 J Clin Aesthet Dermatol. 2021;14(6):49–54.
J Clin Aesthet Dermatol. 2021;14(6):49–54.
by Allyson Brahs, BS; Brigitte Sledge, DO; Heidi Mullen, DO; Andrew Newman, DO; Yebabe Mengesha, MD; and Sarah Estrada, MD
Ms. Brahs is with Western University of Health Sciences, College of Osteopathic Medicine of the Pacific in Pomona, California. Drs. Sledge, Mullen, Newman, Mengesha, and Estrada are with HonorHealth Scottsdale Dermatology Residency Program and Affiliated Dermatology in Scottsdale, Arizona.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Angiolymphoid hyperplasia with eosinophilia (ALHE) is an uncommon, benign inflammatory vasoproliferation. The literature is divided regarding whether it embodies a vascular neoplasm or a reactive process secondary to various stimuli. ALHE presents as solitary or clustered papules or nodules primarily on the head and neck, especially on or around the auricle. Histologically, ALHE is characterized by a proliferation of blood vessels lined by plump epithelioid endothelial cells and a prominent perivascular infiltrate rich in lymphocytes and eosinophils. ALHE follows a benign clinical course, yet treatment is challenging because of its high recurrence rate. We present the case of a 37-year-old Filipino man with lesions located on the central face. Kimura disease was considered due to his age, sex, and ethnicity; however, his clinical features—specifically, the presence of discrete papules and lack of lymphadenopathy—and his histological findings were consistent with ALHE. He reported trauma prior to the onset of the lesions, suggesting a reactive etiology.
Keywords: Angiolymphoid hyperplasia with eosinophilia, epithelioid hemangioma, Kimura disease
As its name suggests, angiolymphoid hyperplasia with eosinophilia (ALHE) is a vascular proliferation with lymphocytic and eosinophilic infiltration. Clinically, it manifests as smooth-surfaced singular or clustered pink to violaceous papulonodules occurring predominantly on the head and neck with a predilection for the auricular area.1 Patients might experience associated pruritus, pain, pulsation, or bleeding.1 When present in a middle-aged Asian male, it is important to consider Kimura disease. In the present case report, we describe a 37-year-old Filipino male with ALHE papules initially located on the upper lip and subsequently progressing to the left nasal sill following trauma. We also conducted a review of relevant literature and highlight key distinguishing features between ALHE and Kimura disease.
Case Presentation
A 37-year-old Filipino male patient with no significant medical history presented to our clinic with the complaint of skin lesions located on the upper lip for three months, extending to the nose. The patient was referred by his primary care physician; no treatment had been performed prior to presentation. He denied associated symptoms, including itching, burning, and pain. He also denied all prodromal symptoms; however, he did admit to trauma a few months prior to lesion onset. The patient stated that his cousin inadvertently struck him in the face with his elbow during a playful wrestling match prior to the start of the facial lesions. He denied a family history of similar lesions.
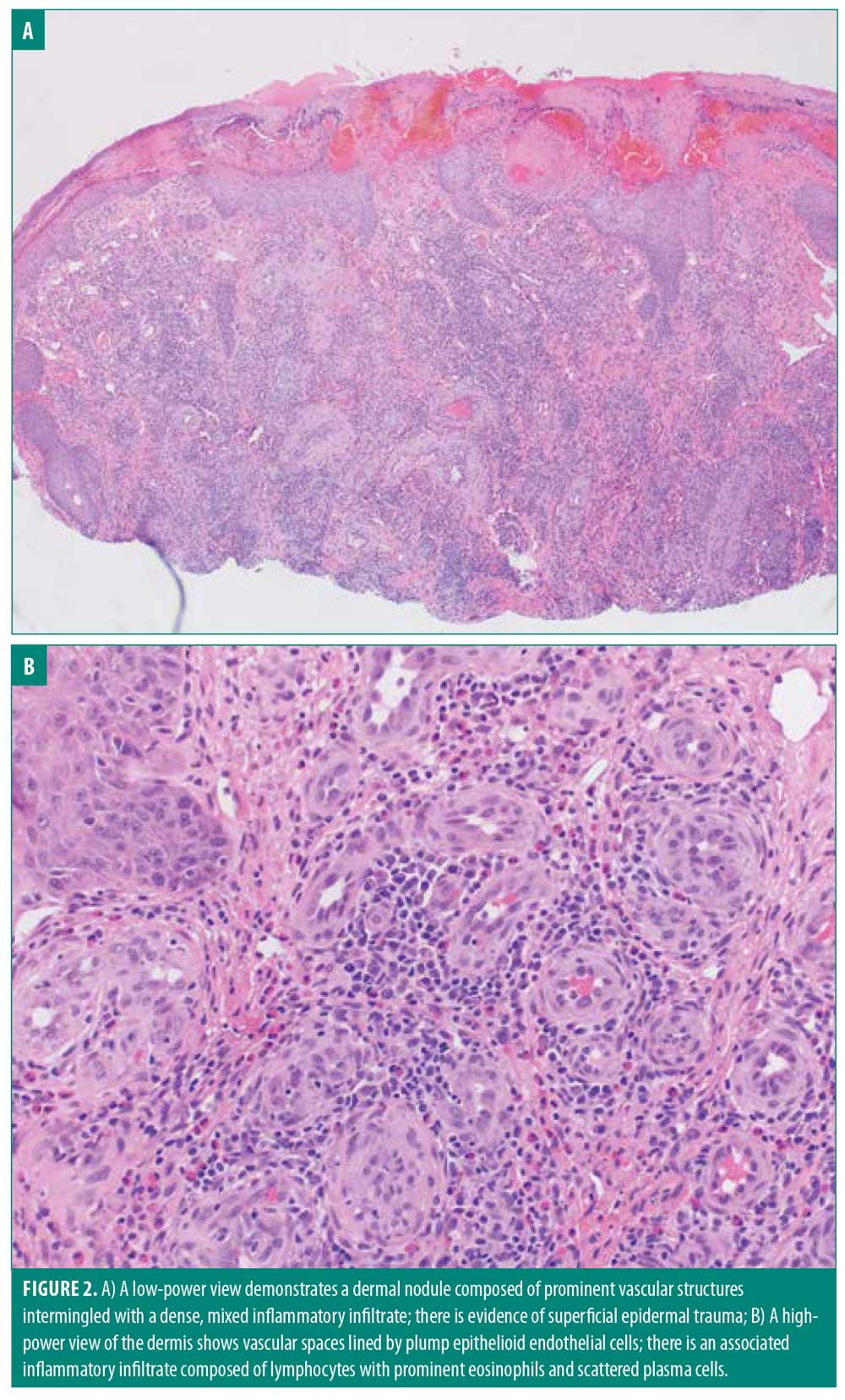
Upon physical examination, numerous flesh-colored and dull red papules were noted clustered adjacent to the nose and upper lip (Figure 1). Multiple shave biopsies revealed dermal expansion by a dense lymphoplasmacytic inflammatory infiltrate that included numerous eosinophils (Figure 2). The overlying epidermis showed evidence of superficial trauma. A background network of vessels was seen in close association with the infiltrate. This combination of findings suggested ALHE. No evidence of neoplasm was seen. Gram staining was negative for bacterial organisms. A multitude of laboratory assessments were performed following histopathologic diagnosis, including human immunodeficiency virus, acute hepatitis panel, urine protein electrophoresis, complete blood count, basic chemistry panel, and urinalysis with microscopy. Work-up was negative with the exception of an elevated eosinophil count, further supporting the diagnosis of ALHE.
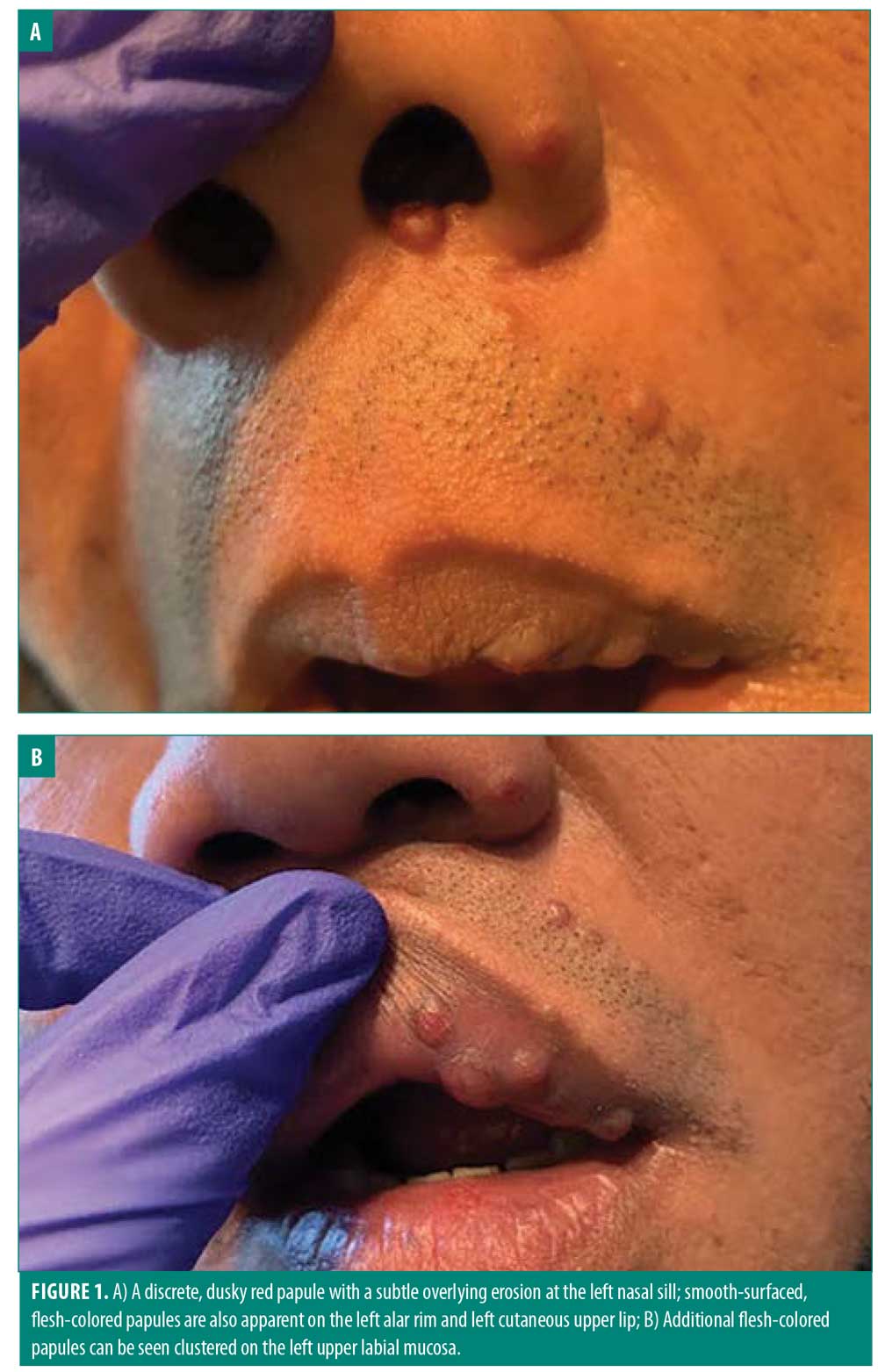
At a follow-up appointment, treatment options were discussed, including cryosurgery, excision, laser, Mohs micrographic surgery (MMS), and intralesional steroids. After careful consideration and given the area of predilection and high recurrence rate, conservative methods were chosen first. The patient received 0.3mL of Kenalog (2.5mg/mL) (Bristol-Myers Squibb, New York, New York) in eight lesions adjacent to the nose and left upper lip. At his follow-up appointment one month later, the patient reported initial mild improvement in affected areas. However, the lesions returned to their original size shortly after treatment. Other invasive measures were discussed in further detail at that time, with emphasis on the difficulty of definitive treatment of ALHE; many therapies are not 100-percent curative. The patient opted for surgical removal and the lesions were removed via shave procedure. Unfortunately, the patient did not follow-up again for further management or treatment.
Discussion
In 1969, Wells and Whimster2 first introduced the term “subcutaneous angiolymphoid hyperplasia with eosinophilia.” Mehregan3 later dropped the “subcutaneous” descriptor of Wells and Whimster’s title in 1971 because the disorder is not confined to the subcutaneous tissue, leaving the designation ALHE that we know today. In 1982, Weiss and Enzinger4 established the terminology “epithelioid hemangioma” to differentiate it from the similar yet potentially malignant “epithelioid hemangioendothelioma.” Many other antiquated names have been used to describe this lesion, including “pseudopyogenic granuloma,” “angiomatous nodule,” and “histiocytoid hemangioma.”5 ALHE and “epithelioid hemangioma” have been the terms favored in the literature, and the persistence of both terms highlights the debated etiology. The name “epithelioid hemangioma” supports the neoplastic hypothesis, while the name “angiolymphoid hyperplasia with eosinophilia” suggests a reactive phenomenon. Since the lesions in our patient arose secondary to trauma and therefore support a reactive process, we favor the term ALHE and will use it from this point forward.
To date, the largest analysis of ALHE is a systematic review of 908 patients conducted by Adler et al.1 This study established a mean age of 37.6 years and revealed an equivalent sex distribution.1 Asian and Caucasian patients compose a large proportion of the ALHE patient population, although the high Asian prevalence could be attributed to the initial inclusion of Kimura disease in early studies of ALHE before it was distinguished as a nosologically distinct entity.1,6 Patients might experience symptoms such as pruritus, throbbing, pain, or bleeding, though lesions may be asymptomatic in 15 percent of cases, as was true in our patient.1 ALHE has been described on most cutaneous sites; however, a majority (approximately 87 percent) of lesions present on the head and neck, especially around the auricle and the scalp.1 These papulonodules are predominantly localized to the dermis and/or subcutis. Extracutaneous involvement has been reported in tissues such as the lacrimal gland,7 bone,8 colon,9 oral mucosa,10 and radial artery.11 Approximately 53.4 percent of ALHE lesions are solitary.1 When there are many, they tend to be confluent or clustered, as was seen in the present case.12 Systemic eosinophilia and regional lymphadenopathy occur in a minority of patients (up to 20% and 10% of cases, respectively).13
Microscopically, ALHE is characterized by two principal components: a distinct vascular proliferation and a mixed inflammatory infiltrate. The vascular component displays marked proliferation of irregularly shaped vessels of varying sizes lined by epithelioid endothelial cells, which are plump endothelial cells with large orthochromatic nuclei and eosinophilic cytoplasm, with occasional “hobnailing” or protrusion into the lumen.14 These cells will sometimes exhibit cytoplasmic vacuoles resembling pseudolumens.5 The perivascular infiltrate is rich in lymphocytes and eosinophils, with occasional mast cells, plasma cells, and histiocytes. There may be nodular aggregates of lymphocytes forming follicles, although these are more commonly seen in early lesions.5,15 The epidermis is generally unaffected but may display acanthosis or erosion secondary to superficial trauma.15 A fibrous and myxoid stroma commonly accompanies the condition.15 Positive nuclear staining for Finkel-Biskis-Jinkins murine osteosarcoma viral oncogene homolog B (FOSB) has recently been shown to help differentiate ALHE from histologically similar malignant lesions.16
ALHE’s clinicopathologic picture shares similarities with many benign and malignant conditions. In a study of 89 biopsy-proven ALHE lesions, the most common prebiopsy diagnoses were epidermal cyst and angioma, with ALHE only suspected in one patient.15 Other conditions in the clinical differential diagnosis for ALHE include angiosarcoma, Merkel cell carcinoma, epithelioid hemangioendothelioma, Kaposi sarcoma, pyogenic granuloma, cylindroma, trichoepithelioma, sarcoidosis, molluscum contagiosum, granuloma faciale, and many more causes of nondescript facial papules. Kimura disease, once thought to exist along the same disease spectrum as ALHE, should be suspected in young to middle-aged Asian males, like our patient, since it is the most common demographic affected by this condition.17,18 Kimura disease shares features with ALHE such as a predilection for the head and neck, tissue eosinophilia, vascular proliferation, and a persistent indolent course. Kimura disease differs in that it frequently demonstrates robust systemic eosinophilia, elevated immunoglobulin E levels, subcutaneous swelling as opposed to discrete papules or nodules, regional lymphadenopathy, eosinophilic abscess formation, and general lack of an epithelioid endothelial lining.19,20 In our patient, the absence of lymphadenopathy, clinical appearance of clustered discrete papules, and histopathological findings favored the diagnosis of ALHE. Of note, our patient did have an elevated eosinophil count, but, as mentioned previously, this can be found in up to 20 percent of ALHE cases.13 Differentiating between ALHE and Kimura disease is an important distinction to make because Kimura disease is thought to be a chronic inflammatory condition often treated with systemic immunosuppressive agents and requires careful long-term follow-up for complications such as nephrotic syndrome.17,18
ALHE’s pathogenesis remains unclear, though many theories have been proposed in divided support of either a reactive versus a neoplastic process. In favor of the reactive hypothesis, many provoking factors have been implicated, including prior trauma,5,21–23 hormonal changes (pregnancy, oral contraceptive pills, menstruation),13,15,24 infectious agents, and arteriovenous shunting.15,25 An association between ALHE and human herpes virus-8 has been suggested in a few reports, yet newer studies show no pathogenetically relevant association.26,27 Human polyomavirus-6, human immunodeficiency virus, and otitis externa have also been noted to coincide with ALHE.15,28,29 In a study of 116 patients, nine percent of lesions were associated with prior trauma.15 Such antecedent trauma that has been reported includes frostbite, incision and drainage, laceration, frictional trauma, blunt trauma, venipuncture, and thermal burn.15,22,23,30 It is thought that these various factors trigger an inflammatory response or local hypoxia generating proliferative stimuli for the vascular endothelium, possibly facilitated by angiogenic mediators such as vascular endothelial growth factor or angiotensin II.15,23,25,31,32 In our patient, the lesions developed secondary to trauma, which, in this case, supports this reactive hypothesis.
Conversely, ALHE’s chronic and recurrent clinical course is more consistent with a neoplastic picture. Some cases of ALHE, often with atypical features, harbor rearrangements of the Fos gene family, leading to oncogenic expression of FOS proteins, which have been implicated as regulators of cellular proliferation, differentiation, and death.33,34 A neoplastic etiology is further supported by a recent study in which 19 of 20 ALHE specimens showed cytoplasmic staining for WT1.35 WT1 has been shown to display a cytoplasmic staining pattern in vascular neoplasms such as infantile hemangioma and tufted angioma.36
Other studies have reported that ALHE, or at least a subset of ALHE, might represent a T-cell lymphoproliferative disorder.27,37 Kempf et al27 found that five of seven ALHE lesions displayed monoclonal T-cell proliferation. In an ALHE patient with concurrent peripheral T-cell lymphoma, the same T-cell receptor gene rearrangement was detected in an affected lymph node and her ALHE lesion.37
The dichotomy in the literature regarding the pathogenesis of this disorder suggests that ALHE can even encompass a heterogeneous group that is yet to be fully distinguished, similar to how ALHE and Kimura disease were once thought to be synonymous. Alternatively, it could represent a combination of reactive and neoplastic processes in which an inciting event stimulates genetic alterations. Further studies are required to determine whether ALHE truly embodies a vascular neoplasm, a reactive vascular hyperplasia in response to a variety of insults, a combination of the aforementioned, a lymphoproliferative process with subsequent angiogenesis, or a heterogeneous group of disorders.
ALHE is considered to have a benign course, so observation is reasonable if the lesions are neither symptomatic nor bothersome to the patient; however, likely secondary to its predilection for the head and neck, treatment is often pursued. Treatment recommendations are largely based on case reports and retrospective studies due to ALHE’s uncommon occurrence. Surgical excision is the standard therapeutic approach and has the lowest failure rate (40.8%) as defined by recurrence or incomplete resolution.1 The recurrence risk is the greatest when ALHE is associated with an earlier age of onset, multiple lesions, bilateral lesions, longer duration of disease, and symptomatic lesions.1 Recurrence is thought to be due to inadequate margins or an underlying arteriovenous shunt.15 To address these concerns, MMS has been used with success and can be considered for application in regions where tissue-sparing is important, such as the most common location, which is the head and neck.38 One author proposed to excise ALHE as if it were a locally malignant tumor with 4-mm safety margins to decreased recurrence rates.39
Multiple other therapeutic modalities have been reported with varying degrees of success. Topical, intralesional, and systemic steroids have all been used, with intralesional steroids showing the lowest rate of treatment failure amongst the three.1 Cryotherapy has been effective in some cases, while showing little benefit in others.14,40 Lasers, including pulsed dye, argon, and carbon dioxide, have provided favorable outcomes as well, especially for poor surgical candidates.41–43 Many additional therapies have been described, including electrocoagulation with photodynamic therapy,44 radiofrequency ablation with sclerotherapy, antibiotics, various chemotherapeutics, beta-blockers, pentoxifylline, retinoids, and many more.1 Despite all these treatment options, none have been shown to be consistently effective. The clinical course is typically chronic, though spontaneous regression has been rseported in around 2.9 percent of cases.1,45 Our patient’s disease course displays the resilient nature of ALHE since the lesions persisted after shave biopsies and subsequent intralesional steroids.
Conclusion
The present report demonstrates the perplexing nature of ALHE with respect to its challenging clinical diagnosis, unclear pathogenesis, and therapy-resistant nature. These factors, in addition to its distribution in sensitive areas such as the central face in our patient’s case, constitute a presumably frustrating condition. Our patient’s findings, along with a review of the literature, suggest that trauma may be an important inciting factor for the development of ALHE, though further research is necessary to settle its debated etiology. Treating ALHE is certainly difficult as demonstrated by the sheer number of treatment modalities reported. The most common and effective treatment to date is standard excision, yet it still boasts a high failure rate of 40.8 percent.1 Due to ALHE’s benign nature, it is reasonable to start with a more conservative approach such as intralesional steroids, then progress to surgery if needed. There is potential that using MMS or aggressive margins may decrease recurrence rates. Future studies are needed to narrow the therapeutic gap and improve the quality of life of patients affected by ALHE.
References
- Adler BL, Krausz AE, Minuti A, et al. Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): a systematic review. J Am Acad Dermatol. 2016;74(3):506–512.e11.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81(1):1–14.
- Mehregan AH, Shapiro L. Angiolymphoid hyperplasia with eosinophilia. Arch Dermatol. 1971;103(1):50–57.
- Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50(5):970–981.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139(5):683–686.
- Henry PG, Burnett JW. Angiolymphoid hyperplasia with eosinophilia. Arch Dermatol. 1978;114(8):1168–1172.
- Sanchez-Acosta A, Moreno-Arredondo D, Rubio-Solornio RI, et al. Angiolymphoid hyperplasia with eosinophilia of the lacrimal gland: a case report. Orbit. 2008;27(3): 195–198.
- Nielsen GP, Srivastava A, Kattapuram S, et al. Epithelioid hemangioma of bone revisited: a study of 50 cases. Am J Surg Pathol. 2009;33(2):270–277.
- Bui MM, Draper NL, Dessureault S, et al. Colonic angiolymphoid hyperplasia with eosinophilia masquerading as malignancy: a case report and review of the literature. Clin Colorectal Cancer. 2010;9(3):179–182.
- Bartralot R, Garcia-Patos V, Hueto J, et al. Angiolymphoid hyperplasia with eosinophilia affecting the oral mucosa: report of a case and a review of the literature. Br J Dermatol. 1996;134(4):744–748.
- Morton K, Robertson AJ, Hadden W. Angiolymphoid hyperplasia with eosinophilia: report of a case arising from the radial artery. Histopathology. 1987;11(9):963–969.
- Requena L, Sangueza OP. Cutaneous vascular proliferation, part II. Hyperplasias and benign neoplasms. J Am Acad Dermatol. 1997;37(6):887–919.
- Bahloul E, Amouri M, Charfi S, et al. Angiolymphoid hyperplasia with eosinophilia: report of nine cases. Int J Dermatol. 2017;56(12):1373–1378.
- Caca-Biljanovska N, Arsovska-Bezhoska I. Angiolymphoid hyperplasia with eosinophilia successfully treated with cryotherapy. Open Access Maced J Med Sci. 2019;7(5):794–796.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia: a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12(5 Pt 1):781–796.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45(6):395–402.
- Fouda MA, Gheith O, Refaie A, et al. Kimura disease: a case report and review of the literature with a new management protocol. Int J Nephrol. 2011;2010:673908.
- Sun QF, Xu DZ, Pan SH, et al. Kimura disease: review of the literature. Intern Med J. 2008;38(8):668–672.
- Kuo TT, Shih LY, Chan HL. Kimura’s disease. Involvement of regional lymph nodes and distinction from angiolymphoid hyperplasia with eosinophilia. Am J Surg Pathol. 1988;12(11):843–854.
- Chun SI, Ji HG. Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: clinical and histopathologic differences. J Am Acad Dermatol. 1992;27(6 Pt 1):954–958.
- Fetsch JF, Sesterhenn IA, Miettinen M, Davis CJ. Epithelioid hemangioma of the penis: a clinicopathologic and immunohistochemical analysis of 19 cases, with special reference to exuberant examples often confused with epithelioid hemangioendothelioma and epithelioid angiosarcoma. Am J Surg Pathol. 2004;28(4):523–533.
- Busquets AC, Sanchez JL. Angiolymphoid hyperplasia with eosinophilia induced by trauma. Int J Dermatol. 2006;45(10):1211–1214.
- Stewart N, Zagarella S, Mann S. Angiolyphoid hyperplasia with eosinophilia occurring after venipuncture trauma. J Dermatol. 2013;40(5):393–395.
- Moy RL, Luftman DB, Nguyen QH, Amenta JS. Estrogen receptors and the response to sex hormones in angiolymphoid hyperplasia with eosinophilia. Arch Dermatol. 1992;128(6):825–828.
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140(6):1153–1156.
- Bhattacharjee P, Hui P, McNiff J. Human herpesvirus-8 is not associated with angiolymphoid hyperplasia with eosinophilia. J Cutan Pathol. 2004;31(9):612–615.
- Kempf W, Haeffner AC, Zepter K, et al. Angiolymphoid hyperplasia with eosinophilia: evidence for a T-cell lymphoproliferative origin. Hum Pathol. 2002;33(10):1023–1029.
- Rascovan N, Monteil Bouchard S, Grob JJ, et al. Human polyomavirus-6 infecting lymph nodes of a patient with an angiolymphoid hyperplasia with eosinophilia or Kimura disease. Clin Infect Dis. 2016;62(11): 1419–1421.
- D’Offizi G, Ferrara R, Donati P, et al. Angiolymphoid hyperplasia with eosinophils in HIV infection. AIDS. 1995;9(7):813–814.
- Tseng HW, Chien SH, Wu CS, et al. Angiolymphoid hyperplasia with eosinophilia developing in an antecedent welding burn: a case report. Kaohsiung J Med Sci. 2010;26(5):266–270.
- Sun ZJ, Zhang L, Zhang WF, et al. A possible hypoxia-induced endothelial proliferation in the pathogenesis of epithelioid hemangioma. Med Hypotheses. 2006;67(5):1133–1135.
- Aoki M, Kimura Y, Kusunoki T, et al. Angiolymphoid hyperplasia with eosinophilia associated with anomalous dilatation of occipital artery: IL-5 and VEGF expression of lesional mast cells. Arch Dermatol. 2002;138(7):982–984.
- Antonescu CR, Chen HW, Zhang L, et al. ZFP36-FOSB fusion defines a subset of epithelioid hemangioma with atypical features. Genes Chromosomes Cancer. 2014;53(11):951–959.
- Huang SC, Zhang L, Sung YS, et al. Frequent FOS gene rearrangements in epithelioid hemangioma: a molecular study of 58 cases with morphologic reappraisal. Am J Surg Pathol. 2015;39(10):1313–1321.
- Tokat F, Lehman JS, Sezer E, et al. Immunoreactivity of Wilms tumor 1 (WT1) as an additional evidence supporting hemangiomatous rather than inflammatory origin in the etiopathogenesis of angiolymphoid hyperplasia with eosinophilia. Dermatol Pract Concept. 2018;8(1):28–32.
- Trindade F, Tellechea O, Torrelo A, et al. Wilms Tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33(6):569–572.
- Gonzalez-Cuyar LF, Tavora F, Zhao XF, et al. Angiolymphoid hyperplasia with eosinophilia developing in a patient with history of peripheral T-cell lymphoma: evidence for multicentric T-cell lymphoproliferative process. Diagn Pathol. 2008;3:22.
- Miller CJ, Ioffreda MD, Ammirati CT. Mohs micrographic surgery for angiolymphoid hyperplasia with eosinophilia. Dermatol Surg. 2004;30(8):1169–1173.
- Labib A, Estawrow M. Angiolymphoid hyperplasia with eosinophilia: new concept to lower recurrence. J Craniofac Surg. 2019;30(5):e386–e388.
- Rongioletti F, Cecchi F, Pastorino C, Scaparro M. Successful management of refractory angiolymphoid hyperplasia with eosinophilia with thalidomide. J Eur Acad Dermatol Venereol. 2016;30(3):527–529.
- Gupta G, Munro CS. Angiolymphoid hyperplasia with eosinophilia: successful treatment with pulsed dye laser using the double pulse technique. Br J Dermatol. 2000;143(1):214–215.
- Hobbs ER, Bailin PL, Ratz JL, Yarbrough CL. Treatment of angiolymphoid hyperplasia of the external ear with carbon dioxide laser. J Am Acad Dermatol. 1988;19(2 Pt 1):345–349.
- Pasyk KA, Elsenety EN, Schelbert EB. Angiolymphoid hyperplasia with eosinophilia-acquired port-wine-stain-like lesions: attempt at treatment with the argon laser. Head Neck Surg. 1988;10(4):269–279.
- Wu L, Li F, She W, et al. Combination of electrocoagulation and photodynamic therapy for angiolymphoid hyperplasia with eosinophilia in the external ear. Photodiagnosis Photodyn Ther. 2019;27: 449–451.
- Lin B, Tan SH, Looi A. Angiolymphoid hyperplasia with eosinophilia of the eyelid with spontaneous regression. Ophthalmic Plast Reconstr Surg. 2008;24(4):308–310.

