 J Clin Aesthet Dermatol. 2020;13(3):28–30
J Clin Aesthet Dermatol. 2020;13(3):28–30
by Beverly Johnson, MD; Samantha Marrone, MD; and Amit Om, MD
Drs. Johnson, Marrone, and Om are with Florida State University in Tallahassee, Florida.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Melasma is a common pigmentation disorder with few satisfactory treatment options. The hyperpigmentation has both an epidermal and dermal component. To date, combination therapies have been observed to yield greater improvements in melasma compared to monotherapies. Cysteamine has been tested and shown to improve epidermal melasma. In this case series, we examined the efficacy of nightly applications of cysteamine cream, washed off after 15 minutes, with monthly in-office laser treatment sessions using a 650-microsecond neodymium-doped yttrium aluminium garnet 1,064-nm laser. The patients all reported satisfaction with the results of this combination therapy. None of the patients experienced irritation with the product nor did they experience discomfort/downtime with the laser sessions. Evaluation of the patients two months after the treatment indicated persisting effects. Our small case series suggests high levels of satisfaction can be achieved using this combined topical and laser approach.
KEYWORDS: Melasma, cysteamine cream, Nd:YAG laser
Melasma is a hyperpigmentation disorder that pathogenetically involves hereditary factors and exposure to sunlight and heat.1 Hydroquinone-based bleaching agents are currently considered the gold standard treatment for melasma. The field of research into better treatment options for melasma is expanding, and the impetus for this might partially be attributed to the concern among some patients that hydroquinone is carcinogenic.2 Additionally, hydroquinone can be very irritating, and is often compounded with fluocinolone acetonide 0.01% and tretinoin 0.05%. The addition of topical steroids and chronic use of these agents causes the epidermis to thin, and thus, be more susceptible to worsening of melasma and increased irritation that can lead to postinflammatory hyperpigmentation. Additionally, there exists the possibility of developing islands of hypopigmentation when using hydroquinone, due to inconsistent application techniques by patients to affected areas. Hydroquinone has been known, in rare cases, to cause exogenous ochronosis. Rebound hyperpigmentation can also occur after the cessation of bleaching agents.1
Cysteamine cream, a more recently developed depigmenting agent, has demonstrated efficacy in both the laboratory and clinical practice for the treatment of melasma.3–6 Additionally, lasers have shown promise in the treatment of melasma, most notably including the Nd:YAG laser.7 In particular, the 650-microsecond 1064-nm laser from Aerolase has been used to treat melasma in all skin types without complications. The safety and efficacy of this laser in the treatment of melasma have been demonstrated in previous studies.8
This small case series describes the cases of three patients treated for melasma with the combination of cysteamine cream and the 650-microsecond 1064nm Nd:YAG laser.
Methods
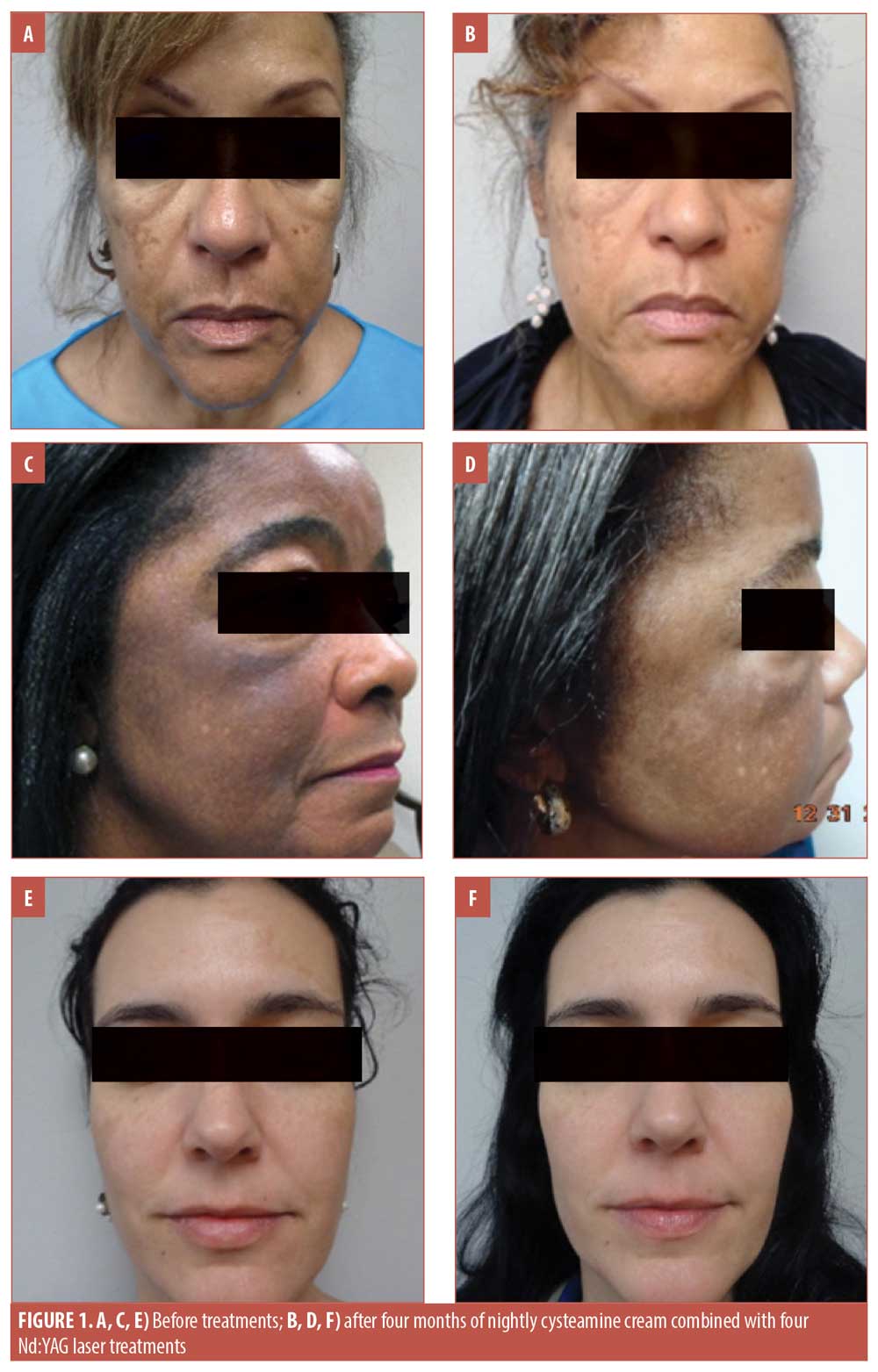
Three patients representing a range of light, medium, and dark complexions, aged 67 years (Figure 1A), 61 years (Figure 1C), and 41 years (Figure 1E), presented to our dermatology clinic for treatment of melasma after having been treated unsuccessfully with hydroquinone in the past. The patients had not used any topical treatments in the year prior to presenting to our clinic and none of the patients had undergone laser treatments before. Signed informed consent and photoconsent was obtained from each patient. Photographs were taken in the clinic prior to starting treatment and at each monthly visit prior to undergoing the laser treatment (Figure 1).
The patients were instructed to apply cysteamine cream nightly to the affected areas and rinse it off after 15 minutes. The patients were instructed not to use any other topical therapies for their melasma, but were given sunscreen with a sun protection factor (SPF) of 50 with instructions to use it every day, regardless of activity or weather. Additionally, each patient underwent monthly treatments with the 650-microsecond 1064nm Nd:YAG laser for four months for a total of four sessions.
Results
The patients all reported satisfaction with the results of this combination therapy. None of the patients experienced irritation with the product nor did they experience discomfort/downtime with the laser sessions. No “halos” of hypopigmentation were observed; evaluation of the patients two months after the treatment indicated persisting effects. The patients were educated on the negative effects of sun exposure to the skin, including the worsening of melasma, and were instructed to continue regular use of sunscreen. All three patients opted to continue the monthly laser treatments. Figure 1 shows the progression of treatment outcomes over four months.
Discussion
The cysteamine cream used in this combination therapy (Scientis Pharma, Geneva, Switzerland) contains 5% cysteamine hydrochloride as the active ingredient and niacinamide 5%. The base is formulated with aqua 75%, paraffinum liquidum 10%, shea butter 5%, lecithin 5%, glyceryl stearate 5%, isopropyl myristrate 5%, cetyl alcohol 5%, and ascorbyl palmitate 1%. Cysteamine is thought to work through the inhibition of melanin synthesis.5
The 650-microsecond 1064-nm laser from Aerolase has been used to treat melasma in all skin types without complications. The use of the LightPod laser (Aerolase Corp., Tarrytown, New York) was recently reviewed.9 It is uniquely suited to application in patients with brown skin, because the 650-microsecond 1064 neodymium-doped yttrium aluminium garnet (Nd:YAG laser) generates the least amount of heat, a factor known to trigger an exacerbation of melasma.2 The interval of treatment every 4 to 6 weeks allows time for the phagocytosis of pigment. Using this technology, we have been able to target deeper dermal melanin and vascular components that topical therapy cannot treat.2
As seen in our case series, this combination approach appears to be effective for patients with a wide range of skin tones and varying severities of melasma, and might best suit patients who are concerned about either the costs or risks associated with hydroquinone or those who have melasma that is refractory to it.
Limitations. Limitations of our study include subjective assessments of patient outcomes, as opposed to histological assessments. Additional research is needed to confirm our findings. Further investigations should involve more subjects, blind the investigators, and corroborate the results using reflectance colorimeter or microscopy.
Conclusion
To our knowledge, this is the first case series on combining topical cysteamine cream with a laser modality for the treatment of melasma. Our small case series suggests high levels of satisfaction can be achieved using this combined topical and laser approach.
References
- Mary Wu Chang. Disorders of Hyperpigmentation. In:Bolognia, Schaffer, Cerroni, eds. Dermatology. 4th ed. Elsevier. 2018: 119–1122.
- Roberts W. Laser treatment of skin of color for medical and aesthetic uses with a new 650-microsecond Nd:YAG 1064 nm laser. J Drugs Dermatol. 2019;18(4):s135–137.
- McGregor D. Hydroquinone: an evaluation of the human risks from its carcinogenic and mutagenic properties. Crit Rev Toxicol. 2007;37(10):887–914.
- Besouw M, van den Heuvel L, van Eijsden R, et al. Increased human dermal microvascular endothelial cell survival induced by cysteamine. J Inherit Metab Dis. 2013;36(6):1073–1077.
- Qiu L, Zhang M, Sturm RA, et al. Inhibition of melanin synthesis by cystamine in human melanoma cells. J Invest Dermatol. 2000;114(1): 21–27.
- Farshi S, Mansouri P, Kasraee B. Efficacy of cysteamine cream in the treatment of epidermal melasma, evaluating by Dermacatch as a new measurement method: a randomized double blind placebo controlled study. J Dermatologl Treat. 2018;29(2):182–189.
- Kasraee B, Mansouri P, Farshi S. Significant therapeutic response to cysteamine cream in a melasma patient resistant to Kligman’s formula. J Cosmet Dermatol. 2019;18(1):293–295.
- Arora P, Sarkar R, Garg V, Arya L. Lasers for treatment of melasma and post-inflammatory hyperpigmentation. J Cutan Aesthet Surg. 2012;5(2):93–103.
- Cook-Bolden F. A novel 0.65 millisecond pulsed 1064 Nd:Yag laser to treat skin of color without skin cooling or anesthetics. J Drugs Dermatol. 2011;10(12 Suppl):s10–s11.

