 J Clin Aesthet Dermatol. 2021;14(6):55–59.
J Clin Aesthet Dermatol. 2021;14(6):55–59.
by Donovan G. Kearns, BA; Shelley Uppal, MD; Vipawee S. Chat, BA; and Jashin J. Wu, MD
Mr. Kearns is with Loma Linda University School of Medicine in Loma Linda, California. Dr. Uppal is with Albany Medical College School of Medicine in Albany, New York. Ms. Chat is with the Medical College of Georgia at Augusta University in Augusta, Georgia. Dr. Wu is with the Dermatology Research and Education Foundation in Irvine, California.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Wu is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America LLC, Zerigo Health. The other authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Interleukin (IL)-17 inhibitors are a newer class of biologic used to treat patients with moderate-to-severe plaque psoriasis and psoriatic arthritis.
Objective. We compared evidence-based clinical practice guidelines (CPGs) from leading dermatological organizations for the use of IL-17 inhibitors in psoriasis.
Methods. Guidelines from the Joint American Academy of Dermatology-National Psoriasis Foundation (AAD-NFP) Guidelines, British Association of Dermatologists guidelines (BAD), and European S3 group (ES3) were all reviewed and compared.
Results. This analysis revealed significant overlap in the recommendations made by experts from each CPG. However, our review highlights differences in routine laboratory recommendations and the relative and absolute contraindications to use with IL-17 inhibitors.
Conclusion. IL-17 inhibitors are an effective treatment option for psoriasis. This analysis and review of guidelines for IL-17 inhibitor use highlights the consensus in treatment protocols and areas of disagreement between CPGs.
Keywords: Psoriasis, IL-17 inhibitors, practice guidelines, secukinumab, ixekizumab, brodalumab
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately three percent of the world’s population.1 While topical therapies are often first-line treatments for mild-to-moderate psoriasis, approximately 1 in 6 individuals have moderate-to-severe disease that requires systemic treatment, such as biologics or small-molecule inhibitors.2 In 2015, secukinumab became the first interleukin (IL)-17 inhibitor approved by the United States (US) Food and Drug Administration (FDA) for use in patients with moderate-to-severe plaque psoriasis. Since then, ixekizumab and brodalumab have also obtained FDA approval for the treatment of psoriasis. In several meta-analyses, IL-17 inhibitors were found to be among the most effective treatments for plaque psoriasis, superseding many of their predecessors, including tumor necrosis-alpha (TNF-alpha) inhibitors.3–5
Several national and regional dermatologic associations have published evidence-based guidelines summarizing consensus recommendations regarding psoriasis treatment with biologics. Each organization includes a panel of experts that evaluate current research, formulate recommendations for proper selection and administration of treatment, as well as assess treatment dosage, duration, adverse effects, and special treatment circumstances. These national organizations have also established up-to-date, evidence-based clinical practice guidelines (CPGs), which are guiding frameworks for clinicians treating psoriasis and are useful in optimizing patient care. This review focuses on the use of IL-17 inhibitor therapy. The American Academy of Academy and National Psoriasis Foundation (AAD-NFP), British Association of Dermatologists (BAD), and European Dermatology Forum in cooperation with the European Academy of Dermatology and Venereology and the International Psoriasis Council (European S3) have all published recent guidelines regarding the use of biologic medications for patients with psoriasis.6–8 Recommendations are frequently updated as knowledge of pathophysiology evolves and new treatment modalities emerge. This literature review will focus on consensus recommendations for IL-17 inhibitor therapy use in patients with moderate-to-severe psoriasis.
Methods
A literature search was conducted in PubMed, OVID, and Google search for CPGs discussing the use of IL-17 inhibitors. International guidelines, including recommendations for the use of secukinumab, ixekizumab, or brodalumab, were included within the present analysis.
Results
Steps to take before the initiation of IL-17 inhibitor treatment. Dermatologists have a duty to educate and inform patients about the benefits and possible adverse effects of IL-17 inhibitor therapy. The AAD-NPF, BAD, and ES3 have all placed an emphasis on clearly disclosing possible benefits and risks of treatment with patients. Proper disclosures include the discussion of all possible treatment modalities with the best interest of the patient in mind. The AAD-NFP also recommends discussing patient preferences prior to selection of a treatment, as some patients might prefer medication with a lower cost, less frequent dosing schedule, or particular route of administration.6
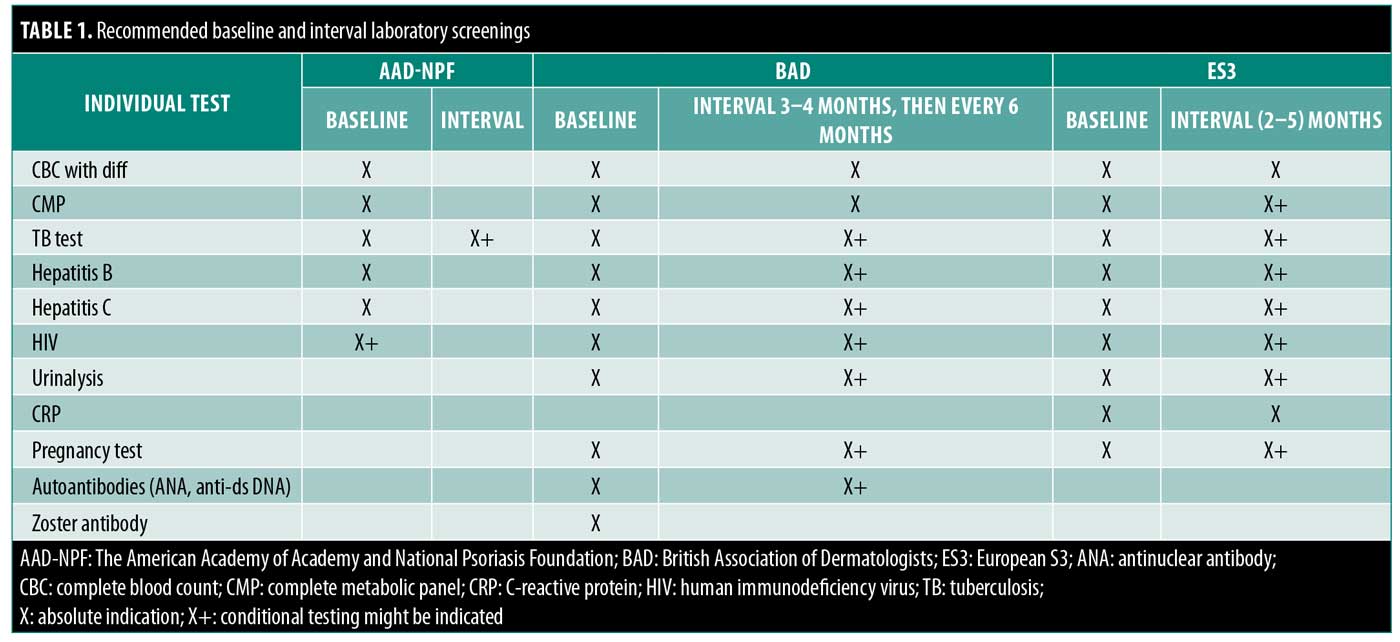
A proper physical examination and complete medical history-taking are recommended by all expert CPGs before initiating IL-17 inhibitor therapy. Psoriasis phenotype, disease course, and medication history should all be assessed. This also includes assessing for a history of prior malignancy, infectious disease, and comorbid conditions. Infectious disease screening for tuberculosis (TB), hepatitis B (HBV) and hepatitis C (HCV) is recommended by all organizations. Human immunodeficiency virus (HIV) screening is fully endorsed by the ES3 and BAD, but is recommended at the practitioner’s discretion by the AAD-NPF.9 The ES3 recommends a baseline objective assessment of disease (e.g., Psoriasis Area and Severity Index [PASI], body surface area [BSA], Physicians’ Global Assessment, presence of arthritis) for all patients. Dermatologists should conduct a reassessment of patient history and physical examination, plans for becoming pregnant, and screening for skin cancer for all patients. The AAD-NPF also recommends periodic assessments for suicidal ideation in patients treated with brodalumab, which could indicate the need for more frequent follow-up visits or referral to psychiatry.10
All CPGs recommend that patients receive a baseline complete blood count with differential, complete metabolic panel, and TB screening (i.e., Mantoux test and T-SPOT test or QuantiFERON-TB Gold test) before and during treatment. Given the immunosuppressive effects of biologics, annual testing for TB should be considered in high-risk patients. Patients with a positive TB test should be referred for chest radiograph. Both the ES3 and BAD recommend gathering a pretreatment baseline pregnancy and urine status results. The BAD also suggests a baseline auto-antibody panel for antinuclear antibody and anti-double-stranded DNA, with a repeat assessment if the patient develops transaminitis or signs of autoimmunity. The ES3 is alone in recommending a baseline C-reactive protein examination with repeat testing every 2 to 5 months. Table 1 includes a list of pretreatment and repeat laboratory recommendations for patients prior to the initiation of therapy. Periodic reevaluation of certain laboratory and infectious markers is recommended by all three of the CPGs on an as-needed basis for at-risk patients.
Due to the immunosuppressive effects of IL-17 inhibitor therapy, it is recommended that patients remain up to date on their vaccinations prior to starting the medication. Both the AAD-NPF and BAD suggest that inactivated vaccinations are safe and permissible during immunosuppressive treatment.6–8,11 The AAD-NPF states that some experts recommend waiting 2 to 3 half-lives before and after administration of live vaccines, while others advise discontinuation of biologics from four weeks before to 1 to 2 weeks after. The half-lives of secukinumab, ixekizumab, and brodalumab are 27, 13, and 11 days, respectively.12 The BAD recommends stopping biologic therapy for 6 to 12 months before the administration of any live vaccinations and to wait four weeks after immunization before resuming IL-17 inhibitor therapy. The most recent set of guidelines from the European S3 does not offer any updated recommendations with regard to vaccinations and IL-17 inhibitor use. The BAD also suggests that live vaccines should be avoided in infants (up to six months of age) whose mothers received biologic therapy beyond 16 weeks of gestation.
All three CPGs recommend that an active or current history of inflammatory bowel disease (IBD) represents a relative contraindication to treatment with secukinumab. The BAD suggests obtaining a gastroenterology consult before initiating treatment with brodalumab, ixekizumab, or secukinumab in patients with IBD. The BAD and S3 consider acute and chronic HBV or HCV infection as possible relative but not absolute contraindications to treatment. According to the AAD-NPF, a history of recent suicidality or suicidal ideation is a relative contraindication to brodalumab treatment specifically. The S3 also mentions malignancies or lymphoproliferative disorders as a relative contraindication to treatment with secukinumab. A summary of this information can be found in Table 2.
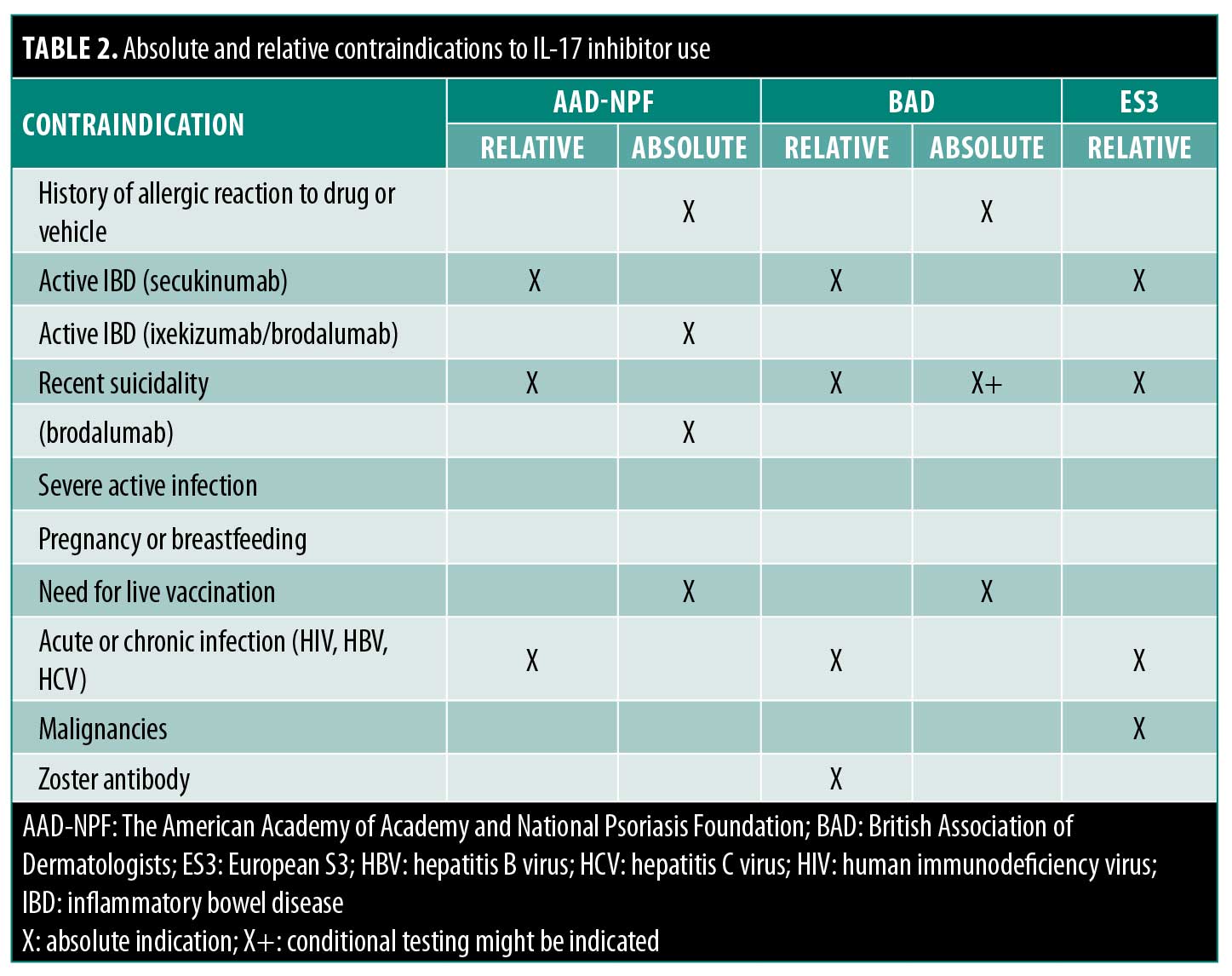
A history of allergic reaction to the therapeutic agents or vehicle or the need for live vaccination are absolute contraindications shared by all three CPGs. The AAD-NPF considers IBD to be an absolute contraindication to treatment with brodalumab or ixekizumab.13 The S3 considers pregnancy or lactation to be an absolute contraindication to secukinumab use, although the AAD-NPF and BAD specify that there have been no human studies evaluating the safety of IL-17 inhibitors in pregnancy or breastfeeding. A summary of this information can be found in Table 2.
Discussion
Management of severe plaque psoriasis. The IL-17 inhibitor class has established itself as one of the most effective medications for the treatment of moderate-to-severe plaque psoriasis, with one meta-analysis revealing ixekizumab, brodalumab, and secukinumab as triggering the greatest improvement in PASI 75, PASI 90, and PASI 100 at Week 16 when compared to other biologic medications.5
As with all of the medications in the IL-17 inhibitor class, secukinumab is recommended for use by patients with moderate-to-severe plaque psoriasis. The same dosing schedule is recommended by all three CPGs: a starting dose of secukinumab of 300mg by self-administered subcutaneous injection at Weeks 0, 1, 2, 3, and 4, followed by a maintenance dose of 300mg every fourth week. A dosage of 300mg was shown in trials to be more effective than a dosage of 150 mg.14
The CPGs provided by the BAD and AAD-NPF offer recommendations for ixekizumab dosing during treatment initiation and maintenance. Patients should be started on an initiation dose of 160mg administered subcutaneously, followed by 80mg every two weeks until Week 12. Following Week 12, a maintenance dose of 80mg every four weeks should be administered.
The CPG provided by the AAD-NPF was the only one of the three reviewed that offered recommendations for brodalumab dosing during treatment initiation and maintenance. The optimal dose of brodalumab is 210mg by self-administered subcutaneous injection on Weeks 0, 1, and 2, followed by 210mg administered every two weeks thereafter.
The AAD-NPF, BAD, and ES3 recommend that clinicians carry out a standardized disease assessment to determine the efficacy of IL-17 inhibitor treatment. The BAD defines minimal response as a at least a 50-percent reduction in baseline disease severity score (e.g., PASI50 preferably or percent BSA affected) and clinically relevant improvements in physical, psychological, or social functioning parameters (e.g., at least a 4-point improvement in Dermatology Life Quality Index or resolution of mood disorders). The AAD-NPF primarily uses PASI response to evaluate treatment response, as it takes into account not only the BSA but also patient symptomatology, such as intensity of redness, scaling, and plaque thickness. The ES3 uses objective measurements (e.g., PASI response, percent BSA involvement, Physicians’ Global Assessment, presence of arthritis) and assessments for health-related quality of life such as the Dermatology Life Quality Indexor Skindex-29 or -17) to assess disease severity. The AAD-NPF specifically recommends that clinicians perform a primary disease assessment after 12 weeks of continuous treatment, while no specific intervals are recommended by the BAD and ES3. According to the BAD, patients who fail to achieve the minimum response criteria (primary failure), initially respond then lose therapeutic response (secondary failure), or develop intolerance or contraindications to treatment should consider taking up an alternative therapy. For patients with a partial treatment response, the AAD-NPF recommends dose escalation or the addition of other treatment modalities, such as topical corticosteroids, methotrexate, or ultraviolet light therapy.15 The ES3 currently does not have recommendations in place for secukinumab-treated patients who develop partial or inadequate treatment responses.
Management of nonplaque forms of psoriasis. The IL-17 inhibitor class has shown benefits in several other psoriasis phenotypes, including scalp, palmoplantar, nail, erythrodermic, inverse, and generalized pustular psoriasis. The AAD-NPF was the only organization to provide recommendations for the treatment of nonplaque psoriasis phenotypes (Table 3).
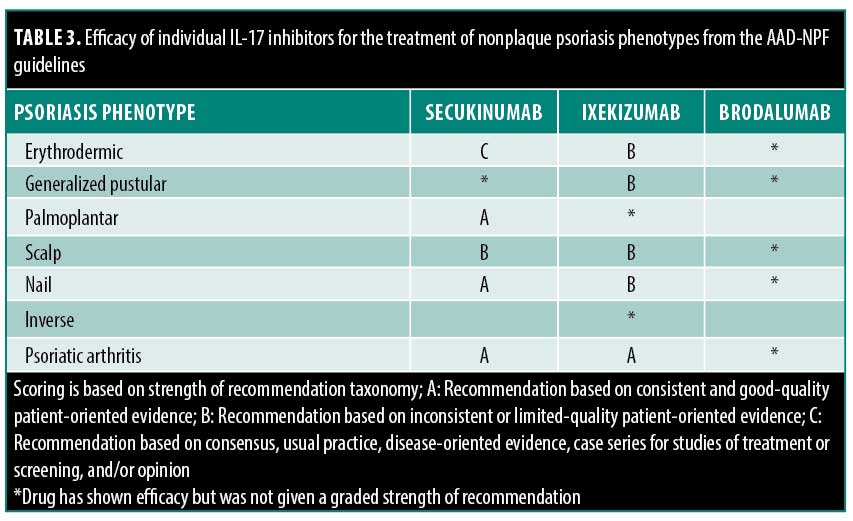
Erythrodermic psoriasis is an uncommon variant that presents with widespread erythema of the skin and requires rapid treatment. The AAD-NPF recommends ixekizumab or secukinumab as monotherapy for patients with erythrodermic psoriasis (Level B and C recommendations, respectively). Brodalumab has also been shown to be effective in treating this condition, although the strength of recommendation for its use has not been provided by the AAD-NPF.
Generalized pustular psoriasis is a rare and clinically distinct variant of psoriasis characterized by widespread sterile pustules. The AAD-NPF endorsed the use of both ixekizumab and brodalumab as monotherapy for patients with generalized pustular psoriasis (Level B recommendation). Secukinumab has also been evaluated and has shown promise in the treatment of generalized pustular psoriasis, although the strength of recommendation for its use has not been provided by the AAD-NPF.
Palmoplantar psoriasis is a variant of the disease that affects the hands and feet. Secukinumab is recommended by the AAD-NPF for use as monotherapy treatment in adult patients with moderate-to-severe palmoplantar pustulosis (Level A recommendation). Ixekizumab has also been evaluated and has shown benefit in patients with palmoplantar pustulosis, although the strength of recommendation for its use has not been provided by the AAD-NPF.
Table 3 summarizes the AAD-NPF’s findings regarding the efficacy of IL-17 inhibitors for the nonplaque psoriasis phenotypes, including scalp, palmoplantar, nail, inverse psoriasis, and psoriatic arthritis.
Special populations and circumstances. Patients with a history of IBD are at increased risk for complications when treated with IL-17 inhibitors. In select patients, IL-17 inhibitors can cause new-onset IBD or reactivate preexisting IBD.13,16 Recommendations for their use in this distinct population by the three CPGs can be found above in the relative contraindications section and in Table 2.
Providers should be cautious when prescribing brodalumab to patients with a history of depression. The AAD-NPF considers a history of suicidal ideation, recent suicidal behavior, or suicidal ideation to be an absolute contraindication for its use due to a small number of completed suicides observed during the drug trials.17 For this reason, brodalumab has a black box warning stating “suicidal ideation and behavior, including completed suicides, have occurred in patients treated during clinical trials.” The medication is solely available through a restricted program under a risk evaluation and mitigation strategy (called the SILIQ risk evaluation and mitigation strategy program). While the nature of the association between brodalumab and suicide is currently uncertain, an analysis of five trials involving the medication suggest the timing of events do not indicate a causal relationship.17 Nonetheless, providers should cautiously prescribe brodalumab in patients with a history of depression or other mood disorders.
Patients with medical conditions that predispose them to an immunosuppressed state (i.e., HIV) require a careful initial evaluation and consideration of the advantages and disadvantages of IL-17 inhibitor therapy. All three CPGs recommend that high-risk patients undergo HIV screening (of presence of HIV-1 and HIV-2 antibodies and HIV-1 antigens) prior to treatment initiation and annual screenings for HIV, HBV, and HCV infection during treatment. HBV vaccination should also be offered when high risk patients screen negative for HBV infection. The AAD-NPF recommends that HIV patients must receive highly active antiretroviral therapy, show a normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection to be eligible for IL-17 therapy. BAD and ES3 recommend that IL-17 inhibitor treatment in HIV patients be considered on a case-by-case basis. Clinicians should be aware that severe psoriasis can be a manifestation of poorly controlled HIV infection and is likely to resolve with highly active antiretroviral therapy.18
Patients with chronic viral infections, such as HCV or HBV infection, necessitate extensive precautions be taken before initiating immunomodulating treatments. All three CPGs recommend that high-risk patients undergo serological screening for HCV and HBV infections (e.g., HB surface Ag, anti-HB surface Ab, anti-HB core Ab, and hepatitis C antibody tests). Screening during treatment is also advised for patients at an increased risk of infection. Both the BAD and ES3 suggest that IL-17 inhibitor treatment in patients infected with HCV or HBV be considered on a case-by-case basis and only with the involvement of a relevant specialist.
Little data are currently available on the risk of IL-17 inhibitors in psoriasis patients during the perioperative period. The AAD-NPF, BAD, and ES3 all suggest that IL-17 inhibitor therapy can be continued in patients undergoing minor procedures resulting in clean wounds. However, for patients undergoing moderate- and high-risk surgical procedures that involve the respiratory, gastrointestinal, or genitourinary tract, discontinuation of the biologic may be considered on a case-by-case basis. Patient characteristics, risk of infection, and psoriasis severity are important factors that must be evaluated. If necessary, the BAD and AAD-NPF recommend discontinuing the medication for three to five times the half-life of the drug and for one to two weeks after elective surgery if there are no postoperative complications (AAD-NPF).
Limitations. This comparison of IL-17 inhibitor guidelines was dependent on the original recommendations put forth by the AAD-NPF, BAD, and ES3. Because the ES3 guideline was published in 2017, its analysis did not include information regarding the use of ixekizumab or brodalumab, as they became established therapies after secukinumab (in 2016 and 2017, respectively).
Conclusion
IL-17 inhibitors are an extremely effective treatment option for patients with moderate-to-severe psoriasis with a good safety profile. A careful analysis of guidelines set forth by the AAD-NPF, BAD, and ES3 revealed a significant overlap in expert treatment recommendations. Recommendations regarding the suggested baseline and interval laboratory screening tests as well as the classification of relative and absolute contraindication to therapy varied between the three CPGs. CPGs remain reliable, evidence-based sources of treatment recommendations for clinicians to effectively and safely treat patients. This review and comparison of consensus guidelines provides dermatologists with excellent recommendations to safely treat moderate-to-severe psoriasis with IL-17 inhibitors.
References
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179.
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14(8):e0220868.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269.
- Warren RB, Gooderham M, Burge R, et al. Comparison of cumulative clinical benefits of biologics for the treatment of psoriasis over 16 weeks: results from a network meta-analysis. J Am Acad Dermatol. 2019;82(5):1138–1149.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Nast A, Spuls PI, van der Kraaij G, et al. European S3-guideline on the systemic treatment of psoriasis vulgaris – Update Apremilast and Secukinumab—EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2017;31(12):1951–1963.
- Smith CH, Yiu ZZ, Bale T, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020—a rapid update. Br J Dermatol. 2020;183(4):628–637.
- Chiricozzi A, Saraceno R, Cannizzaro MV, et al. Complete resolution of erythrodermic psoriasis in an HIV and HCV patient unresponsive to antipsoriatic treatments after highly active antiretroviral therapy (ritonavir, atazanavir, emtricitabine, tenofovir). Dermatology. 2012;225(4):333–337.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23(2):1–3.
- Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23(1): 50–74.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113.
- Reich K, Leonardi C, Langley RG, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: a presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017;76(3):441–448.e442.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Ryoo JY, Yang HJ, Ji E, Yoo BK. Meta-analysis of the efficacy and safety of secukinumab for the treatment of plaque psoriasis. Ann Pharmacother. 2016;50(5):341–351.
- Wright S, Alloo A, Strunk A, Garg A. Real-world risk of new-onset inflammatory bowel disease among patients with psoriasis exposed to interleukin 17 inhibitors. J Am Acad Dermatol. 2020;83(2):382–387.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78(1):81–89.e85.
- Vittorio Luigi De Socio G, Simonetti S, Stagni G. Clinical improvement of psoriasis in an AIDS patient effectively treated with combination antiretroviral therapy. Scand J Infect Dis. 2006;38(1):74–75.

