 J Clin Aesthet Dermatol. 2020;13(3):31–36
J Clin Aesthet Dermatol. 2020;13(3):31–36
by Vanessa Ziying Lim, MBBS; Angeline Anning Yong, MBBS; Wee Ping Melissa Tan, MBBS; Xiahong Zhao, Bsc (Hons), PhD; Maria Vitale, MD; and Chee Leok Goh, MBBS
Drs. Lim, Yong, Tan, Zhao, and Goh are with the National Skin Centre in Singapore. Dr. Vitale is with the Medical Department, Cantabria Labs in Madrid, Spain.
FUNDING: This work was supported by grants from Cantabria Labs.
DISCLOSURES: Dr. Goh is an advisory board member for Cantabria Labs. Dr. Vitale is an employee of Cantabria Labs. The other authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Two extracts derived from the gastropod Cryptomphalus aspersa have been shown to have dermal regeneration properties: SCA® (secretion filtrate) with fibroblast growth factor-like activity and IFC®-CAF (cellular activating factor), a snail egg extract with skin stem cell activation activity.
Objective. The objectives of this study were to evaluate the synergic antiaging activity and tolerability of SCA and IFC-CAF in a combined regimen compared to vehicle as a placebo control.
Methods. A three-month, single-center, double-blinded, randomized, vehicle-controlled trial assessed the effects of a daily skincare routine divided into two treatment phases, as follows: intensive (1 month) and maintenance (2 months). Fifty women, aged 45–65 years, with signs of photoaging were randomized to receive either the active ingredients (n=30) or vehicle (n=20). Clinical evaluations included objective measurements of barrier function and skin hydration, elasticity, and color/brightness. Subjective assessments were conducted according to the Rao-Goldman and Glogau scales for wrinkles, the Patient Global Assessment (PGA) scale and Investigator Global Assessment (IGA) scale.
Results. Subjects in the active treatment group experienced reductions in transepidermal water loss and significant improvements in skin roughness, firmness, and elasticity. Both groups showed significant improvements in fine lines and wrinkles. PGA and IGA assessments indicated greater improvement in the active treatment group.
Conclusion. The active snail extract treatment appears to be effective in improving signs of skin aging in women 45 to 65 years old. Larger randomized, controlled studies are needed to confirm our results.
KEYWORDS: Cryptomphalus aspersa, snail secretion, snail egg extract, skin aging, photoaging
Skin aging includes intrinsic aging, a process attributable to the passage of the time, and extrinsic aging, which involves changes induced by exogenous factors, such as pollution and solar radiation. Both types of aging demonstrate abnormal elastin synthesis and collagen degradation. Degeneration of the extracellular matrix (ECM) is accelerated due, in part, to the increase in number and transcription rate of matrix metalloproteinases (MMPs).1 These proteolytic enzymes are synthesized by fibroblasts and, to date, more than 20 different types have been identified. Aging skin shows an increased expression of MMPs, mainly MMP-1 and MMP-3.2 Modifications are sometimes detected in the tissue inhibitors of MMPs that bind to these enzymes to prevent them from degrading the matrix.1
In the skin, specific growth factors (GF) regulate vital cellular activities, including mitogenesis, motogenesis, angiogenesis, chemotaxis, and the formation of the ECM. GFs are involved in wound healing, through their abillity to reverse the effects of collagenases, increase collagen levels, and decrease tissue inflammation.3 Clinical studies have demonstrated that topical application of human or animal-derived GFs or the injection of autologous GFs can increase dermal collagen synthesis, thus reducing signs of skin aging, such as fine lines and wrinkles.3
Two extracts derived from the gastropod Cryptomphalus aspersa have demonstrated dermal regeneration properties: SCA® (secretion filtrate) with fibroblast growth factor-like activity, and IFC®-CAF (cellular activating factor), a snail egg extract that activates skin stem cells.4–6 These extracts are commercially available under the trade name Endocare, manufactured by Cantabria Labs(Industrial Farmacéutica Cantabria SA, Madrid, Spain). The primary objective of this study was to evaluate the synergistic antiaging activity of SCA (secretion filtrate) and IFC-CAF (cellular activating factor) in a combined cosmeceutical regimen, compared to vehicle as placebo control. The secondary objective was to evaluate the tolerability of this combined regimen.
Methods
This was a three-month, single-center, double-blinded, randomized, placebo-controlled trial designed to assess the effects of a daily skin care routine comprising an intensive phase (1 month in duration) and a maintenance phase (2 months in duration) conducted at the National Skin Centre in Singapore. Fifty women, aged 45 to 65 years old, with Fitzpatrick Skin Phototypes II to V and signs of photoaging (Rao-Goldman wrinkle score7 of 2–4) were recruited. Subjects were enrolled after being informed regarding research participation and potential risks, benefits of participation, and confidentiality concerns; informed consent forms (translated into four languages) were received from all participants. Subjects included in this study agreed to discontinue their own existing facial and eye moisturizers, but were allowed to continue other skincare products, such as cleansers and cosmetics. Exclusion criteria included presence of any uncontrolled systemic disease, a significant history or current evidence of a medical, psychological, or other condition that, by the investigator’s discretion, would preclude enrollment into the study, known hypersensitivity to any of the components of the study products, use of any systemic steroids six months prior to the study period, previous treatment with botulinum toxin, filler, or biostimulatory molecule injections to the facial area, facial dermabrasion, laser treatments, microdermabrasion, or chemical peels six months prior to the study, recent excessive facial exposure to sunlight or artificial ultraviolet light (e.g., tanning beds), and recent history or active presence of any facial skin condition that might interfere with the diagnosis or evaluation of study parameters (e.g., moderate-to-severe acne vulgaris, atopic dermatitis, psoriasis, rosacea, seborrheic dermatitis, and excessive facial hair or coloration).
This study was approved by the institutional ethics committee. Subjects were randomized to receive either the active ingredients (n=30) or vehicle only (n=20).
Study products. Endocare Tensage Serum (Cantabria Labs, Madrid, Spain) is a cosmeceutical product based on the secretion of the mollusc Cryptomphalus aspersa (SCA) and other active ingredients, including Tensderm, vitamins C and E, nicotinamide, dismutin, and mimosa extract. Endocare Cellage/CellPro products are based on a combination of IFC-CAF, Retinsphere® technology, and Wharton Gel Complex® (WGC), with additional ingredients added to enhance antioxidant, anti-inflammatory and antiglycation activity.5 A new Endocare Cellage/CellPro Concentrate formulation for the treatment of skin aging contains a higher concentration of the active ingredients IFC-CAF and WGC. Endocare Cellage/CellPro products have demonstrated clinical improvement in signs of skin aging.8 Because the main trigger for photoaging is sun exposure, the treatment regimen also included a photoprotector sun protection factor (SPF) 50 (Heliocare Gel SPF50; NeoAsia, Singapore), which was used daily by all study subjects.
Study regimen. In the intensive phase (i.e., first month), participants were instructed to apply Endocare Tensage Serum or vehicle in the morning in sufficient quantity to cover the entire face. After complete absorption of the serum, participants applied the sunscreen provided. At night, participants applied approximately 2 to 3 pumps of the intensive Endocare Cellage/CellPro Concentrate or vehicle.
During the maintenance phase (second and third months), participants were instructed to apply the Endocare Tensage Serum or placebo vehicle in the mornings in sufficient quantity to cover the entire face. After complete absorption, they were instructed to apply the sunscreen provided. At night, they were instructed to apply 2 to 3 pumps of Endocare Cellage/CellPro Cream or placebo vehicle to their face.
All patients were educated on the need for sun protection and instructed to stop all other topical facial products other than their own cleansers and cosmetics.
Assessment methods. Clinical evaluation included subjective assessments by patients and physician investigators for wrinkles, Investigator (IGA) and Patient Global Assessments (PGA), and a self-assessment patient questionnaire. Objective skin measurements included transepidermal water loss (TEWL), skin hydration, skin elasticity, and skin brightness.The Rao-Goldman Five-Point Scale7 is scored by physicians and assesses wrinkles at the perioral and periocular areas. The Glogau Four-Point scale9 is also scored by physicians and assesses the presence of wrinkles at rest or in motion.
Physician perception of subject improvement was evaluated according to the IGA scale and the Investigator Skin Condition evaluation, which included the parameters of skin roughness, lack of skin brightness, lack of elasticity, pore prominence, mottled pigmentation, and lines and wrinkles at baseline (T0) and during the two follow-up visits (T30 and T90). Physicians also assessed tolerability based on symptoms of stinging, burning, itching, erythema, desquamation, and edema at both follow-up visits (T30 and T90).
Subject-reported assessment of their own overall skin condition was evaluated at each study visit according to a PGA scale and a Self-Assessment of Facial Skin Quality Questionnaire.
TEWL was measured on the cheek area by the Tewameter® 300 (Courage+Khazaka, electronic GmbH, Köln, Germany). A decreased TEWL index value signifies an improvement of skin barrier functions.10 Skin hydration was also measured on the cheek area by the Corneometer® CM 825 (Courage+Khazaka, electronic GmbH, Köln, Germany). The Corneometer® measures the change in the dielectric constant as the stratum corneum hydration level affects the capacitance of a precision capacitor. A higher hydration index value reflects greater hydration.11
The Cutometer® (Courage+Khazaka, electronic GmbH, Köln, Germany) quantitatively evaluates the mechanical and viscoelastic parameters of skin firmness (R0) and elasticity (R2). The firmness of skin is determined at baseline, and elasticity refers to the resistance to mechanical force versus the ability of skin to return to its original state after suction pressure is applied.12
The quantitative evaluation of whitening/brightening was measured by the Mexameter® MX18 (Courage+Khazaka, electronic GmbH, Köln, Germany) on the cheek. With this device, a high-power halogen lamp illuminates the skin through optical fibers. The light is analyzed and a melanin index of the skin pigmentation is calculated. The melanin index readings are expected to reduce proportionately with skin whitening.13
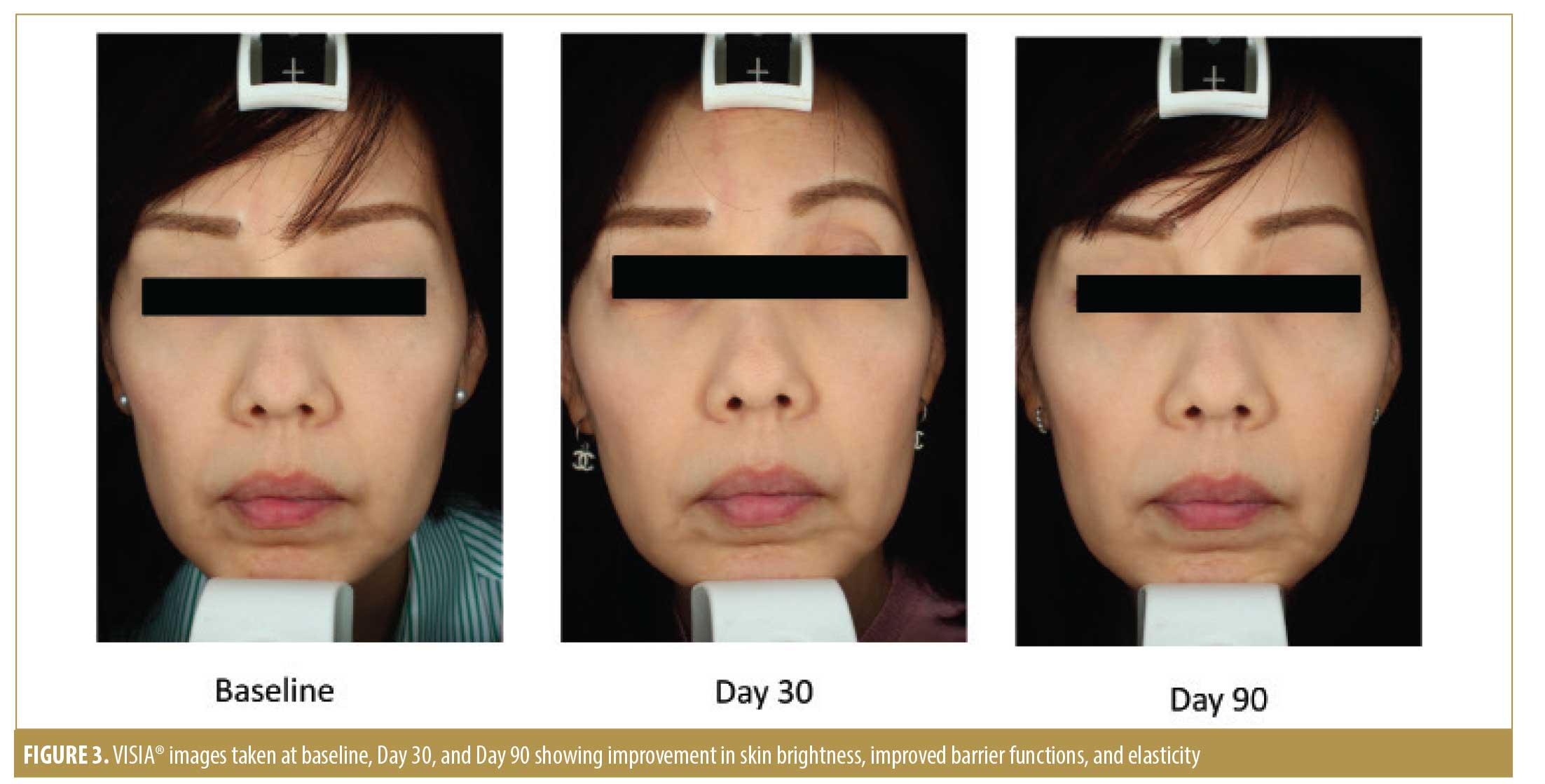
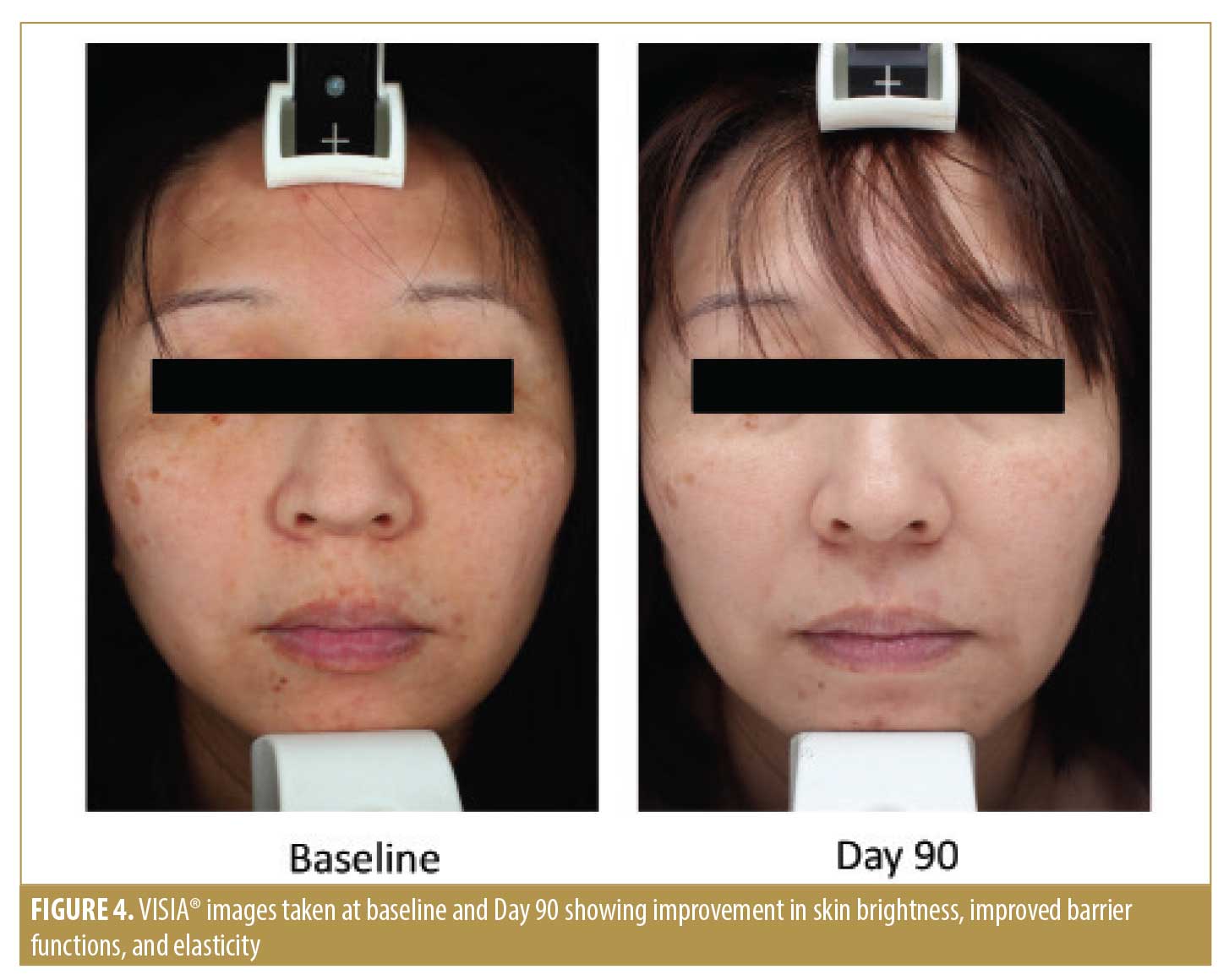
Photographic documentation with the VISIA® camera system (software version 6.4.2; Canfield Scientific, Parsippany, New Jersey) was performed at baseline (T0) and during the follow-up examinations (T30 and T90). Images were taken with standardized camera settings and standardized positioning of the subject.
Statistical analysis. A sample size of 50 patients (n=20, placebo group; n=30, active treatment group) was estimated assuming a power of 80 percent and a level of significance of five percent (p<0.05), based on variability of the parameters found in previous clinical studies. To test the possible significant differences in the study outcomes evaluated at baseline (T0), one month (T30), and three months of treatment (T90), generalized linear mixed-effect models with a within-subjects factor to account for repeated measurements taken from each individual, a treatment factor (active vs. vehicle), time factor (T90 vs. T30 vs. T0), and an interaction term between the treatment factor and time factor were performed. For ordinal study outcomes, a ordinal logistic link function was applied, whereas a linear link function was applied for study outcomes reported using an interval/ratio scale. Descriptive statistics were summarized using means and standard deviations (SDs) for interval/ratio variables and medians and ranges for ordinal variables. The age of participants between treatment groups was compared using a two-sample Student’s t-test. Skin tolerability parameters, PGA score, and IGA score between treatment groups at each study visit were compared using the Mann-Whitney U test. Significance was tested at a level of 0.05. All statistical analyses were conducted using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).14
Results
The mean ages were 54.3 years (range: 45–54.3, SD: 6.0) and 55.8 years (range: 45–65, SD: 5.1) in the vehicle and active groups, respectively. There was no statistical difference in age between the vehicle (mean: 54.3; SD: 6.0 years) and active group (mean: 55.8; SD: 5.1 years) (p=0.361).
Objective measurements at the follow-up visits are summarized in Figure 1. Subjects in the active treatment group experienced significantly greater improvement in the reduction of TEWL compared to the vehicle group at T90 (p=0.026). Subjects in the active treatment group also demonstrated significantly greater improvement in skin firmness, measured by the Cutometer®, at both the 30-day (p=0.012) and 90-day (p=0.005) follow-up visits and in skin elasticity at T90 (p=0.024), compared to the vehicle group. The active treatment group demonstrated increased hydration index values according to the Corneometer® at both T30 and T90, although the improvement was not significantly different between the active treatment and vehicle groups. Both the active treatment and vehicle groups showed a borderline nonsignificant reduction in melanin index values measured with the Mexameter® after 30 days of treatment (p=0.051).
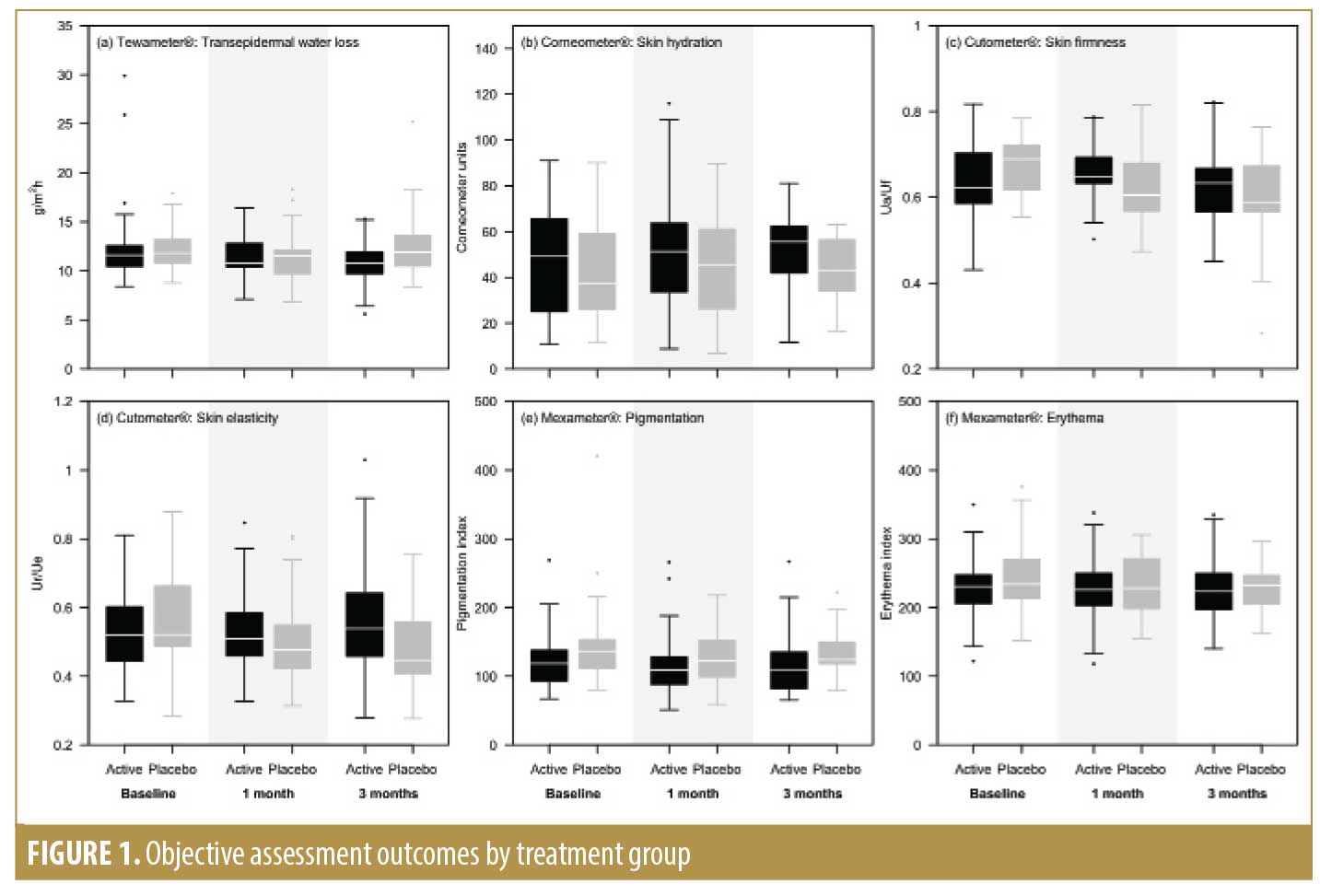
Based on the Rao-Goldman Five-Point Scale, the active and control groups both showed significant improvement in periocular (relative risk [RR]: 0.052; p=0.021) and perioral (RR: 0.106; p=0.033) wrinkles at the end of treatment without significant differences between the groups; results on the Glogau scale were similar.
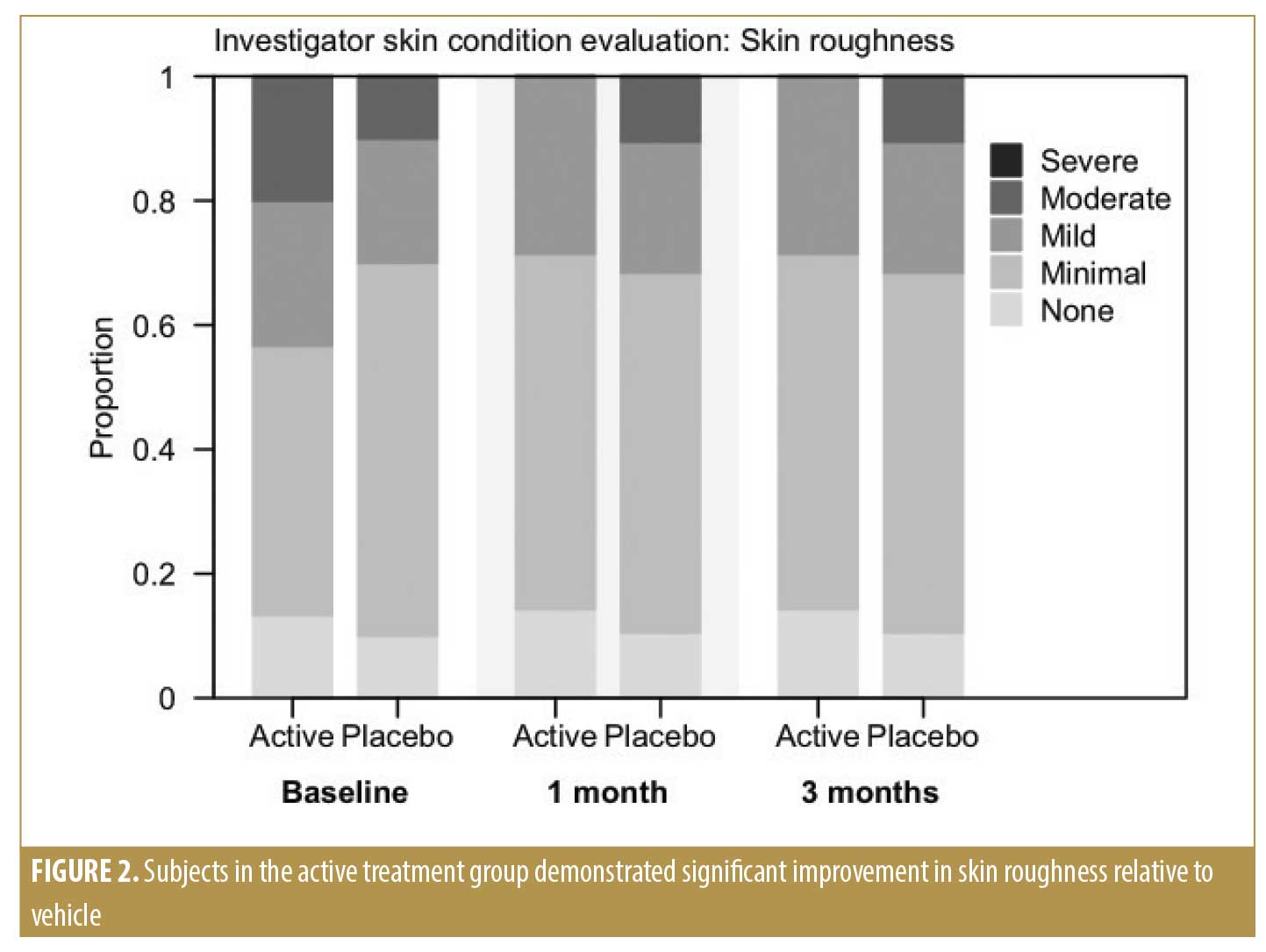
Per the Investigator Skin Condition evaluation, our physicians assessed the parameters of skin roughness, lack of skin brightness, lack of elasticity, pore prominence, mottled pigmentation, and lines and wrinkles at baseline and during the two follow-up visits. Subjects in the active treatment group demonstrated significantly greater improvement in skin roughness compared to those in the vehicle group at the end of the study (p=0.002) (Figure 2). Subjects in both treatment groups experienced significant improvement in skin brightness (p<0.001), elasticity (p=0.019), mottled pigmentation (p=0.027), and lines and wrinkles (p=0.004) at the end of the study, but there was no significant difference between the active and vehicle-treated groups (Figures 3 and 4).
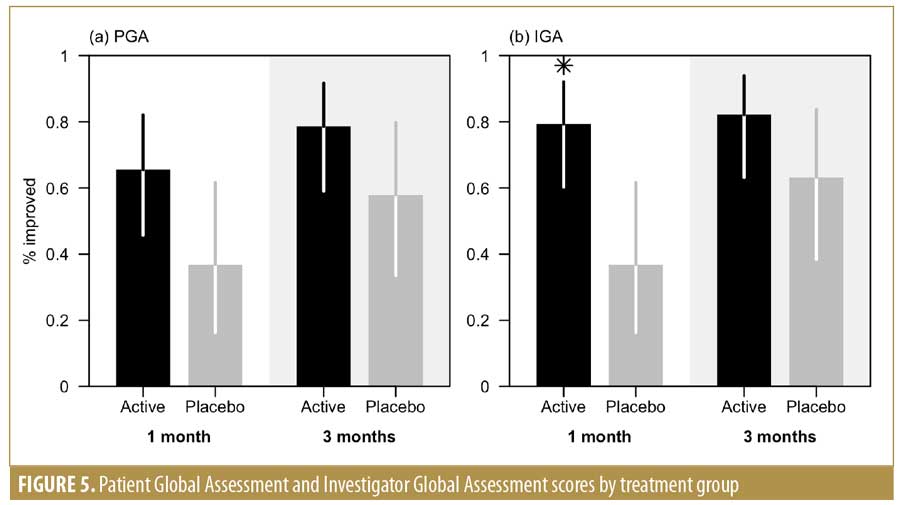
The global assessment performed by the researcher (IGA) greater improvement in the active group compared to the vehicle group at one month after treatment and at the last follow-up visit. The statistical significance was reached after one month of treatment (79.3% vs. 36.8%; p=0.005) (Figure 5). The improvement assessed using the PGA was greater in the active group at both visits compared to the vehicle group. The results of both PGA and IGA were in concordance.
There was a total of three withdrawals from the study, including one subject from the active treatment group who withdrew after one month (T30) due to the development of facial herpes zoster unrelated to the study products. The other two other subjects, one from the active treatment group and one from the vehicle group, withdrew due to the development of contact dermatitis, but we were unable to ascertain if the development was directly related to the study products or to other reasons, due to lack of availability of ingredients required to conduct confirmatory patch testing.
The active treatment group reported significantly less stinging (p=0.002), burning (p=0.002), and erythema (p=0.034) compared to the vehicle group by the end of the study, but there was no significant difference in reports of itching, desquamation, and edema between the active treatment and vehicle groups; hence, the overall tolerability was equal or better in the active group than in the vehicle group.
Discussion
In-vitro studies have demonstrated several key changes in photoaged skin, including accumulation of degenerated elastic fibers, decreased fibronectin activity, and reduced collagen synthesis, which results in dermal thinning and a loss of structural support.15–17 SCA has GF-like activity that increases the proliferation, migration, and activity of fibroblasts and keratinocytes, improving the cell structure and assembly of ECM.4–6,18 Several studies have previously demonstrated that SCA is effective in the treatment of photoaged skin.4–6,19 SCA has been shown to prevent elastosis in aged skin and accelerate the processes of skin regeneration and wound healing, as evidenced by its use in injured skin (e.g., radiodermatitis). SCA has demonstrated faster skin regeneration after dermatological procedures like laser or peeling.20–22
IFC-CAF is a derivative of Cryptomphalus aspersa eggs that has regenerative properties. It activates skin stem cells, stimulating differentiation and migration, and the synthesis of ECM components.4–6 In addition, IFC-CAF promotes improvements in skin structure by aiding in the organization of the cytoskeleton, which promotes epithelial tissue regeneration,4–6 and increasing collagen and fibronectin synthesis in the dermis. Moreover, MMP-release is downregulated.4–6 Of note, in-vitro studies have demonstrated that IFC-CAF induces the differentiation of stem cells to cutaneous cell lineages, the migration of a human keratinocyte cell line (HaCaT cells), primary dermal fibroblasts, and senescent dermal fibroblasts, organizes the cytoskeletal proteins, and enhances the production of ECM fibronectin and collagen I to prevent cutaneous aging.6 These effects suggest that IFC-CAF might have a clinical impact on skin rejuvenation and regeneration of wounded tissues and serves as a therapeutic agent for the treatment of skin aging. This evidence suggests that SCA and IFC-CAF might work synergistically to firm and densify skin while reducing lines and wrinkles.
Retinsphere, a combination of a retinoid ester, hydroxipinacolone retinoate, and retinol glycospheres, aids in the normalization of epidermal proliferation and facilitates the penetration of other active ingredients.23 Retinsphere technology has demonstrated efficacy in improving photoaging and melasma.23
WGC is an active ingredient derived from the ECM of porcine umbilical cord. It is rich in glycosaminoglycans and growth factors (insulin-like GF-1, transforming GF-beta, fibroblast GF-1, fibroblast GF-2) and has been shown to increase human primary fibroblast proliferation while preserving their viability and morphology.4–6 WGC has also been shown to promote fibroblast migration, exhibiting a high chemotactic capacity. The analysis of ECM components suggests that WGC can increase the levels of collagen I and fibronectin fibers and the synthesis of collagen III and VII and hyaluronic synthases 1 and 3.4–6
Endocare Cellage/CellPro formulations combine IFC-CAF, Retinsphere, and WGC and have shown significant improvement in both investigator and patient assessments in overall photoaging.4–6 These findings were related to the increase in epidermal thickness observed histologically and the greater levels of dermal ECM fibers, Collagen Types I and III, elastin density, and fibronectin expression.4–6 The increase in the expression of these various components of the ECM is thought to be responsible for the objective and subjective improvements in aging. This combination therapy is also well-tolerated.4–6
Our study suggests that the synergic activity of SCA and IFC-CAF in Endocare Tensage and Cellage/CellPro products can produce overall improvement in TEWL, skin roughness, firmness, and elasticity in aging skin compared to vehicle controls. There was also subjective improvement in the overall appearance of skin aging, based on the IGA and PGA assessment scores. In particular, the results of this study suggest that the Endocare Cellage/CellPro regimen is well-tolerated in the Asian skin type. In several efficacy parameters, this regimen demonstrated superiority over the vehicle, which contains ingredients with hydration and moisturization efficacy but without the active ingredients.
Conclusion
Subjects who received the active gastropod extract treatment showed significant improvement in a number of skin aging parameters, including TEWL, skin elasticity, wrinkles, skin roughness, and overall IGA and PGA assessments versus subjects who received the vehicle. In addition, the active ingredients were generally well tolerated by subjects in the active treatment group. The combination of the two extracts applied using the intensive and maintenance treatment regimens appear to be an effective antiaging treatment regimen. Larger, randomized, controlled trials are needed to confirm our findings.
References
- Uitto J, Fazio MJ, Olsen DR. Molecular mechanisms of cutaneous aging. Age-associated connective tissue alterations in the dermis. J Am Acad Dermatol. 1989;21(3 Pt 2):614–622.
- Truchuelo MT, Jiménez N, Miguel-Gomez L, et al. Histological and immunohistochemical evaluation of the efficacy of a new cosmetic formulation in the treatment of skin photoaging. Dermatol Res Pract. 2017;2017:8407247.
- Fabi S, Sundaram H. The potential of topical and injectable growth factors and cytokines for skin rejuvenation. Facial Plast Surg. 2014;30(2):157–171.
- Fabi SG, Cohen JL, Peterson JD, et al. The effects of filtrate of the secretion of the Cryptomphalus aspersa on photoaged skin. J Drugs Dermatol. 2013;12(4):453–457.
- Espada J, Salazar N, Damian A, et al. Cryptomphalus aspersa mollusk eggs extract promotes migration and regenerative behavior of human keratinocytes and mesenchymal stem cells in vitro. J Invest Dermatol. 2014;134(8):S1–S9.
- Espada J, Matabuena M, Salazar N, et al. Cryptomphalus aspersa mollusc eggs extract promotes migration and prevents cutaneous ageing in keratinocytes and dermal fibroblasts in vitro. Int J Cosmet Sci. 2015;37(1):41–55.
- Carruthers J, Donofrio L, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of facial fine lines. Dermatol Surg. 2016;42 Suppl 1(Suppl 1):S227–S234.
- Draelos, Z. The role of a natural mollusk egg derived ingredient in facial appearance. J Drugs Dermatol. 2017;16(7):611–614.
- Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996;15(3): 134–138.
- Berardesca E, Loden M, Serup J, et al. The revised EEMCO guidance for the in vivo measurement of water in the skin. Skin Res Technol. 2018;24(3): 351–358.
- O’goshi K, Serup J. Inter-instrumental variation of skin capacitance measured with the Corneometer. Skin Res Technol. 2005;11(2):107–109.
- Ahn S, Kim S, Lee H, et al. Correlation between a Cutometer and quantitative evaluation using Moire topography in age-related skin elasticity. Skin Res Technol. 2007;13(3):280–284.
- Matias AR, Ferreira M, Costa P, et al. Skin colour, skin redness and melanin biometric measurements: comparison study between Antera(®) 3D, Mexameter(®) and Colorimeter(®). Skin Res Technol. 2015;21(3):346–362.
- R Core Team, R Foundation for Statistical Computing. R: A language and environment for statistical computing. Available at: https://www.R-project.org/. 28 Feb 2020.
- Bernstein EF, Chen YQ, Tamai K, et al. Enhanced elastin and fibrillin gene expression in chronically photodamaged skin. J Invest Dermatol. 1994;103(2):182–186.
- Knott A, Drenckhan A, Reuschlein K, et al. Decreased fibroblast contractile activity and reduced fibronectin expression are involved in skin photoaging. J Dermatol Sci. 2010;58(1):75–77.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168(6):1861–1868.
- Brieva A, Philips N, Tejedor R, et al. Molecular basis for the regenerative properties of a secretion of the mollusk Cryptomphalus aspersa. Skin Pharmacol Physiol. 2008;21(1):15–22.
- Cruz MC, Sanz-Rodríguez F, Zamarron A, et al. A secretion of the mollusk Cryptomphalus aspersa promotes proliferation, migration and survival of keratinocytes and dermal fibroblasts in vitro. Int J Cosmet Sci. 2012;34(2):183–189.
- Abad R. Treatment of experimental radiodermatitis (single irradiation hyperdose) with a regenerative glucoproteic mucopolysacharid complex. Dermatol Cosmet. 1999;9(2):53–57.
- Truchuelo MT, Vitale M. A cosmetic treatment based on the secretion of Cryptomphalus aspersa 40% improves the clinical results after the use of nonablative fractional laser in skin aging. J Cosmet Dermatol. 2019;19(3):1–7.
- Sisto T, Bussoletti C, Celleno L. Valutazione half-face dell’efficacia rigenerativa e riparativa di un siero a base di secreto di Cryptomphalus aspersa al 15% versus placebo, in due gruppi di pazienti con acne e fotoinvecchiamento trattati con la tecnica peeling. Esperienze Dermatologiche. 2013;15(4):181–184.
- Cameli N, Mariano M, Abril E, et al. Clinical evaluation of the efficacy of a new retinoic-complex (Retinsphere) product for the treatment of skin photoaging. Esperienze Dermatologiche. 2012;14:29–34.

