 J Clin Aesthet Dermatol. 2021;14(6):E61–E65
J Clin Aesthet Dermatol. 2021;14(6):E61–E65
by Irma Bernadette S. Sitohang, MD, PhD; Retno W. Soebaryo, MD, PhD and Mpu Kanoko, MD, PhD
Dr. Sitohang and Prof. Soebaryo are with the Department of Dermatology and Venereology, Faculty of Medicine, Universitas Indonesia in Jakarta, Indonesia. Prof. Kanoko is with the Department of Pathological Anatomy, Faculty of Medicine, Universitas Indonesia in Jakarta, Indonesia.
FUNDING: This study was supported and funded by Grant from Universitas Indonesia Grant number (letter No. 1537/UN2.R12/PPM.00/2015 and Recipient Name: Retno Widowati Soebaryo).
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Clincal trial ID: NCT04206631
Background. Acne vulgaris is a polymorphic skin condition comprising inflamed and noninflamed lesions. In addition to topical retinoids, systemic antibiotics play a role as a main therapy for acne with inflamed papules and cysts. However, due to the increasing tendency for bacterial resistance, alternatives to antibiotics are needed.
Objective. The aim of this study was to evaluate the effectiveness of acne lesion extraction compared to oral doxycycline for moderate acne vulgaris and to explore the impact of both treatments on Hypoxia-inducible factor (HIF)-1 alpha.
Methods. This randomized clinical trial was conducted in two teaching hospitals in 2016. Subjects with moderate acne vulgaris (N=140) were divided into two groups. Each subject in both groups received 0.05% tretinoin cream, applied to the entire face each night, and 2.5% benzoyl peroxide gel, applied to the acne lesions in the morning and afternoon. One group was also treated with oral doxycycline 100mg once daily and the other was treated with acne lesion extraction performed on all facial lesions every two weeks; the patients were evaluated via patient-reported self-assessment and lesion counts every two weeks for six weeks. HIF-1 alpha expression of the biopsied lessions was examined via immunohistochemistry.
Results. 128 subjects completed the study. Among these 128 subjects there was a prominent decrease in inflamed lesions at Week 6 in the lesion extraction group compared to the oral doxycycline group (p<0.05). HIF-1 alpha expression of the biopsied lesions was found in 7 of 9 samples taken from the oral doxycycline group, while 3 of 4 samples in the lesion extraction group were found negative.
Conclusion. According to our results, acne lesion extraction appeared to be more effective than oral doxycycline in treating this sample of patients with moderate acne vulgaris. Additionally, HIF-1 alpha expression appeared to be decreased after acne lesion extraction.
Keywords: Acne lesion extraction, Hypoxia-inducible factor-1 alpha, moderate acne vulgaris, oral doxycycline
Acne vulgaris (AV) is a chronic inflammatory disease of the pilosebaceous unit and is characterized by open and closed comedones, erythematous papules, pustules, nodules, and pseudocysts.1 In Indonesia, the currently used AV classification system is based on Lehman et al2 and includes mild, moderate, and severe grading. The first-line treatment for moderate AV, based on the Global Alliance recommendation, is a combination of a topical retinoid and an oral antibiotic with or without topical benzoyl peroxide.3, 4 Sitohang et al5 reported that for AV to improve from moderate to mild, a four-week average duration of oral clindamycin 300mg as first-line treatment was needed. Oral antibiotics can be discontinued if moderate AV has improved to mild AV. Parsad et al6 reported that the use of cream tretinoin 0.05% and doxycycline 100mg per day for 12 weeks yielded a treatment efficacy of 70 percent in patients with moderate AV.6
The unsatisfactory results of AV therapies and the presence of some side effects encourage clinicians to research the possibility of replacing oral antibiotics with other adjuvant treatment modalities for moderate AV.7 Acne lesion extraction is one form of adjuvant therapy that can be done safely, economically, and conveniently by applying mild pressure with a device to extract the contents of pilosebaceous follicles.8 Although this technique has been widely used for a long time, reports regarding its efficacy and side effects remain limited.9
Keratinocyte hyperproliferation and excessive sebum within the duct of patients with AV cause widening of the duct, which can lower the oxygen pressure (i.e., hypoxia) and encourage the growth of Propionibacterium acnes. Hypoxia has been thought to induce the expression of hypoxia-inducible factor (HIF)-1.10 We hypothesized that lesion extraction opens the oxygen passage into the pilosebaceous follicles and can reduce hypoxia in the acne lesions, minimizing the creation of the anaerobic environment. This study aimed to evaluate the efficacy and side effects of acne lesion extraction compared to oral doxycycline as the first-line method of management for moderate AV and to determine whether acne lesion extraction can reduce the presence of HIF-1 alpha expression as a marker of hypoxia.
Methods
Study design. This was an experimental study with a randomized, controlled trial design. Block randomization was prepared and performed by an independent statistician without clinical involvement in the study. The same statistician disclosed the code at the end of the study. Patients aged 15 to 50 years with moderate AV according to Lehmann grading of acne vulgaris, with 20 to100 comedones and 15 to 50 inflammatory lesions, or with a total of 30–125 AV lesions, were selected consecutively and asked to participate as a research.
Exclusion criteria was as follows:
- Oral antibiotic use within two weeks prior to the study
- Topical retinoid use within two weeks prior to the study
- Systemic retinoid use within three months prior to the study
- Pregnancy or lactation
- Use of oral contraception during the study period
- History of medication allergies or skin abnormalities secondary to first-line treatment for moderate AV
Study protocol. Each subject in both groups received 0.05% tretinoin cream, applied to the entire face each night, and 2.5% benzoyl peroxide gel, applied to the acne lesions in the morning and afternoon. Lesion extraction was performed on facial lesions every two weeks in the acne lesion extraction group. Extraction was performed on all acne lesions, both inflammatory and noninflammatory lesions. Meanwhile, oral doxycycline 100mg once daily was administered without lesion extraction to the doxycycline group.
Outcome measurements. Both groups were evaluated subjectively, using patient’s self-assessment, and objectively, by counting the number of lesions of all types during visits at Week 2, 4, and 6. Primary objective assessment was done by counting the total number of acne lesions at every visit compared with the total number of initial lesions. Clinical improvement was categorized as “good” if the total number of lesions was reduced by more than 50 percent from initial assessment. The subjective self-assessment was performed by asking patients to report the perceived effectiveness of the therapy every two weeks. Side effects of both treatments were recorded. Additionally, expression of HIF-1 alpha was completed by immunohistochemistry analysis.
Statistical analysis. At the end of the study, the collected data were analyzed using the Statistical Package for the Social Sciences version 17 software program (IBM Corp., Armonk, New York). Demographic data were analyzed using the chi-square and Mann–Whitney U tests as appropriate. Treatment efficacy data were analyzed using general linear mixed model tests. Finally, side effects were analyzed using the chi-square test. The significance level was set at 0.05.
Biopsy procedure. At the beginning of the study, biopsies of lesions located on the back were performed on volunteers from both groups. The lesions biopsied from the back had the same characteristics as those extracted from the face. In the lesion extraction group, a representative erythematous papule on the back was selected for extraction and then was left for five minutes because, in a normoxia condition, the HIF-1 alpha protein is extremely labile, with a half-life of less than five minutes.11–13 Afterwards, a 4-mm punch biopsy was performed. In the doxycycline group, the biopsy was performed on an erythematous papule prior to treatment. The biopsy tissues were sent for immunohistochemistry evaluation for HIF-1 alpha expression.
Immunohistochemistry. The preparation began with deparaffinization of tissue sections with xyol three. Peroxidases were used for quenching of endogenous enzyme. We then applied protein blockers (Background Sniper, Biocare; Pacheco, California) to block non-specific binding sites. Next, we dried the protein blocker and dripped it with the primary HIF-1 alpha antibody solution. After 24 hours of incubation, the secondary antibody was used (Trekkie Universal Link, Biocare; Pacheco, California). Futhermore, we added chromogen substrate (betazoid diaminobenzidine chromogen) and washed the preparation with running water. Finally, the preparation was counterstained with hematoxycillin, dehydrated, and covered with a glass slide. The last stage of the preparation was observation under a microscope using objective lens at 10x and 40x.14–16
Interpretation of immunohistochemistry results. Immunohistochemistry evaluation for HIF-1 alpha antibody was reported qualitatively and quantitatively. The qualitative result was positive if there was brown-stained nucleus or cytoplasm of keratinocytes and pilosebaceous follicle sebocytes, which implied the presence of HIF-1 alpha. Mammary gland cells were used as the negative control and mammary invasive ductal carcinoma cells were used as the positive control (Figure 1).
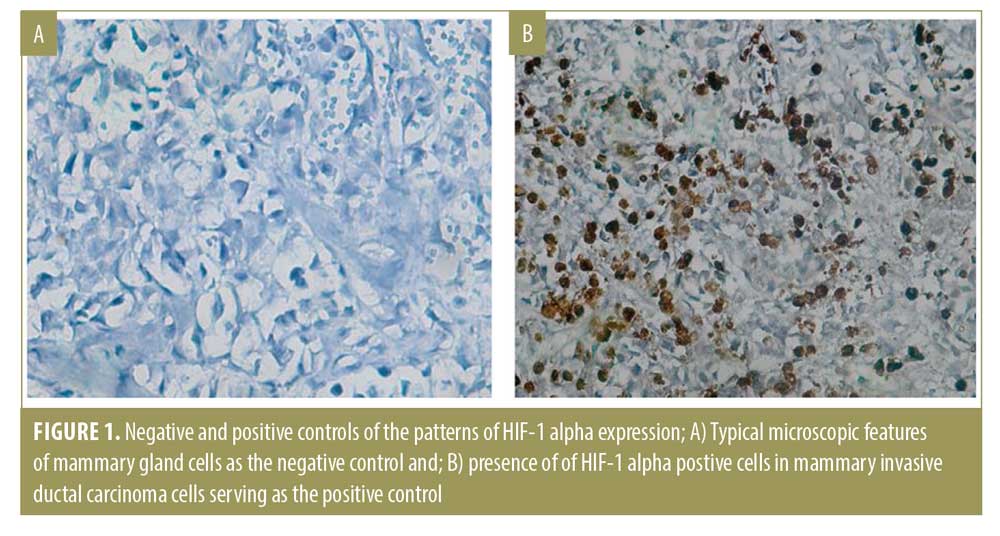
Quantitative measures were performed by a board certified anatomical pathologist who did not participate in the research, who manually counted the numbers of nuclei or cytoplasm of pilosebaceous duct keratinocytes and sebocytes that expressed HIF-1 alpha, using the software ImageJ in two fields of view.17 H-score was used to quantitatively establish the degree of hypoxia in the AV lesions with following formula:
H score=(I + 1) × P1
I indicated staining intensity, which was the positivity value of each cell in one field of view; this was determined subjectively and assigned a value between one and three.
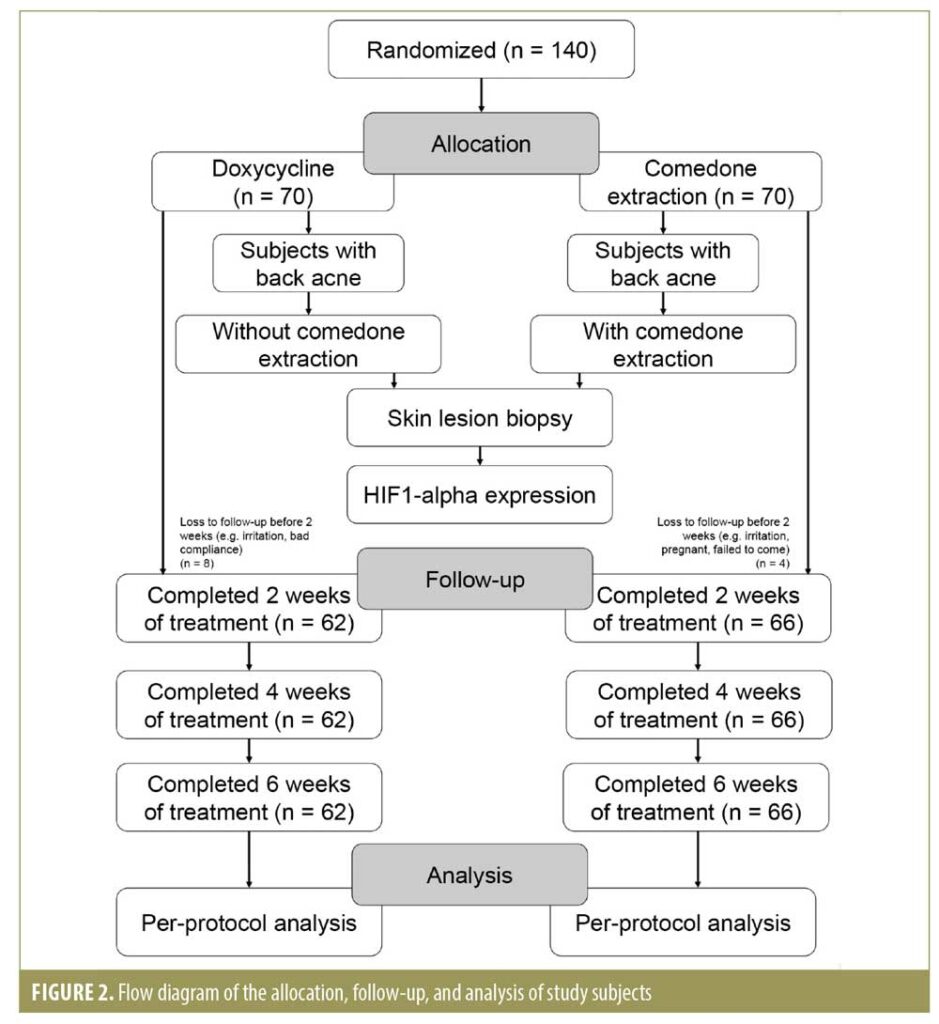
P1 indicated percentage of positively stained cells (varied between 5% and 100%). The H value range was 0 to 400 and was assigned as follows: 0–9=negative, 10–200=weak (+1), 201–300=moderate (+2), and 301–400=strong (+3). The H value represented the degree of positivity; HIF-1 alpha expression was considered positive when the value was greater than 10.18, 19 The allocation, follow-up, and analysis of study subjects is shown in Figure 2.
Ethics approval and consent to participate. This study was performed according to the principles of the Declaration of Helsinki and was approved by the ethics committee of the Faculty of Medicine, Universitas Indonesia (No. 213/UN2.F1/ETIK/2015) Approval Date: March 23rd, 2015. Written consent was obtained from each study subject.
Results
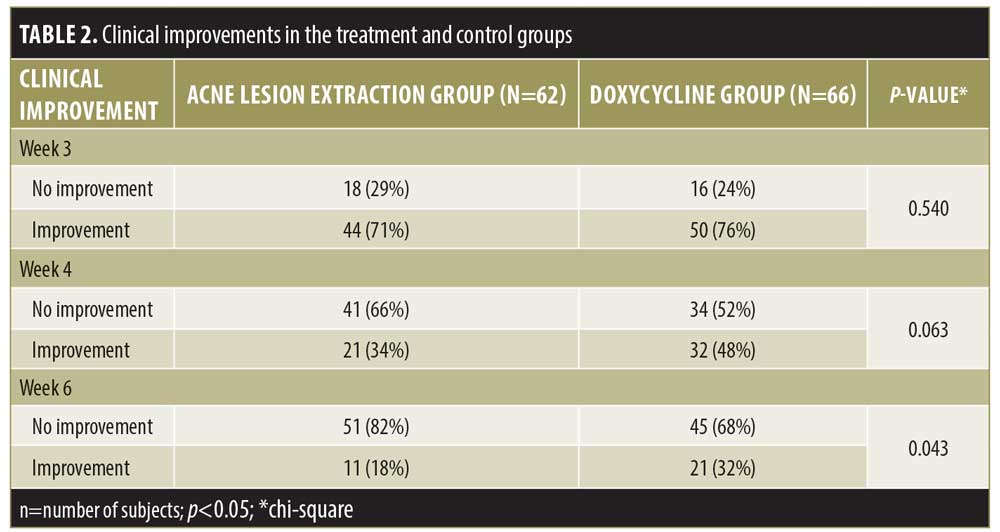
Out of 140 subjects, 128 completed the research and 12 dropped out from the study. Subjects discontinued the study due to either heavy irritation, pregnancy, and/or failure to show up for evaluation at Weeks 2, 4, and 6. The sociodemographic characteristics of the subjects, including sex and age, were similar in the two study groups (Table 1). The clinical improvement in the Weeks 4 and 6 showed noticeable improving trends, especially in the lesion extraction group, but the difference was only significant in Week 6. (Table 2). During Week 2, 4, and 6 visits, therapy was evaluated subjectively using patient self-assessment. The patient self-assessment for both groups demonstrated positive trends after Week 4 and Week 6. However, the result was not statistically significant.
Side effects. The side effects observed in the doxycycline group were nausea, vomiting, and abdominal discomfort. Meanwhile, the side effects in the lesion extraction group included erythema, local edema, localized bleeding that stopped spontaneously, and a weal that disappeared after 24 hours. During Week 2, one subject in the acne lesion extraction group experienced erythema that resolved within approximately one hour. During Week 4, two subjects in the extraction group had erythema, and one subject had a weal; these side effects also resolved within approximately one hour.
HIF-1 alpha expression on immunohistochemistry analysis. A total of 15 volunteer subjects (9 in the doxycycline group and 6 in the lesion extraction group) consented to undergo biopsy.
Qualitative measurement of HIF-1 alpha expression. In the doxycycline group, positive results (indicating HIF-1 alpha expression in the keratinocyte and pilosebaceous follicle sebocytes) were found in 7 out of 9 subjects. In the acne lesion extraction group, two specimens could not be assessed due to the absence of pilosebaceous follicular duct, three specimens showed negative results, and one specimen demonstrated positive results.
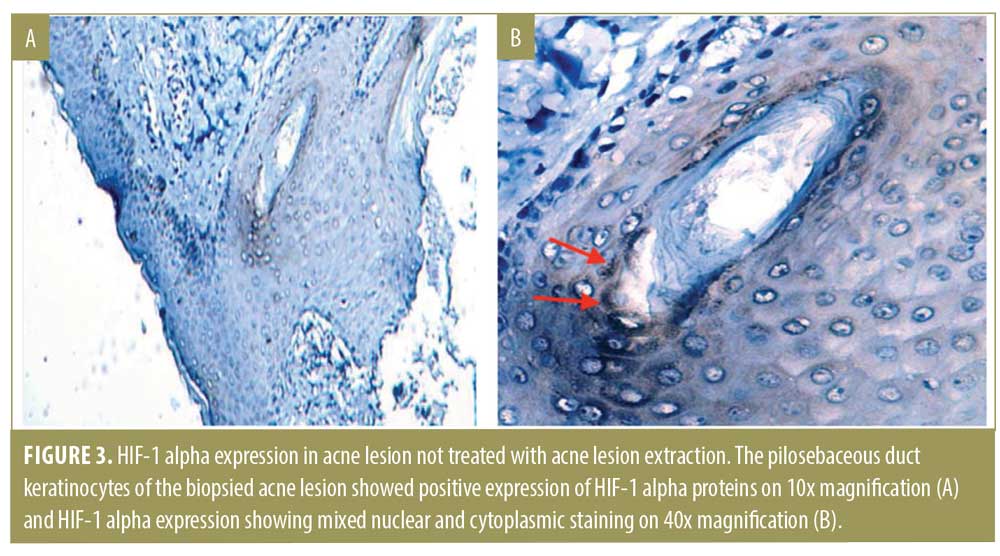
Quantitative measurement of HIF-1 alpha expression. Seven out of nine specimens from the doxycycline group expressed HIF-1 alpha (Figure 3). H value appears in various degrees, with three weak-positive specimens, three moderate-positive specimens, and one strong-positive specimen. The pilosebaceous duct keratinocytes of the biopsied acne lesion showed positive expression of HIF-1 alpha proteins on 10x magnification (Figure 3A) and HIF-1 alpha expression showing mixed nuclear and cytoplasmic staining on 40x magnification (Figure 3B).
Discussion
In our study group, lesion extraction appeared to be more effective than doxycycline in reducing acne lesion for moderate AV. Lesion improvement started during Week 4 and was significant in Week 6 in the lesion extraction group.
AV starts with microcomedones, as a result of follicular epithelial hyperproliferation.20 Hyperproliferation of basal keratinocytes that line the wall of the infrainfundibulum plays the main role in this accumulation and can further evolve into noninflammatory comedones or inflammatory lesions.21–23 Lesion extraction of the contents of a blocked pilosebaceous follicle can be carried out by simple mechanical pressure using an extractor, thereby reducing the number of inflammatory and noninflammatory lesions and temporary improvement of most noninflammatory lesions.21,24,24 Although this procedure has long been used widely, very little data on its effectiveness and side effects have been reported.9 This procedure is known to increase the risk for tissue damage, worsen cystic lesions, and potentially provoke inflammation by rupturing the contents of a comedone through the base of the follicle and into the dermis.9 However, we did not observe these in the present study, possibly because the subjects were on topical retinoid therapy that can lead to collagen formation and prevent the formation of scars.26
Danby10 reported that the accumulation of sebum and hyperproliferating keratinocytes in the pilosebaceous ducts of an acne lesion increases the pressure of the ducts. This can increase the size of the ducts, decrease the oxygen pressure, and lead to hypoxia or anoxia. This hypoxic/anoxic condition can trigger the expression of the transcription factor HIF-1.10, 27
Unicellular and multicellular organisms have a mechanism to detect oxygen pressure and respond to hypoxia by increasing gene expression, which is important for cell and tissue adaptation. HIF-1 alpha is one of the regulators of oxygen homeostasis and is a heterodimeric transcription factor that is expressed in tissues.28,29 The response of the transcriptional program to a hypoxic challenge extensively varies among different cell types.30,31
In this study, in nine doxycycline group preparations, we found three moderate-positive specimens and one strong-positive specimen. Three weakly positive HIF-1 alpha expression was possibly because oxygen was still present in the vessels in the dermis; this process is a physiologic stress response to achieve homeostasis in the pilosebaceous follicular duct.32,33 According to Cramer et al,33 in-vivo overexpression of HIF-1 in myeloid cell metabolism resulted in increased localized inflammation and promoted bactericidal responses.33, 34 In this study, we did not measure localized inflammation secondary to HIF-1 alpha. In the group that received acne lesion extraction, two out of six preparations could not be assessed because of the absence of pilosebaceous follicular ducts in each field. Of the remaining four preparations that contained pilosebaceous follicular ducts, one appeared brownish in color and implied the presence of HIF-1 alpha expression, whereas the other three preparations had no brownish color in the nucleus and/or cytoplasm. This might happen because the path of follicle went oblique.
This study demonstrated that hypoxia/anoxia in AV lesions might be decreased or eliminated by lesion extraction, which likely allowed the oxygen to enter and reduce the anaerobic environment in acne lesions. As higher oxygen tension occurred after lesion extraction, it may have suppressed the HIF-1 alpha which is known to stimulate lipid accumulation and induce inflammatory cytokines.10,35 Moreover, P. acnes is an anaerobic microorganism that grows well in low oxygen pressure (hypoxia/anoxia) environments, which can cause keratinocyte production of proinflammatory cytokines and enhance the bactericidal activity of phagocytic and epithelial cells. Thus, with lesion extraction, the growth of P. acnes may be suppressed.12,36
Limitations. This study had some limitations. First, in the extraction group, HIF-1 alpha expression could not be examined in two biopsy samples, probably because all pilosebaceous follicle units may have been removed during biopsy. One biopsy sample was positive for HIF-1 alpha expression, possibly because the follicle route was skewed or the duct was not extracted. Lastly, HIF-1 alpha as a marker of hypoxia could not be confirmed statistically because of limitation of biopsy sample sizes.
Conclusion
Compared to oral antibiotics, acne lesion extraction appeared to be more effective than oral doxycycline after six weeks of treatment for moderate AV and had fewer adverse events. HIF-1 alpha expression as a prominent regulator in acne formation was decreased after acne lesion extraction. Further studies with larger sample sizes are needed to confirm statistical significance of HIF-1 alpha as a marker of hypoxia in acne lesion.
Acknowledgments
We acknowledge the Faculty of Medicine Universitas Indonesia—Dr. Cipto Mangunkusumo Hospital as well as the health personnel of the Department of Dermatology and Venereology, the Department of Pathological Anatomy, the Department of Histology, and the Department of Biochemistry and Molecular Biology. We also acknowledge Presidential-Army Central Gatot Soebroto Hospital and the health personnel of the Department of Dermatology and Venereology, who contributed to the management of the study subjects. We also acknowledge Prof. Dae Hun Suh from Seoul National University Hospital for support.
References
- D Cunliffe W, Simpson N. Diseases of sebaceous glands. In: Champion R BJ, Burns D, Breathnach S, eds. Textbook of Dermatology. 6th ed. Oxford: Blackwell Science; 2012. 1927–1982.
- Lehmann HP, Robinson KA, Andrews JS, et al. Acne therapy: a methodologic review. J Am Acad Dermatol. 2002;47:231–240.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(1 Suppl):S1–S37.
- Divisi Dermatologi Kosmetik Departemen Ilmu Kesehatan Kulit dan Kelamin FKUI/RSCM J. Panduan Pelayanan Medis RSCM. 2005–2012.
- Sitohang I, Makes W. Lama penggunaan klindamisin oral pada pasien akne vulgaris sedang (AVS) yang memberikan perbaikan hasil terapi di Departemen Ilmu Kesehatan Kulit dan kelamin RS Dr. Cipto Mangunkusumo Jakarta tahun 2009. MDVI. 2011;38:113–117.
- Parsad D, Pandhi R, Nagpal R, Negi KS. Azithromycin monthly pulse vs daily doxycycline in the treatment of acne vulgaris. J Dermatol. 2001;28:1–4.
- Haedersdal M, Togsverd-Bo K, Wulf HC. Evidence-based review of lasers, light sources and photodynamic therapy in the treatment of acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:267–278.
- Khunger N. Standard guidelines of care for acne surgery. Indian J Dermatol Venereol Leprol. 2008;74 Suppl:S28–S36.
- Bowe WP, Shalita AR. Procedural treatments for acne. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne vulgaris. London: Informa; 2011:208–217.
- Danby FW. Ductal hypoxia in acne: is it the missing link between comedogenesis and inflammation? J Am Acad Dermatol. 2014;70:948–949.
- Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540.
- Otrock ZK, Hatoum HA, Awada AH, Ishak RS, et al. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009;70:93–102.
- Chun Y-S, Kim M-S, Park J-W. Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci. 2002;17:581–588.
- Herzer S, Englert DF. Nucleic acid hybridization. In: Gerstein AS, ed. Molecular biology problem solver: a laboratory guide. Hoboken: John Wiley & Sons. 2001:399–460.
- Kim SW, Roh J, Park CS. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med. 2016;50:411–418.
- Kroshinsky D, Shalita AR. Tropical retinoids. In: Webster GF, Rawlings A, eds. Acne and its therapy. New York: Informa Healthcare; 2007:130–132.
- Grishagin IV. Automatic cell counting with ImageJ. Anal Biochem. 2015;473:63–65.
- Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review. Diagn Pathol. 2014;9:221.
- Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878.
- Norris JFB, Cunliffe WJ. A histological and immunocytochemical study of early acne lesions. B J Dermatol. 1988;118:651–659.
- Heughebaert C, Shalita AR. Comedogenesis. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne vulgaris. London: Informa; 2011:28–42.
- Holmes RL, Williams M, Cunliffe WJ. Pilosebaceous duct obstruction and acne. B J Dermatol. 1972;87:327–332.
- Plewig G, Fulton JE, Kligman AM. Cellular dynamics of comedo formation in acne vulgaris. Arch Dermatol Forsch. 1971;242:12–29.
- Lowney ED, Witkowski J, Simons HM, Zagula ZWJ. Value of comedo extraction in treatment of acne vulgaris. JAMA. 1964;189:1000-1002.
- Plewig G. Follicular keratinization. J Invest Dermatol. 1974;62:308–315.
- Plewig G, Kligman AM. Acne and rosacea. 3rd ed. Berlin: Springer-Verlag Berlin Heidelberg. 2019; 558–565.
- Leire E, Olson J, Isaacs H, et al. Role of hypoxia inducible factor-1 in keratinocyte inflammatory response and neutrophil recruitment. J Inflamm. 2013;10:28.
- Haddad JJ, Harb HL. Cytokines and the regulation of hypoxia-inducible factor (HIF)-1alpha. Int Immunopharmacol. 2005;5:461-83.
- Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–242.
- D’Alessandro S, Magnavacca A, Perego F, et al. Effect of hypoxia on gene expression in cell populations involved in wound healing. Biomed Res Int. 2019;2019:2626374.
- Gardner LB, Corn PG. Hypoxic regulation of mRNA expression. Cell Cycle. 2008;7:1916-24.
- Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26.
- Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57.
- Ziello JE, Jovin IS, Huang Y. Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80:51–60.
- Choi K, Jin M, Zouboulis CC, Lee Y. Increased lipid accumulation under hypoxia in SZ95 human sebocytes. Dermatology. 2021;237(1):131–141.
- Befani C, Liakos P. The role of hypoxia-inducible factor-2 alpha in angiogenesis. J Cell Physiol. 2018;233:9087–98.

