J Clin Aesthet Dermatol. 2021;14(12):E84–E94.
J Clin Aesthet Dermatol. 2021;14(12):E84–E94.
by Lee Walker, BDS, MFDS, RCPSG, MJDF, RCS, ENG; Cormac Convery, MB ChB, MSc, MASLMS; Emma Davies, RN, INP; Gillian Murray, MPharm, PG Dip Clin Pharm, INP; and Brittony Croasdell, MS,FNP-BC, APRN CANS
Dr. Walker is with B City Clinic in Liverpool, England. Dr. Convery is with The Ever Clinic in Glasgow, Scotland. Ms. Davies is Clinical Director of Save Face in Cardiff, United Kingdom. Ms. Murray is with Clinical Academic Kings College in London, England. All authors are founding board members of the Complications in Medical Aesthetics Collaborative (CMAC).
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
There are multiple treatment strategies proposed for the management of vision loss related to the injection of soft tissue fillers. Currently, there is no internationally accepted consensus on the immediate management of soft tissue filler induced vision loss (STFIVL). A recent systematic review of the literature concluded that there is not enough evidence to support retrobulbar hyaluronidase, and alternative treatments require exploration. The available literature demonstrates the inconsistent and unproven success of retrobulbar and peribulbar hyaluronidase in reversal of soft filler induced vision loss. Various therapeutics have been used to aid the reversal of vision loss but with mixed outcomes. The current evidence base does not support the use of retrobulbar and peribulbar hyaluronidase. The use of retrobulbar hyaluronidase for reversing soft tissue filler induced vision loss is controversial. Its efficacy remains unproven and there is mixed evidence within the literature. The current evidence suggests that there may be an increased risk of introducing severe adverse events associated with retrobulbar hyaluronidase and may even exacerbate the problem for those clinicians who are not ophthalmology trained. Therefore, we recommend two alternative treatment pathways for ophthalmology and non-ophthalmology trained practitioners. The suggested goal of this publication is to understand the pathophysiology of STFIVL, recognize signs and symptoms, and to propose algorithms to manage vision loss for both non-ophthalmology and ophthalmology trained clinicians. Clinicians must act swiftly and arrange immediate transfer to an emergency department or ophthalmology specialist setting to give the patient the best chance of vision restoration. The focus of any intervention for non-ophthalmology trained clinicians should be based around the immediate use of non-invasive techniques.
Keywords: Vision loss, blindness, hyaluronic acid, filler, complications, soft tissue, ophthalmology, central retinal artery, ophthalmic artery, hyaluronidase, risk, algorithm.
Vision loss after facial injection is an extremely rare event, estimated at 0.001 percent1 but we must assume the incidence is higher due to under-reporting. Since the first 98 cases documented in 2015, there has been an increase of 49 percent based on the most recent global review in 2019.2 Furthermore, the incidence has evolved from mainly autologous fat injections to a more recent rise in hyaluronic acid filler related cases.3 Since the incidence of this potentially catastrophic complication is on the rise with the use of hyaluronic acid dermal fillers, the responsible clinician must be competent to mitigate known risk factors, recognize the symptoms, and instigate safe, evidence-based management strategies.
Pathophysiology
There are three factors required for blindness to occur: injection pressure exceeding systolic pressure, sufficient amount of material within the lumen of the vessel, and retrograde and subsequent anterograde passage of material into the ophthalmic circulation.
1) Injection pressure exceeding systolic pressure.The average injection pressure required to embolize the ophthalmic artery is 166.7 mmHg. Monitoring of the injection pressure showed that it is easy for the injector to exert a pressure well above 200mmHg within 2 to 3 seconds.4
2) Sufficient amount of material within the lumen of the vessel. Findings indicate that only a small amount of filler is required to occlude branches of the ophthalmic artery. The average entire volume of the supratrochlear artery from the glabella to the orbital apex is 0.085mL (range 0.04–0.12mL), and injection volume should not exceed 0.085mL in critical injection points.1,5
3) Retrograde and subsequent anterograde passage of material into the ophthalmic circulation. The pressure during the injection is likely to be dispersed to multiple vessels rather than transmitted to one artery.4 Terminal cutaneous branches of the ophthalmic artery, namely the supraorbital and supratrochlear, supply the medial forehead, and anastomoses between these vessels and the terminal branches of the angular artery are well documented. Similarly, anastomoses with the superficial temporal arteries and the orbit have also been demonstrated. Injection of filler material into one of these vessels can lead to retrograde flow to beyond the point of the origin of the ophthalmic artery. When pressure from the plunger is released, systolic pressure drives the product forward to enter the ophthalmic artery and specifically, the central retinal artery, resulting in visual loss.1,5
Risk zones
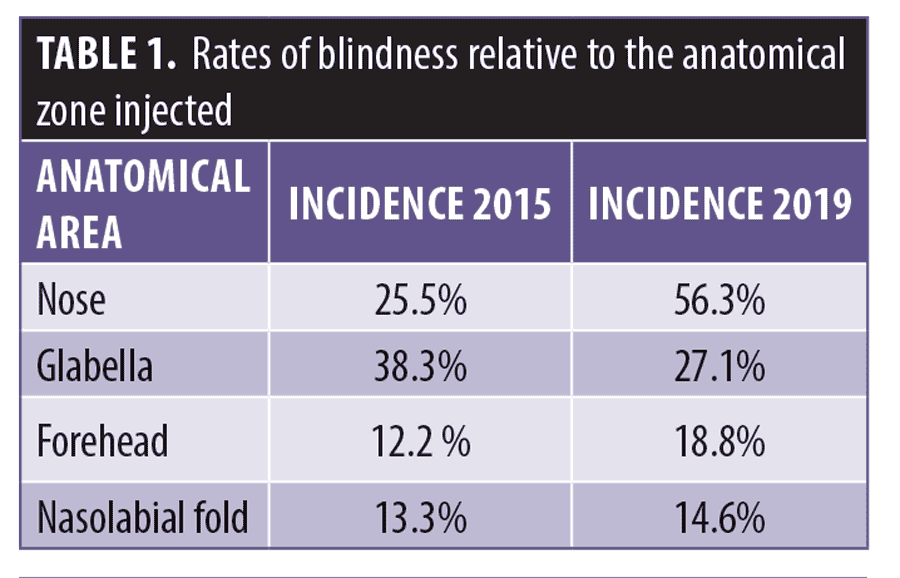
There are areas of the face with a higher incidence of vision loss. Table 1 shows the rates of blindness relative to the anatomical zone injected. The nasal region now has the highest associated incidence of vision loss, overtaking the glabella as the highest risk area for soft tissue filler injections.2 The risks have been zoned according to treatment indications, by a 2020 consensus, as illustrated in Figure 1.

Types of blindness
Several types of blindness/vision loss have been described in the literature.1,6,7 The degree of subsequent visual disturbance relates to the type of soft tissue filler, the volumes injected, cohesivity, particle size, and the branches of the ophthalmic artery affected.1
There are various classifications based on the artery occluded, the presentation of symptoms, and the nature of the onset.
Occluded artery (Figure 2). Based on which artery is occluded, vision loss/blindness can be classified into six subtypes.3,8
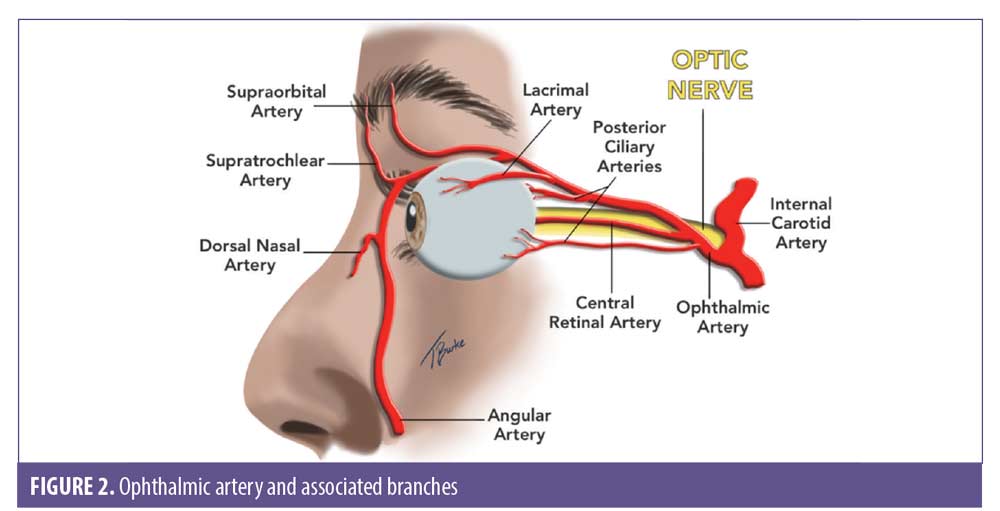
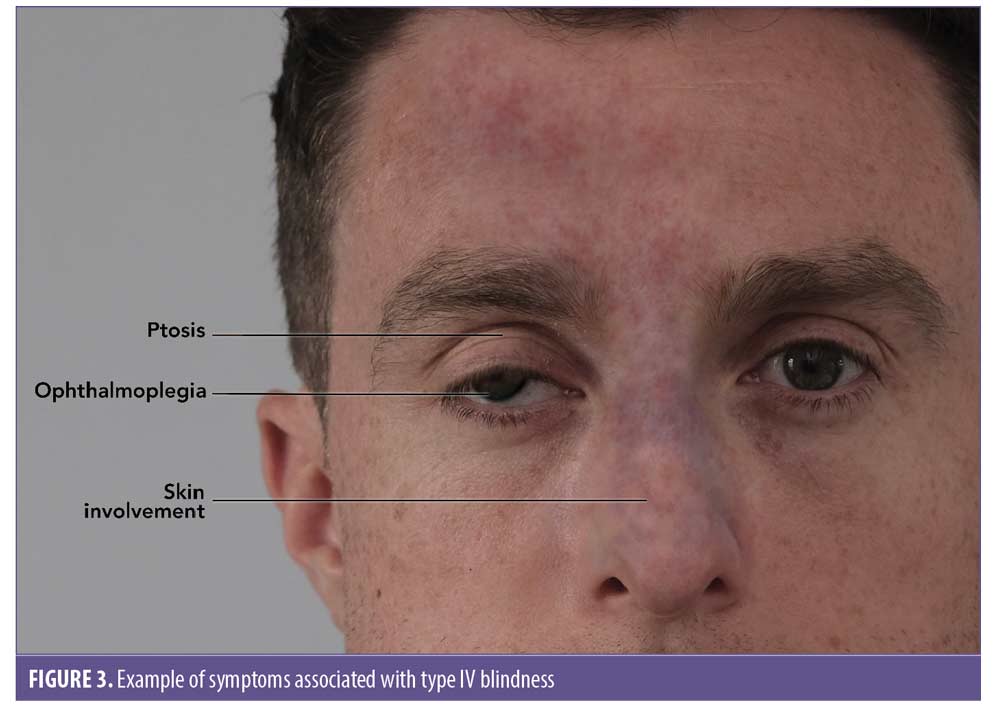
- Ophthalmic artery occlusion (OAO). Total obstruction of the ophthalmic artery would tend towards visual loss, ptosis, and ophthalmoplegia
- Generalized posterior ciliary artery occlusion with relative central retinal artery sparing (PCAO)
- Central retinal artery occlusion (CRAO). Isolated central retinal artery occlusion would tend towards visual loss alone
- Branch retinal artery occlusion (BRAO). Branch retinal artery occlusion may cause a partial visual loss
- Anterior ischaemic optic neuropathy (AION)
- Posterior ischaemic optic neuropathy (PION)
Combinations of these patterns can occur.1 Each type of occlusion carries a distinct prognosis, which is worse in the diffuse occlusion group.7
Classification of blindness/vision loss based on presentation of symptoms. Table 2 shows four presentation subtypes of periocular complications associated with blindness following cosmetic filler injection.6 Figure 3 shows an example of Type IV blindness.
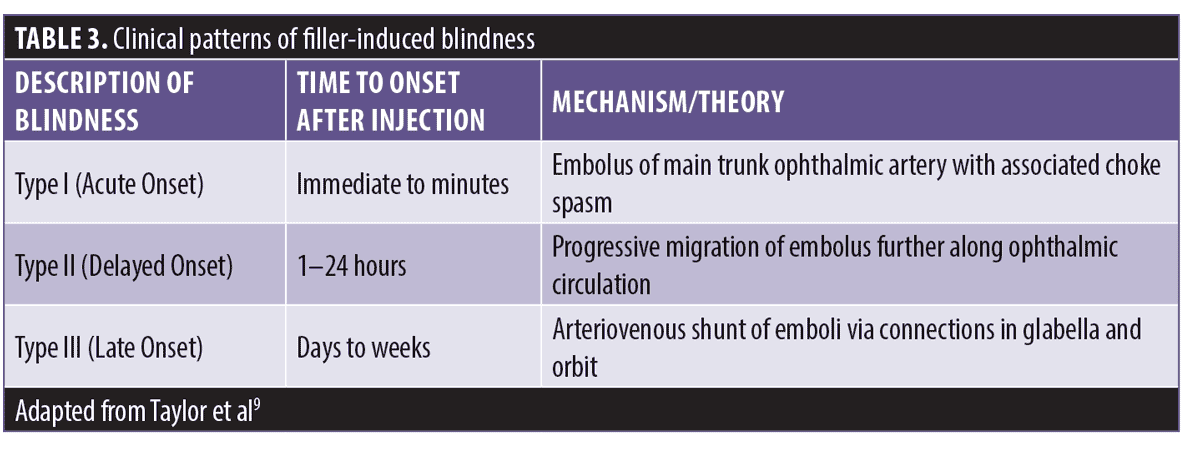
Classification of blindness/vision loss based on time to onset. In a publication by Taylor et al,9 blindness induced by filler fell into three distinct clinical patterns and is summarized in Table 3.
Prognosis
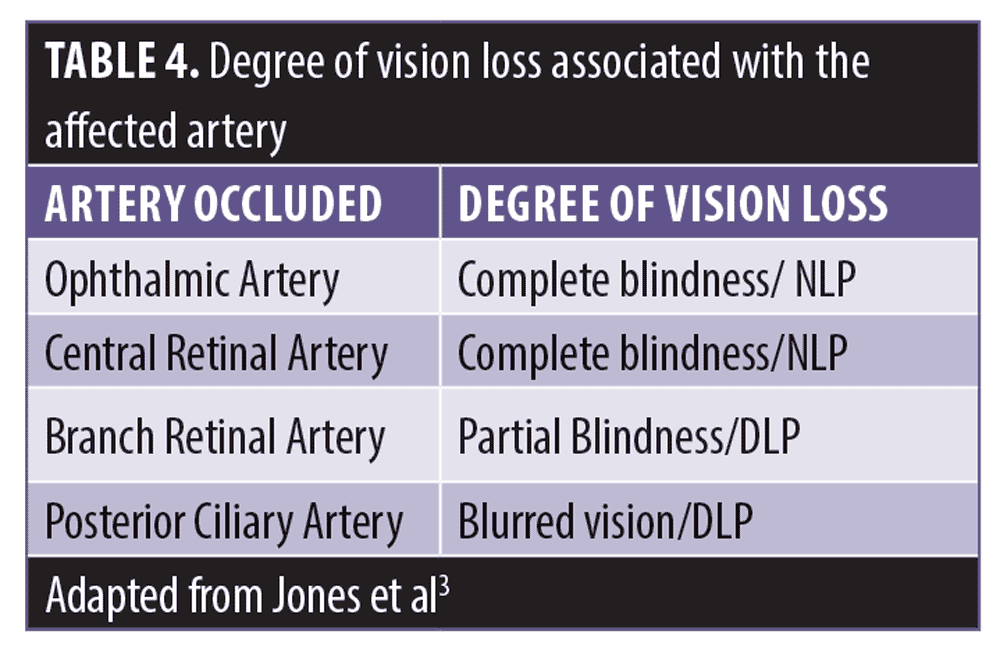
Degree of vision loss predicts the location of the embolus, which might be the most important prognostic factor.3 Eighty percent of fat emboli present as complete vision loss, whereas 50 percent of HA emboli present as partial vision loss, accounting for its better prognosis. Presentation with complete vision loss/blindness (i.e., no light perception [NLP]) is most often associated with ophthalmic artery occlusion (OAO) or central retinal artery occlusion (CRAO), and most do not recover.3
Presentation with partial vision loss (i.e., blurry vision to diminished light perception [DLP]) is less commonly associated with OAO/CRAO, and more often involves more distal branches of both the posterior ciliary arteries or the central retinal artery, likely due to smaller emboli. Partial vision loss after HA filler has a better prognosis than complete vision loss.3
Branch retinal artery occlusion (BRAO) has the most favourable prognosis. In the literature it shows that all fully and partially recovered vision loss patients received some form of treatment.3 Table 4 summarizes the affected artery and degree of vision loss associated.
Signs and Symptoms
The signature features of periocular embolism are instant-onset and simultaneous blindness and ocular pain, which contrasts with ischemia of the facial skin that manifests as blanching followed by delayed pain.10
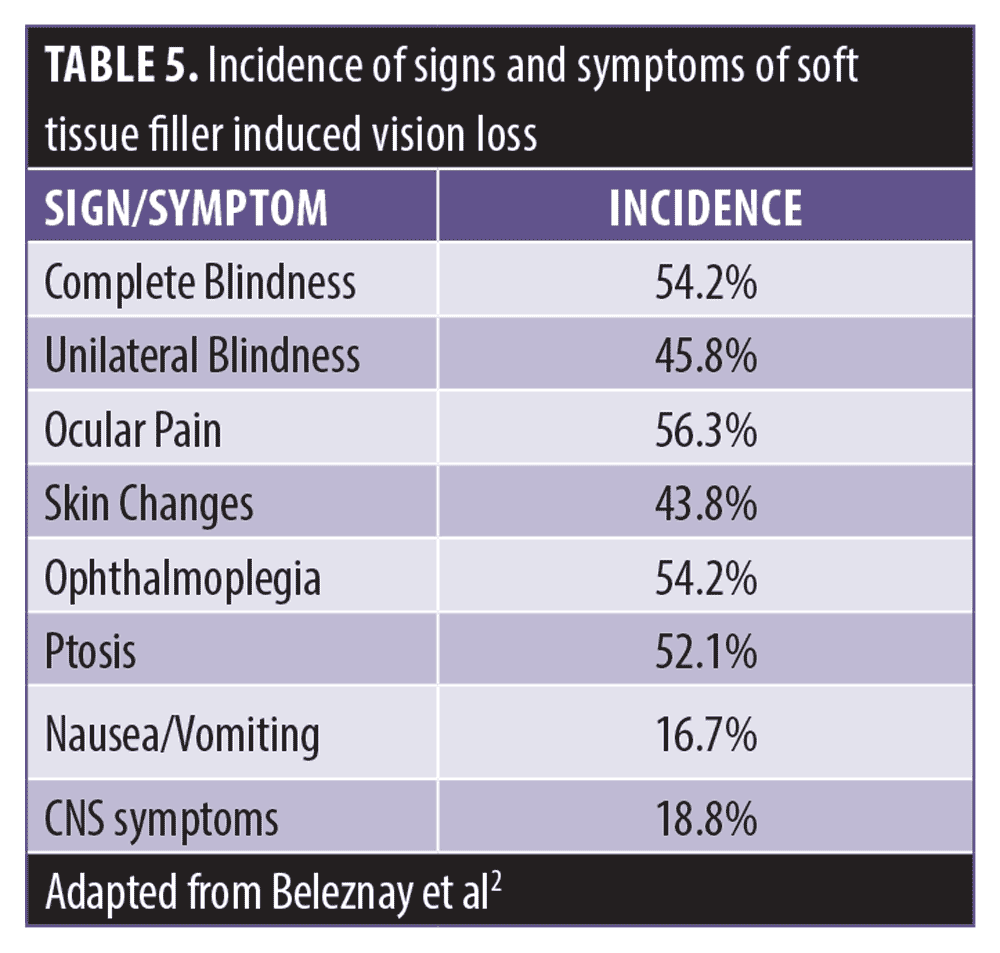
In an updated review of the world literature of 48 new cases of vision loss, 26 cases (54.2%) were found to have complete vision loss, whereas the remaining cases had complete unilateral vision loss.2 In 27 cases (56.3%) pain was reported as one of the initial symptoms, described as periorbital, ocular, periocular, orbital, eye pain, or headache. In 21 cases (43.8%) associated skin changes, commonly described as erythematous to violaceous mottling or skin necrosis, were reported. Ophthalmoplegia (i.e., decreased extraocular muscle movement) was reported in 26 cases (54.2%) and ptosis was seen in 25 cases (52.1%). Most commonly, the ophthalmoplegia and ptosis recovered completely. Nausea and/or vomiting were described as a presenting symptom in eight cases (16.7%). Among the 48 cases, there were nine cases (18.8%) of central nervous system (CNS) complications, including stroke-like features, such as unilateral weakness or evidence of brain infarction on imaging.2 Table 5 summarizes the incidence of signs and symptoms of STFIVL.
Treatment options in the immediate management of STFIVL
There are numerous therapeutic options for the immediate management of STFIVL. The key for clinicians is not to delay any intervention which might reverse vision loss. One big challenge in evaluating potential therapies for STFIVL involves the uncertainty regarding retinal tolerance time or the duration of retinal ischemia prior to irreversible damage. The evidence is uncertain when it comes to how long the retina can survive hypoxia.
Research in primates demonstrated retinal survival if perfusion is restored within 90 to 240 minutes following occlusion.11 However, evidence now suggests that human retinal ischemic survival time might be considerably shorter.12–14 A shorter retinal tolerance time undermines the result of some studies which have purported visual benefit of CRAO treatments given up to 24 to 48 hours after occlusion occurs.12 The actual range is likely to be 15 to 90 minutes.13 In a publication by Tobalem et al14 in 2018, it was suggested that retinal infarction is most likely to occur after only 12 to 15 minutes of complete CRAO. This may help to explain why delayed therapeutic interventions for CRAO are often ineffective. Therefore, interventional time management is critical for any chance of vision restoration.
Management strategies reported in the literature usually include polypharmacy, which makes it difficult to identify key effective interventions.2 Some important interventions require specialist knowledge and skills and access to advanced diagnostic imaging, therefore it is in the patient’s best interests that the treating clinician is able to administer first aid and transfer to specialist care without delay.
Treatment options and management strategies should be divided in two distinct categories: interventional options for non-ophthalmology trained clinicians, and interventional options for ophthalmology trained clinicians.
Most of these therapeutic treatment options are derived and extrapolated from ophthalmic literature for the management of central retinal artery occlusion.2,15,16
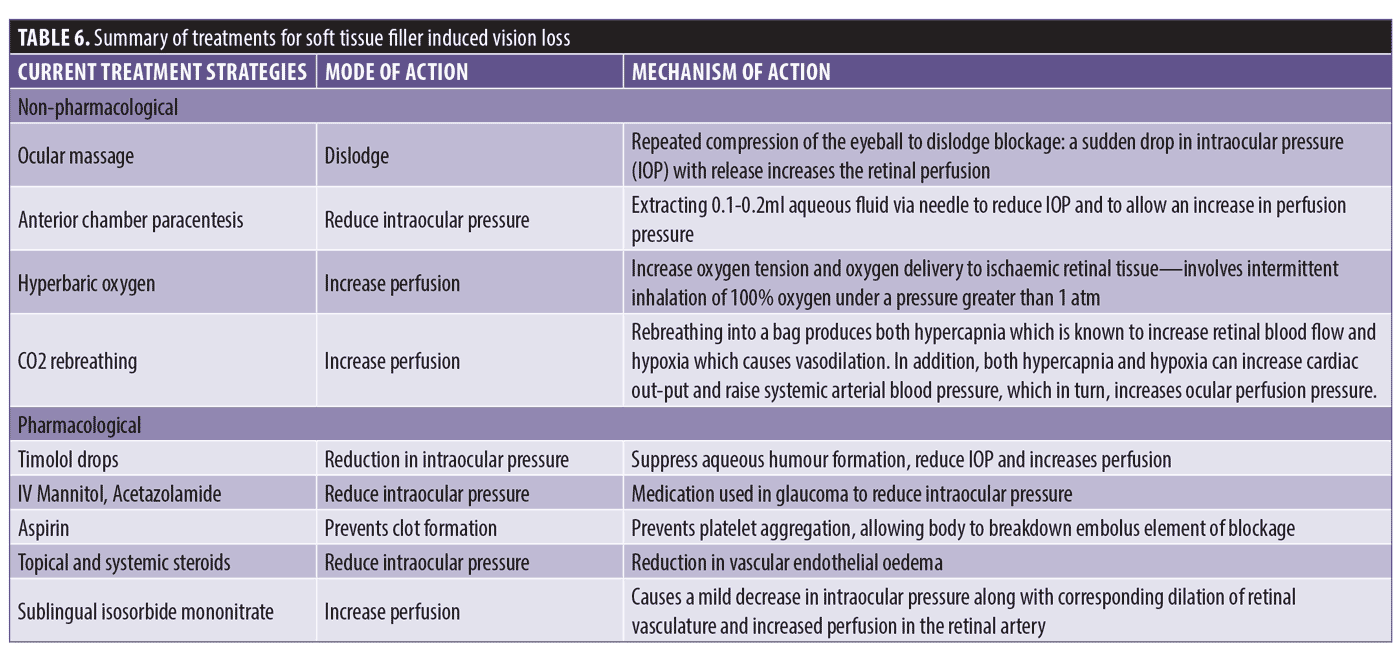
Table 6 shows the pharmacological and non-pharmacological options described within the literature. These options have now been adapted in aesthetic medicine to aid retinal perfusion, reduce intraocular pressure, reduce retinal edema, aid the lysis of hyaluronic acid embolus, and restore vision loss in STFIVL.
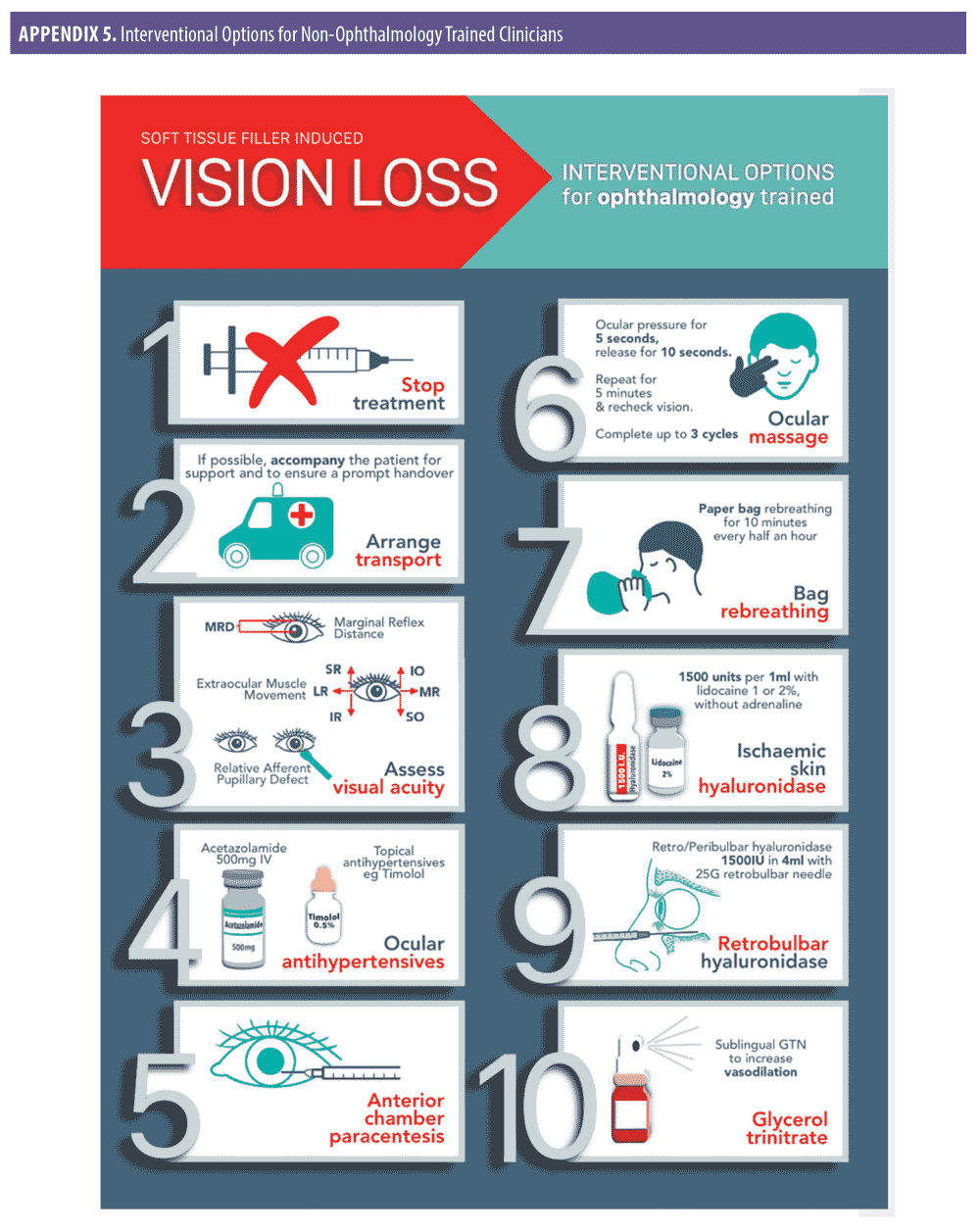
Interventional options for non-ophthalmology trained clinicians (Appendix 1).
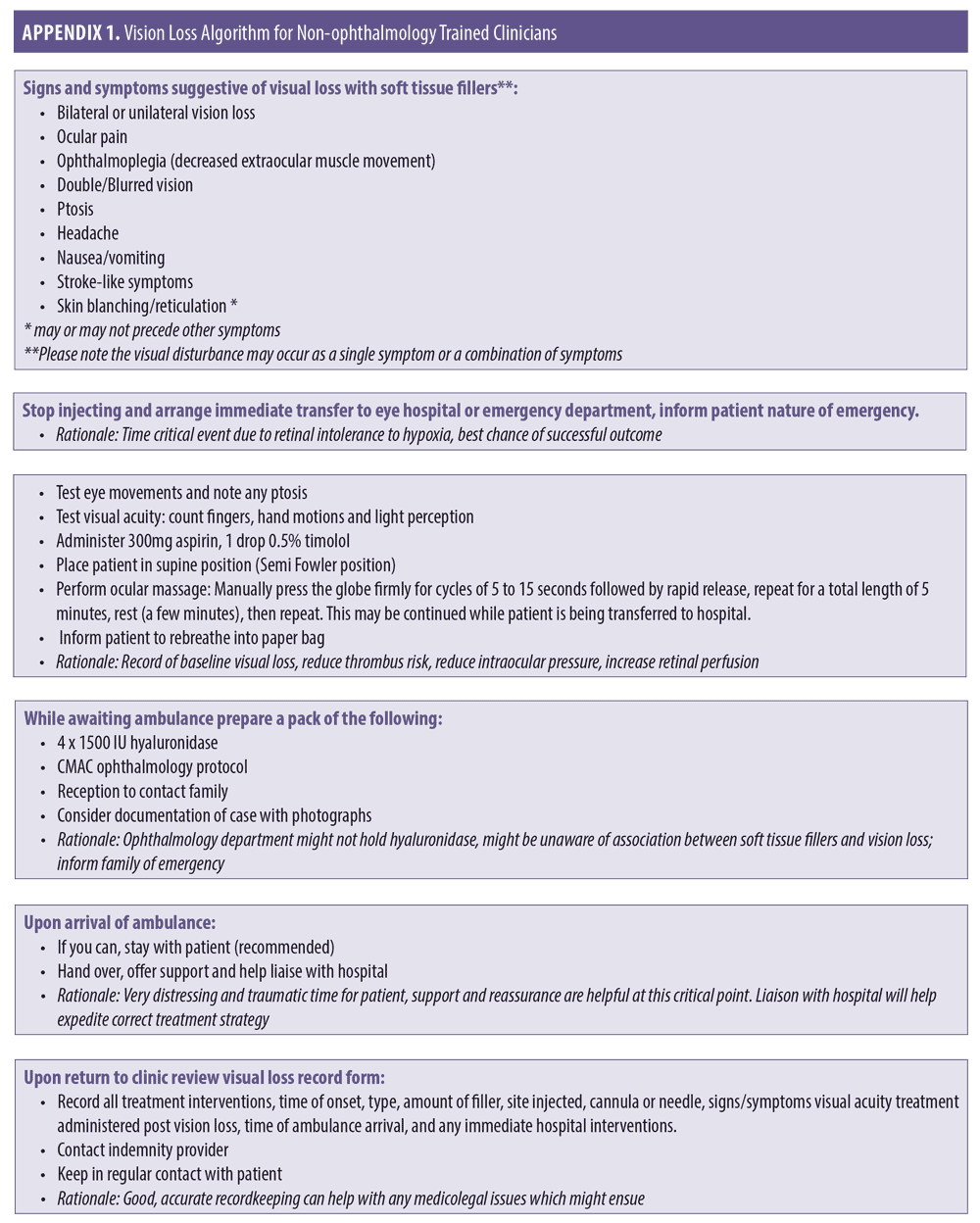
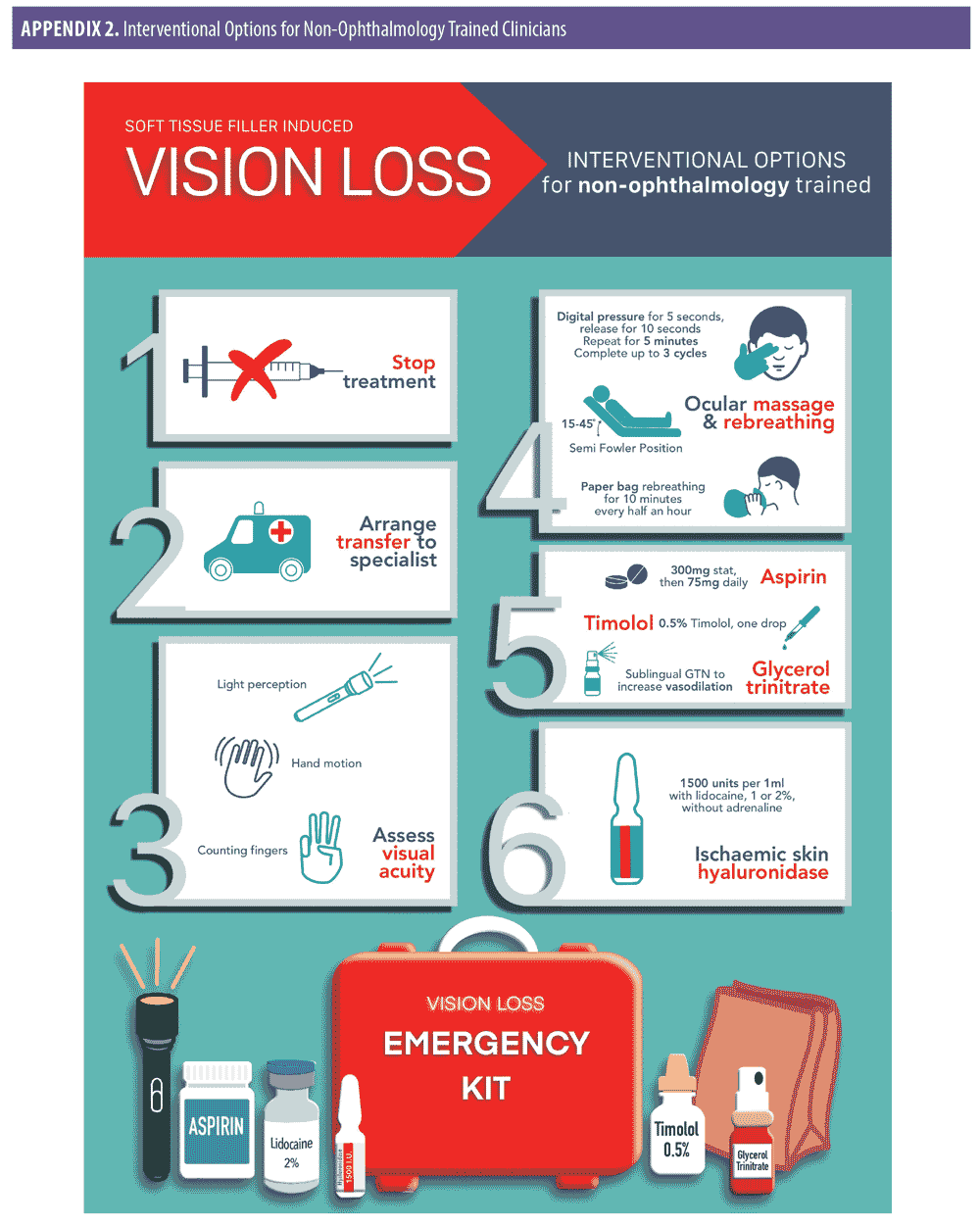
Stop treatment immediately.
The best chance of vision restoration is in a hospital/specialist setting.1 Specialist treatment at an appropriate facility should be instituted as soon as possible to optimize all possible treatment pathways.17 Therefore, the treating clinician should arrange immediate transfer of the patient by calling an ambulance or driving the patient directly to hospital. The authors strongly recommend that the treating clinician accompanies the patient. Accompanying the patient may assist with liaison and prompt hand over in the specialist setting, while also providing support to the patient during this difficult time.
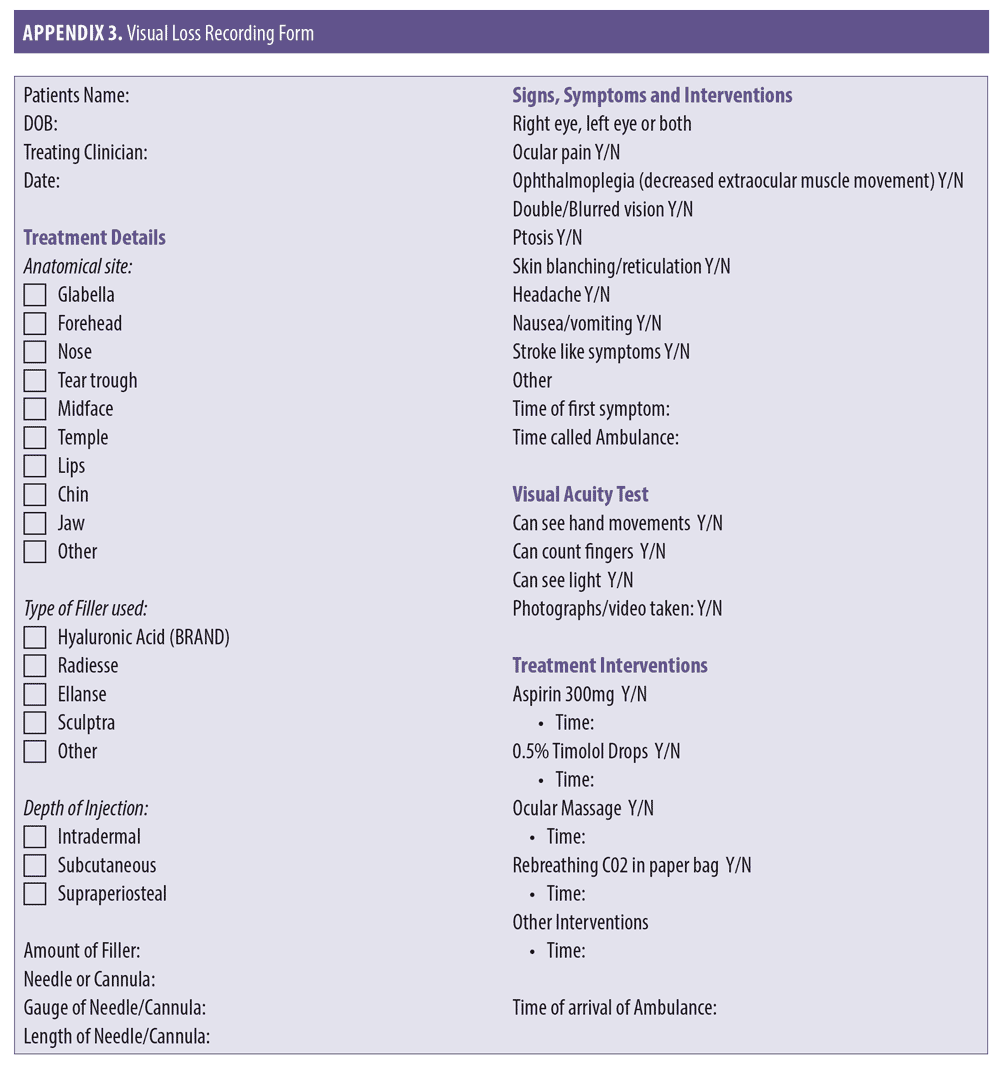
While awaiting transfer, test and document visual acuity.1 Using simple methods (i.e., counting fingers, hand motions and light perception via a hand torch or mobile phone light) can provide useful information to the treating ophthalmologist on hand over.18 Recording vision from worst to best may also prove valuable to the treating specialist. The order of worst to best regarding vision is as follows: No light perception, light perception, hand motion, counting fingers.18
Give the patient 300mg aspirin. There is no direct evidence that aspirin prevents platelet aggregation in the event of a hyaluronic acid-related occlusion; however, it is reasonable, based on the extrapolation of the evidence from acute coronary syndrome, to prescribe 300mg as an immediate dose with 75mg daily until the occlusion is dissolved and vision has been restored. Safety must be considered in the context of the individual patient, and if the patient is allergic to aspirin, clopidogrel at a dose of 300mg immediately, then 75mg daily may be used.19
Reduce intraocular pressure by administering 0.5% timolol drops.18 One drop is administered to the affected eye while awaiting transfer to the hospital. The authors recommend that timolol should form part of a vision loss kit that is readily available at the clinician’s place of work.
Begin ocular massage. Ocular massage is known to cause retinal arterial dilatation and large fluctuations in IOP,20 promoting the dislodgment of the causative embolus. The embolus then either disintegrates or migrates into a peripheral portion of the retinal vasculature, allowing for retinal reperfusion.12 Place the patient in a supine position with the head raised 15 to 45 degrees (Semi Fowler Position), perform digital ocular massage by applying direct pressure through the closed eyelid of the affected eye for five seconds and then releasing and waiting for 10 seconds before applying pressure for another five seconds. The procedure should be repeated in a pulsatile fashion for five minutes, after which vision should be checked. A total of three sets of this pressure/release can be performed if necessary, so that the patient has a total of 15 minutes of pressure/release.18
Instruct the patient to re-breathe into a paper bag for approximately 10 minutes every half an hour.18 This assumes that an increase in carbon dioxide promotes dilation of arterioles.21
If the skin is ischemic in the area of injection, consider prompt administration of hyaluronidase. The use of hyaluronidase in dissolving cross-linked hyaluronic acid is highly effective and has been shown to prevent tissue necrosis. Rapid deployment might rescue threatened tissue. The authors recommend using 1,500 units per 1mL with lidocaine 1% or 2% without adrenaline, employing a high-dose pulsed model of dosing.19
If patient has no medical contraindications, the use of sublingual glycerol trinitrate is a quick and effective method for increased vasodilatation.1
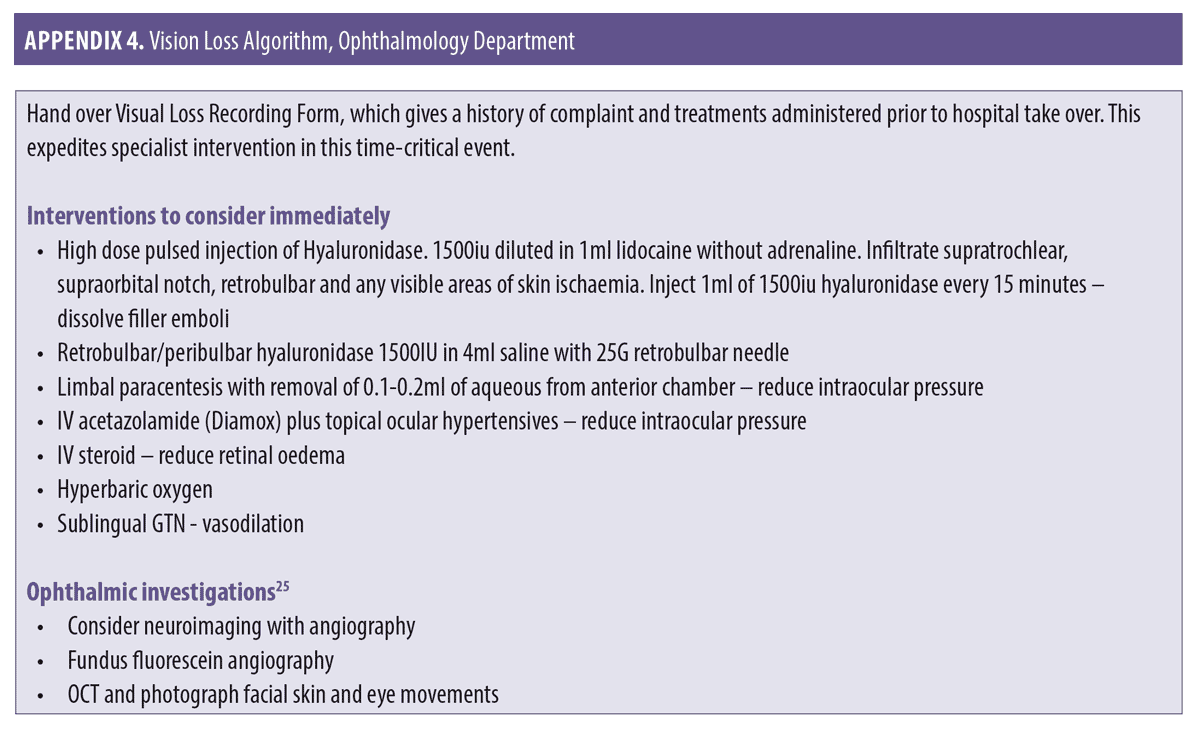
Interventional options for the ophthalmology trained clinician. For the ophthalmology trained clinician, the primary focus is also based around reduction of intraocular pressure and increasing retinal perfusion. Other supportive therapies which have a more invasive nature can be considered; these are suitable for those with an ophthalmology background. HA filler emboli degradation can also be attempted using retrobulbar/peribulbar hyaluronidase. In the eventuality of a patient presenting with immediate visual loss, the course of action of the practitioner should be as follows:
Stop treatment immediately.
The best chance of vision restoration is in a hospital/specialist setting.1 Specialist treatment at an appropriate facility should be instituted as soon as possible to optimize all possible treatment pathways.17 Therefore, the treating clinician should arrange immediate transfer of the patient by calling an ambulance or driving the patient directly to hospital. The authors strongly recommend that the treating clinician accompanies the patient. Accompanying the patient may assist with liaison and prompt hand over in the specialist setting while also providing support to the patient during this difficult time.
While awaiting transfer, test and document visual acuity.1 These more detailed tests should include relative afferent pupillary defect (RAPD) which is an objective measurement of relative optic nerve damage. RAPD is tested by the “swinging light test,” assessing for pupillary constriction on exposure to light.22 Extraocular muscle movement assessment for ophthalmoplegia and ptosis assessment, including measurement of marginal reflex distance, must also be recorded.22
If available, preferably intravenous rather than oral acetazolamide (Diamox) 500mg bolus plus topical ocular antihypertensives (e.g., timolol) to reduce intraocular pressure should be administered.23
Perform anterior chamber paracentesis. This technique has been demonstrated to effectively reduce intraocular pressure with subsequent increase in retinal perfusion rate up to 20 percent. It should be noted that this technique entails the intrinsic risk of intraocular complications.24
Start ocular massage. Ocular massage is known to cause retinal arterial dilatation and large fluctuations in IOP.20 Use a Goldman lens or transpalpebral two-finger approach.24
Instruct the patient to re-breathe into a paper bag for approximately 10 minutes every half an hour.18 This assumes that an increase in carbon dioxide promotes dilation of arterioles.21
If the skin is ischemic in the area of injection, consider prompt administration of hyaluronidase. The use of hyaluronidase in dissolving cross-linked hyaluronic acid is highly effective and has been shown to prevent tissue necrosis. Rapid deployment might rescue threatened tissue. The authors recommend using 1,500 units per 1mL with lidocaine 1% or 2% without adrenaline, employing a high-dose pulsed model of dosing.19
Consider retrobulbar/peribulbar hyaluronidase 1,500IU in 4mL saline with 25G retrobulbar needle.23,25 A systematic review of the literature reported that hyaluronidase was used in 86 percent of vision loss cases; however, only 11.1 percent of cases were successful.26 There are case reports of success using retrobulbar/peribulbar hyaluronidase,27–30 so there may be value in the ophthalmology trained clinician attempting this procedure. An initial volume of 7mL is recommended to avoid orbital compartment syndrome.18
If the patient has no medical contraindications, the use of sublingual glycerol trinitrate is a quick and effective method for increased vasodilatation.1
Conclusion
Vision loss due to soft tissue filler injection is a rare, but catastrophic event. When vision loss occurs, early recognition and prompt treatment are critical. There is currently no internationally accepted gold standard for the treatment of vision loss, and even though there have been consensus recommendations, no specific guidelines are available that have been universally successful in reversing this complication.31 The best chance for vision restoration is a hospital or specialist setting.1
However, it should be noted that a recent survey of British oculoplastic consultants demonstrated a limited awareness of visual vascular complications with dermal fillers and their emergency management. The majority (88%) did not have local management guidelines in place, nor were they aware of guidance to manage the complication (75%).32 A similar situation has been observed in the United States, where a survey of 45 institutions found that only 20 percent had a formal policy, guideline, or white paper to standardize the approach to CRAO treatment.18
The current evidence suggests that there might be an increased probability of introducing severe risks associated with retrobulbar hyaluronidase and may even exacerbate the problem for those clinicians who are not ophthalmology trained. Expert opinion and the reviewed case reports has suggested that many non-ophthalmology trained clinicians would not be adequately prepared or equipped to accurately diagnose and document a CRAO and address these complications should they arise. Therefore, the authors suggest two alternative treatment pathways for the ophthalmology and non-ophthalmology trained practitioner.
Acknowledgments
All illustrations and figures in this article (Figures 1–3, Appendix 3 and 5) were created by Dr. Toni Burke.
Complications in Medical Aesthetics Collaborative (CMAC ) is an organization set up to provide support and education to medical aesthetic clinicians. Members are part of a collaboration, and through working with the membership, CMAC aims to capture data to help improve patient safety. For more details, please see https://www.cmac.world/.
References
- Goodman GJ, Magnusson MR, Callan P, Roberts S, et al. A Consensus on Minimizing the Risk of Hyaluronic Acid Embolic Visual Loss and Suggestions for Immediate Bedside Management. Aesthet Surg J. 2020;40(9):1009–1021.
- Beleznay K, Carruthers JDA, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662–674.
- Jones DH, Fitzgerald R, Cox SE, et al. Preventing and Treating Adverse Events of Injectable Fillers: Evidence-Based Recommendations From the American Society for Dermatologic Surgery Multidisciplinary Task Force. Dermatol Surg. 2021;47(2):214–226.
- Cho KH, Dalla Pozza E, Toth G, et al. Pathophysiology Study of Filler-Induced Blindness. Aesthet Surg J. 2019;39(1):96–106.
- Khan TT, Colon-Acevedo B, Mettu P, et al. An Anatomical Analysis of the Supratrochlear Artery: Considerations in Facial Filler Injections and Preventing Vision Loss. Aesthet Surg J. 2017;37(2):203–208.
- Yujin M, Sangjun Y, Jeong JH, et al. The classification and prognosis of periocular complications related to blindness following cosmetic filler injection. Plastic and Recon Surg. 2017;140(1):61–64.
- Paap MK, Milman T, Ugradar S, et al. Examining the Role of Retrobulbar Hyaluronidase in Reversing Filler-Induced Blindness: A Systematic Review. Ophthalmic Plast Reconstr Surg. May/Jun 2020;36(3):231–238.
- Szantyr A, Orski M, Marchewka I, et al. Ocular Complications Following Autologous Fat Injections into Facial Area: Case Report of a Re-covery from Visual Loss After Ophthalmic Artery Occlusion and a Review of the Literature. Aesthetic Plast Surg. 2017;41(3):580–584.
- Taylor GI, Shoukath S, Gascoigne A, et al. The Functional Anatomy of the Ophthalmic Angiosome and Its Implications in Blindness as a Complication of Cosmetic Facial Filler Procedures. Plast Reconstr Surg. 2020;146(4):745.
- Lee W, Oh W, Ko HS, et al. Effectiveness of Retrobulbar Hyaluronidase Injection in an Iatrogenic Blindness Rabbit Model Using Hyaluronic Acid Filler Injection. Plastic and reconstructive surgery, 2019;144(1):137–143.
- Hayreh, SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):376–391.
- Sharma RA, Newman NJ, Biousse V. Conservative treatments for acute nonarteritic central retinal artery occlusion: Do they work? Taiwan J Ophthalmol. 2021;11(1):16.
- DeLorenzi C. Commentary on: Blindness After Facial Filler Injections: The Role of Extravascular Hyaluronidase on Intravascular Hyaluronic Acid Embolism in the Rabbit Experimental Model. Aesthetic Surg J. 2020:40(3):327–329.
- Tobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion—rethinking retinal survival time. BMC Ophthalmology. 2018;18(1):101.
- Prado G, Rodriquez-Feliz J. Ocular pain and impending blindness during facial cosmetic injections: is your office prepared? Aesth Plast Surg. 2017;41:199–203
- Feltgen N, Neubauer A, Jurklies B, et al; EAGLE-Study Group. Multicenter study of the European As-sessment Group for Lysis in the Eye (EAGLE) for the treatment of central retinal artery occlusion: design issues and implications. EAGLE Study report no. 1 : EAGLE Study report no. 1. Graefes Arch Clin Exp Ophthalmol. 2006;244(8):950–956.
- Humzah MD, Ataullah S, Chiang C, et al. The treatment of hyaluronic acid aesthetic interventional induced visual loss (AIIVL): A consensus on practical guidance. J Cosmet Dermatol. 2019;18(1):71–76.
- Barbarino S, Banker T, Fezza, J. Standardized approach to treatment of retinal artery occlusion after intraarterial injection of soft tissue fillers: EYE-CODE. J Am Acad Dermatol. 2020;S0190-9622(20)33245-X. Online ahead of print.
- Murray G, Convery C, Walker L, Davies E. Guideline for the Management of Hyaluronic Acid Filler-induced Vascular Occlusion. J Clin Aesthet Dermatol. 2021;14(5):E61–E69.
- Ffytche TJ. A rationalization of treatment of central retinal artery occlusion. Trans Ophthalmol Soc U K. 1974;94(2):468–479.
- Frayser R, Hickham JB. Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations. Invest Ophthalmol. 1964;3:427–431
- Arlette JP, Ashenhurst M, Hill V, and Jiang K. Prevention and Management of Filler Induced Iatrogenic Stroke of the Eye. J Cutan Med Surg. 2021;25(5):543–552.
- Murthy R, Roos J. PMFA Journal Site. Is there a role for retrobulbar hyaluronidase in hyaluronic acid vascular embolism related vision loss? January 2021. https://www.thepmfajournal.com/features/features/post/is-there-a-role-for-retrobulbar-hyaluronidase-in-hyaluronic-acid-vascular-embolism-related-vision-loss.
- Graue, G, Ochoa Araujo, DA, Plata Palazuelos, C, et al. The M.A.STE.R.S algorithm for acute visual loss management after facial filler injection. J Cosmet Dermatol. 2020; 19:2859– 2866.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097–11
- Sorensen EP and Council ML. Update in Soft-Tissue Filler-Associated Blindness. Dermatol Surg. 2020;46(5):671–677.
- Sharudin SN, Ismail MF, Mohamad NF, et al. Complete recovery of filler-induced visual loss following subcutaneous hyaluronidase injection. Neuroophthalmology. 2019;43: 102–106.
- Goodman GJ, Clague MD. A rethink on hyaluronidase injection, intraarterial injection, and blindness: is there another option for treatment of retinal artery embolism caused by intraarterial injection of hyaluronic acid? Dermatol Surg. 2016;42:547–549.
- Chesnut C. Restoration of visual loss with retrobulbar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44:435–437.
- Wibowo A, Kapoor KM, Philipp-Dormston WG. Reversal of post-filler vision loss and skin ischaemia with high-dose pulsed hyaluronidase injections. Aesthetic Plast Surg. 2019;43:1337–1344.
- Chatrath V, Banerjee PS, Goodman GJ, et al. Soft-tissue Filler-associated Blindness: A Systematic Review of Case Reports and Case Series. Plast Reconstr Surg Glob Open. 2019;7(4):e2173.
- Joganathan V and Shah-Desai S. Awareness of management of hyaluronic acid induced visual loss: A British National Survey. Eye (Lond). 2020;34(12):2280–2283.