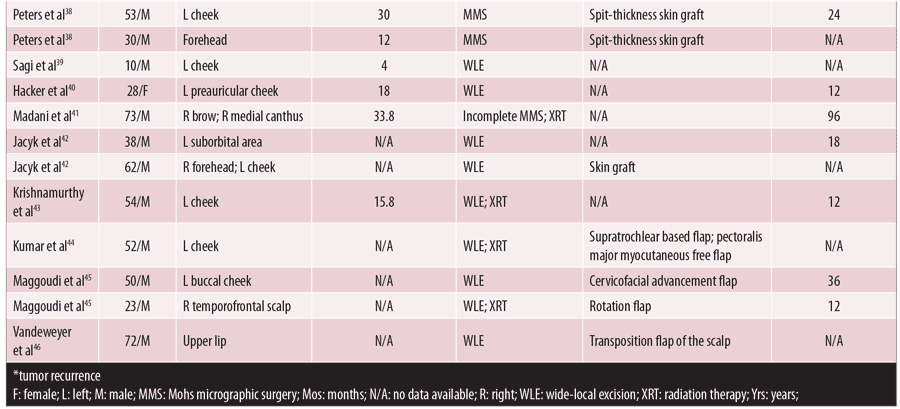
In the entire cohort, only 10 patients (21.7%) had neurologic functional status reported, with four of these patients exhibiting postoperative facial muscle paralysis4,13–18 (Table 2). The neurologic deficits were noted to be transient in two patients, one of whom received Mohs while the other underwent WLE.4 Eight of these patients had a final defect size reported ranging from 17.8 to 100cm2. However, there was no significant difference in the mean surface area of final surgical defect between patients with and without neurologic deficits.
Discussion
DFSP is a locally aggressive, infiltrating tumor most commonly affecting the trunk and extremities but involving the head and neck in up to 10 to 15 percent of cases.11 However, the propensity for deep tissue infiltration places the neurovascular structures of the head and neck at risk, whether by direct involvement with tumor, or during surgical extirpation.19,20 Additionally, a rare variant of DFSP, termed subcutaneous DFSP (scDFSP), was found to be more commonly located on the head and neck, with a tendency for deeper tissue invasion (i.e., into muscle/periosteum), further raising concern for neurologic involvement.12 Branches of the facial nerve might be encountered during surgery, particularly when lesions involve the temporal or zygomatic cheek “facial danger zones.” Injury to the temporal branch of the facial nerve can precipitate brow ptosis and visual field obstruction due to motor innervation of the frontalis, orbicularis oculi, corrugator supercilii, and superior auricular muscles.19,20 Fortunately, interconnected rami of the nerve branches often help restore function by six months following injury.20 Injury to the buccal and zygomatic branches of the facial nerves is likely to impact smile symmetry and oral competence, although both the buccal and zygomatic branches tend to undergo spontaneous recovery of nerve function owing to significant interconnections.19,20
Despite numerous reports in the literature detailing cases of facial DFSP located near these “danger zones,” there is a paucity of neurological functional outcomes reported. Of the 46 cases among our research results, only 10 detailed neurologic outcomes, although there was an additional report of a left supraclavicular DFSP complicated by six months of transient neurapraxia of the spinal accessory nerve.24 Four patients were noted to have postoperative deficits, including facial paralysis and neurapraxia, after undergoing MMS (n=2) and WLE (n=2).4,21–23 Fortunately, these symptoms were transient in 50 percent of these patients, with resolution of neurologic deficits occurring within six months.
Facial nerve injury is a common adverse event of oncologic surgery of the head and neck, and various facial nerve injury grading scales exist, including the House–Brackmann Scale, Facial Nerve Grading Scale 2.0, and the Sunnybrook Facial Grading Scale. These scales attempt to standardize comparison of functional neurologic outcomes of the facial nerve, although they all suffer from varying degrees of interobserver reliability.25 The House–Brackmann scale, and it’s contemporary revision, the Facial Nerve Grading Scale 2.0, are most commonly used.25 However, no evidence of any form of consistent nerve injury grading scale was noted in the published literature on facial DFSP. Further efforts to categorize the neurologic functional outcomes of surgical treatment of DFSP are needed to better counsel patients about their postoperative risk.
MMS has been increasingly favored as the treatment of choice for DFSP, related to tissue-sparing outcomes, enhanced peripheral and deep histologic margin assessment, and a lower recurrence rate as compared with WLE.3,5,8,9,10 Among our cohort, we noted similar recurrence rates to those in the literature (0% vs. 13.3%, respectively; p=0.13), albeit only trending toward significance, likely due to our small sample size. There was no difference in neurologic functional outcomes between patients treated with MMS and WLE, although, as noted above, there was a lack of substantial data in the literature. While there was no significant difference in mean surface area of postoperative surgical defects between patients treated with WLE and MMS in our cohort, a recent retrospective case study at the Mayo Clinic revealed significantly smaller surgical defects resulted from MMS as compared with WLE.10 This could be important, particularly in cosmetically sensitive areas, such as the head and neck.
Originally described in 1979,26 iMMS, combining the complete deep and peripheral margin assessment of Mohs and the extirpative and oncologic reconstruction of head and neck surgery, has been shown to be effective for large cutaneous tumors of the head and neck. Seth et al27 demonstrated improved margin control with iMMS and a decreased need for adjunct radiation therapy, including in cases of large tumors of the central face and those with nerve involvement. In areas where the potential for nerve injury is suspected, such as in head and neck DFSP, a multidisciplinary approach between dermatologic and head and neck or oncologic surgeons might be beneficial.
Conclusion
Facial DFSP, although uncommon, presents significant challenges for both dermatologists and surgeons. A multidisciplinary approach between dermatologic surgeons and otolaryngology—head and neck surgeons, as well as intraoperative Mohs micrographic surgery, could be beneficial in treating these patients. Further reporting, ideally with standardized metrics, of facial nerve injury and neurologic outcomes is needed to better counsel potential future surgical candidates.
References
- Gloster HM, Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3 Pt 1):355–374.
- Heuvel ST, Suurmeijer A, Pras E, et al. Dermatofibrosarcoma protuberans: recurrence is related to the adequacy of surgical margins. Eur J Surg Oncol. 2010;36(1):89–94.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34(8):728–736.
- Longo B, Paolini G, Belli E, et al. Wide excision and anterolateral thigh perforator flap reconstruction for dermatofibrosarcoma protuberans of the face. J Craniofac Surg. 2013;24(6):e597–599.
- Lemm D, Mugge LO, Mentzel T, et al. Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin. 2009;135(5):
653–665. - Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protruberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42(2):261–264.
- Tom WD, Hybarger CP, Rasgon BM. Dermatofibrosarcoma protuberans of the head and neck: treatment with Mohs surgery using inverted horizontal paraffin sections. Laryngoscope. 2003;113(8):1289–1293.
- Snow SN, Gordon EM, Larson PO, et al. Dermatofibrosarcoma protuberans: A report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. Cancer. 2004;101(1):28–38.
- Loghdey MS, Varma S, Rajpara SM, et al. Mohs micrographic surgery for dermatofibrosarcoma protuberans (DFSP): a single-centre series of 76 patients treated by frozen-section Mohs micrographic surgery with a review of the literature. J Plast Reconstr Aes. 2014; 67(10):
1315–1321. - Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43(1):98–106.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78(6):1125–1134.
- Llombart B, Serra-Guillén C, Rubio L, et al. Subcutaneous dermatofibrosarcoma protuberans, a rare subtype with predilection for the head: a retrospective series of 18 cases. J Am Acad Dermatol. 2017;77(3):503–511.e1.
- de Villalain L, de Vicente JC, Astudillo A, et al. Management of facial dermatofibrosarcoma protuberans: case report, justification of aggressive surgical treatment, and reconstruction with an anterolateral thigh flap. J Oral Maxil Surg. 2010;68(8):1994–1999.
- Mehrany K, Swanson NA, Heinrich MC, et al. Dermatofibrosarcoma protuberans: a partial response to imatinib therapy. Dermatol Surg. 2006;32(3):456–459.
- Mori S, Di Monta G, Marone U, et al. Half forehead reconstruction with a single rotational scalp flap for dermatofibrosarcoma protuberans treatment. World J Surg Oncol. 2012;10:78.
- Angouridakis N, Kafas P, Jerjes W, et al. Dermatofibrosarcoma protuberans with fibrosarcomatous transformation of the head and neck. Head Neck Oncol. 2011;3:5.
- Sartore L, Venezia ED, Della Puppa A, et al. Reconstructive strategies for dermatofibrosarcomas of the face: role of regenerative dermal templates. Head Neck. 2015;37(1):E8–E11.
- Nara K, Oue T, Yoneda A, et al. A case of childhood dermatofibrosarcoma protuberans of the face. J Pediatr Surg. 2011;46(7):1438–1441.
- Brown SM, Oliphant T, Langtry J. Motor nerves of the head and neck that are susceptible to damage during dermatological surgery. Clin Exp Dermatol. 2014;39(6):677–682.
- Roostaeian J, Rohrich RJ, Stuzin JM. Anatomical considerations to prevent facial nerve injury. Plastic Reconstr Surg. 2015;135(5):1318–1327.
- Chawla B, Pushker N, Sen S, et al. Recurrent bilateral dermatofibrosarcoma protuberans of eyelids. Ophthal Plast Recons. 2011;27(6):
e167–168. - Thiele OC, Seeberger R, Bacon C, et al. Recurrent craniofacial dermatofibrosarcoma protuberans: long-term prognosis after close surgical removal. The J Craniofac Surg. 2009;20(3):844–846.
- Eguzo K, Camazine B, Milner D. Giant dermatofibrosarcoma protuberans of the face and scalp: a case report. Int J Dermatol. 2014;53(6):767–772.
- Axibal E, Terella A, Ricci D, et al. Neuropraxia of the spinal accessory nerve after Mohs micrographic surgery. Dermatol Surg. 2017;43(9):1192–1195.
- Fattah AY, Gurusinghe ADR, Gavilan J, et al. Facial nerve grading instruments: systematic review of the literature and suggestion for uniformity. Plast Reconstr Surg. 2015;135(2):569–579.
- Levine H, Bailin P, Wood B, et al. Tissue conservation in treatment of cutaneous neoplasms of the head and neck. Combined use of Mohs’ chemosurgical and conventional surgical techniques. Arch Otolaryngol. 1979;105(3):
140–144. - Seth R, Revenaugh PC, Vidimos AT, et al. Simultaneous intraoperative Mohs clearance and reconstruction for advanced cutaneous malignancies. Arch Facial Plast Surg. 2011;13(6):404–410.
- Simman R, DeFranzo A, Sanger C, et al. Dermatofibrosarcoma protuberans of the face: surgical management. J Craniofac Surg. 2005;16(3):439–443.
- Haas AF, Sykes JM. Multispecialty approach to complex dermatofibrosarcoma protuberans of the forehead. Arch Otolaryngol. 1998;124(3):324–327.
- Goshe JM, Lewis CD, Meine JG, et al. Primary dermatofibrosarcoma protuberans invading the orbit. Ophthal Plast Recons. 2012;28(3):e65–67.
- Agostini T, Lazzeri D, Agostini V, et al. Anterolateral thigh flap as the ideal flap to full-thickness cheek reconstruction. J Craniofac Surg. 2010;21(6):
1897–1898. - Bryar P, Sun R, Ebroon D. Nasolacrimal duct obstruction secondary to dermatofibrosarcoma protuberans. Ophthalmology. 2004;111(7):
1398–1400. - Li J, Ge X, Ma JM, et al. Dermatofibrosarcoma protuberans. Ophthalmology. 2012;119(1):
e191–e193. - Schittkowski MP, Wrede A. Dermatofibrosarcoma protuberans with primary orbital manifestation. Orbit. 2013;32(2):117–119.
- Brazzo BG, Saffra N. Dermatofibrosarcoma protuberans of the brow and eyelid. Ophthal Plast Recons. 2004;20(4):332–334.
- Delay E, Lucas R, Jorquera F, et al. Composite cervicofacial flap for reconstruction of complex cheek defects. Ann Plas Surg. 1999;43(4):347–353.
- Erickson BP, Henry C, Alabiad CR. Recurrent dermatofibrosarcoma protuberans masquerading as a lacrimal sac neoplasm: a case report and review. Ophthal Plast Recons. 2015;31(5):
e135–138. - Peters CW, Hanke CW, Pasarell HA, et al. Chemosurgical reports. Dermatofibrosarcoma protuberans of the face. J Dermatol Surg Onc. 1982;8(10):823–826.
- Sagi A, Ben-Yakar Y, Mahler D. A ten-year-old boy with dermatofibrosarcoma protuberans of the face. J Dermatol Surg Onc. 1987;13(1):82–83.
- Hacker SM, Ford MJ. Dermatofibrosarcoma protuberans of the face. International J Dermatol. 1994;33(8):568–569.
- Madani S, Huilgol SC, Carruthers A. Unplanned incomplete Mohs micrographic surgery. J Am Acad Dermatol. 2000;42(5 Pt 1):814–819.
- Jacyk WK, Pasnikowski T. Dermatofibrosarcoma of the face. Report of two cases from Nigeria. Dermatologica. 1977;155(1):45–49.
- Krishnamurthy A, Majhi U. Fibrosarcomatous dermatofibrosarcoma protuberans: an unusual tumor in the facial skin. Indian J Dermatol. 2014;59(1):105.
- Kumar L, Bhandari V, Singh S, et al. Giant dermatofibrosarcoma protuberans: a rare presentation over face. J Canc Res Ther. 2015;11(4):1038.
- Maggoudi D, Vahtsevanos K, Psomaderis K, et al. Dermatofibrosarcoma protuberans of the face: report of 2 cases and an overview of the recent literature. J Oral Maxil Surg. 2006;64(1):140–144.
- Vandeweyer E, Seyeidi JV, Deraemaecker R. Dermatofibrosarcoma protuberans of the upper lip: an overview and a case report. Eur J Surg Oncol. 1997;23(3):275–277.

