J Clin Aesthet Dermatol. 2021;14(11):14–17
Interaction of skin with fractional picosecond laser in Asian patients
Dear Editor:
There is increasing data supporting the use of fractional picosecond laser (FXL-PS) application to achieve skin rejuvenation.1,2 Intra-epidermal cavities resulting from areas of laser-induced optical breakdown (LIOB) have been reported to be associated with the deposition of new dermal collagen, elastic tissue, and mucin.1 Habbema et al3 induced dermal injury using LIOB by Nd:YAG laser with sub-nanosecond light pulses, while leaving an intact epidermis, which led to new collagen growth identified with the Herovici staining technique. Here, we present a small study of three Asian patients who demonstrated LIOB in the epidermis and the dermis following FXL-PS.
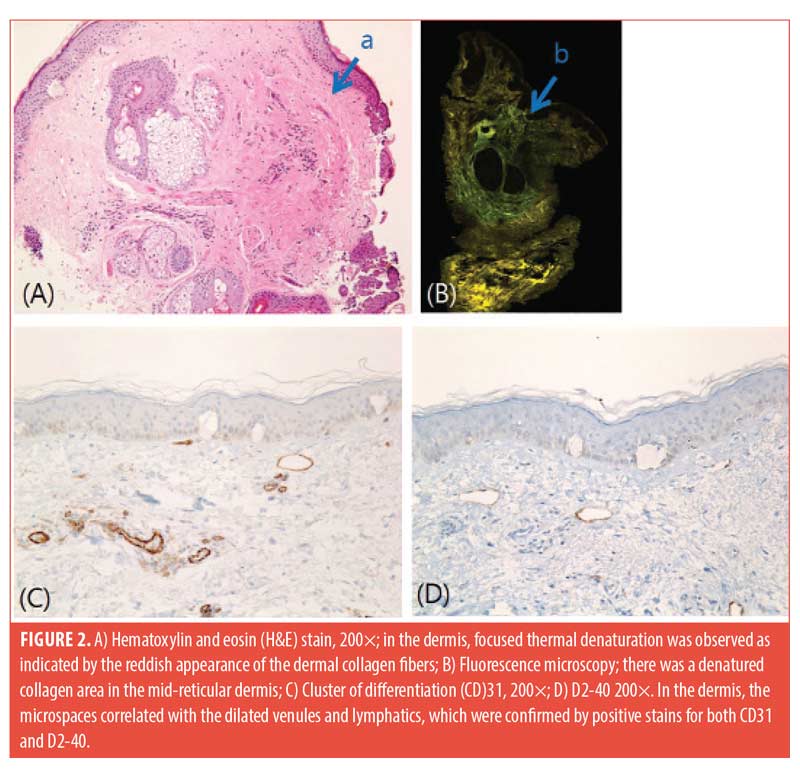
Three patients with Skin Types III and IV participated in this study. We used a 1,064-nm picosecond laser (PICOCARE; WonTech, Daejeon, South Korea) with a microlens array (MLA) with a spot size of 4mm and fluence range of 2.2 to 2.8 J/cm2 applied on the malar area of the face for two non-overlapping passes. Biopsies with a 2-mm punch were performed immediately after treatment. The biopsies were stained with hematoxylin and eosin, and immunohistochemical staining for cluster of differentiation (CD)31 and D240 was performed. Histology revealed a few variably sized intra-epidermal microcavities by coagulation degeneration and necrosis, evidenced by pyknotic nucleus and the shrinkage of cytoplasm of keratinocytes lining the inner sides of microcavities. Extravasation of erythrocytes was found in the papillary dermis (Figure 1). A focal reddish appearance of collagen fibers in the upper dermis with mild edematous changes was noted. Dilated venules and lymphatics were observed in upper dermal microspaces (Figure 2). 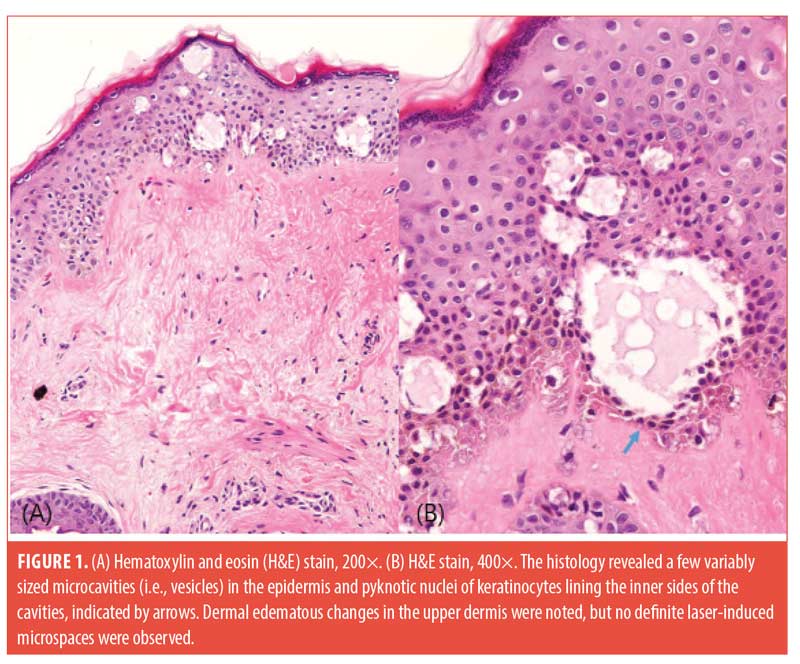
The initiation mechanism of LIOB begins from the production of free seed electrons using a laser pulse through multiphoton absorption or thermionic emission.4 Subsequently, electron density increases to form plasma at the focal area. This results in cavitation bubbles in the skin, which have been suggested to expand, disrupt tissue, and generate shockwaves to further disrupt the colocated tissues to generate microscopic vacuolar tissue reactions.2
Locations of LIOB have been reported differently,2–5 and these differences might be explained by the engineering characteristics of the optical element used to generate the laser micro-injury pattern.5 In the study by Habbema et al,3 dermal LIOB was achieved through two-dimensional scanning of a focused laser beam, but a MLA was used in the study by Tanghetti2 that demonstrated epidermal LIOB. In an ex-vivo study by Chun et al,6 a 1,064-nm MLA with a 4-mm spot size and a laser fluence of 2.8 J/cm2 generated patterns of cavitation and vacuolization formation, Melan-A-positive, CD31-negative cystic cavitation in the epidermis, and noticeable CD31-positive, Melan-A-negative cystic lesions in the dermis. This study demonstrated that cystic cavitation lesions in the dermis can arise in microvascular components, collagen bundles, and perivascular loose connective tissue, and dermal cystic cavitation lesions could be generated independent of the absorption properties of chromophores.6 According to Tanghetti et al,2 as the melanin content of the epidermis is reduced, the size, location, and prevalence of vacuoles decrease. The location, size, and density of the areas of cystic cavitation seem to inversely correlate with the depth of pigments and vascular components as a result of a loss of laser energy due to tissue scattering.6 Hemorrhagic vacuoles within the epidermis or superficial papillary dermis suggest that LIOB can either disrupt the neighboring blood vessels or be initiated by hemoglobin.3 Previous studies have described several factors that affect dermal remodeling, despite the primary damage to the epidermis. Fibroblast activation, which is stimulated by an epidermal injury-induced cytokine release, leads to dermal remodeling.7 Dermal remodeling, including fibroblast proliferation and angiogenesis, could also be stimulated by the traveling shockwave that is initiated in the epidermis.8,9
The results of our small study suggests that epidermal LIOB was attained, but the outcome of dermal LIOB seemed to be nonsignificant. However, we hypothesized that dermal LIOB disrupted neighboring blood vessels and lymphatics. In addition to epidermal injury-induced cytokines, changes around the vessels might have had a dermal-remodeling effect. Our results and conclusion are limited by the very small patient number. A randomized, controlled trial with a much larger patient sample is needed before any firm conclusions can be made.
With regard,
Hye-Jin Ahn, MD; Dong Hye Suh, MD, PhD; In-Hye Kang, MD; Sang Jun Lee, MD, Phd; Min Kyung Shin, MD, PhD, and Kye Yong Song, MD, PhD
Affiliations. Drs. Suh and Lee are with Arumdaun Nara Dermatologic Clinic in Seoul, Korea. Drs. Ahn, Kang, and Shin are with the Department of Dermatology, College of Medicine at Kyung Hee University in Seoul, Korea. Dr. Song is with the Department of Pathology at Kuro Sungsim Medical Center in Seoul, Korea.
Funding. No funding was provided for this article.
Disclosures. The authors declare no conflicts of interest related to the content of this article.
References
- Brauer JA, Kazlouskaya V, Alabdulrazzaq H, et al. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015;151(3):278–284.
- Tanghetti EA. The histology of skin treated with a picosecond alexandrite laser and a fractional lens array. Lasers Surg Med. 2016;48(7):646–652.
- Habbema L, Verhagen R, Van Hal R, et al. Minimally invasive non-thermal laser technology using laser-induced optical breakdown for skin rejuvenation. J Biophotonics. 2012;5(2):194–199.
- Varghese B, Bonito V, Jurna M, et al. Influence of absorption induced thermal initiation pathway on irradiance threshold for laser induced breakdown.
Cutaneous sarcoid-like reaction on tattooed skin after recovery from coronavirus disease 2019
Dear Editor:
The dermatologic manifestations of COVID-19 are complex and incompletely understood. According to the American Academy of Dermatology’s COVID-19 registry, the most commonly reported cutaneous findings include morbilliform rash, pernio-like acral lesions, urticaria, macular erythema, vesicular eruption, papulosquamous eruption, and retiform purpura.1 Here, we describe a patient with recurrent cutaneous sarcoid-like reaction within a tattoo whose most recent flare occurred in the setting of prior COVID-19 infection.
A 42-year-old male patient presented with a three-week history of raised, mildly pruritic papules and nodules within the black ink of his extensive tattoos and an asymptomatic nodule within non-tattooed skin of the right forehead. The patient’s history was significant for a COVID-19 diagnosis four months prior, with mild respiratory and flu-like symptoms lasting for five weeks after diagnosis, suggesting an extended period of illness.
The patient had a similar episode of granulomatous skin reaction occurring three years prior at an outside facility, which resolved with a prednisone taper. He was seen in our clinic approximately one year later with another flare, at which time he was treated with topical triamcinolone 0.1% ointment twice daily, and a 15-day prednisone taper. A biopsy of the forehead and right arm was taken at that time, revealing non-caseating granulomas throughout the dermis with sparse lymphocytic infiltrate (Figure 1). Acid-fast bacilli, Fite, and Grocott’s Methenamine Silver stains were negative. A chest x-ray obtained during this time period was negative for an acute cardiopulmonary process. The patient was then lost to follow-up until his current presentation.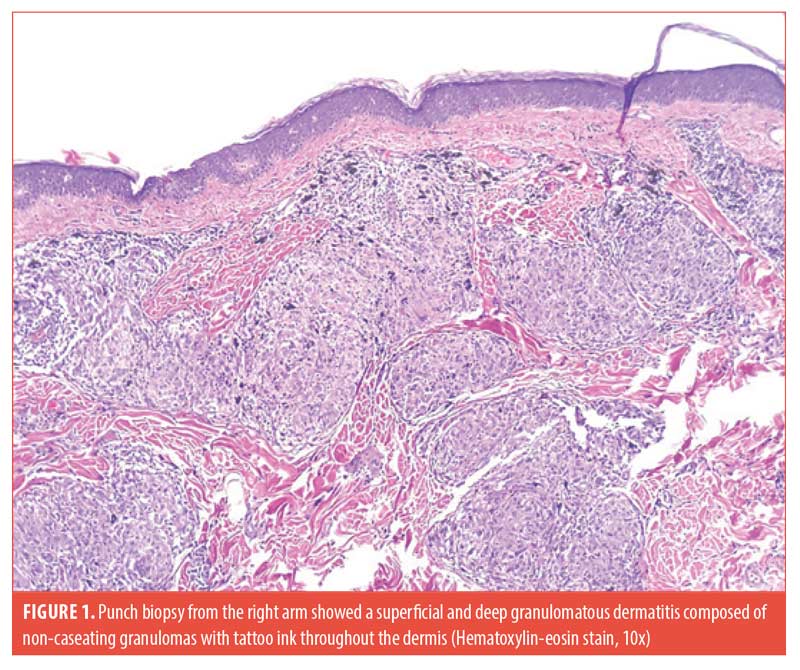
On examination, firm linear nodules and firm papules were visible within the black ink of tattoos on the bilateral arms, bilateral legs, chest, and back (Figure 2). The right forehead showed an erythematous firm nodule with prior biopsy site changes. The remainder of the physical examination was unremarkable, and the patient did not report any systemic symptoms. Serum angiotensin-converting enzyme levels and a repeat chest x-ray were unremarkable. 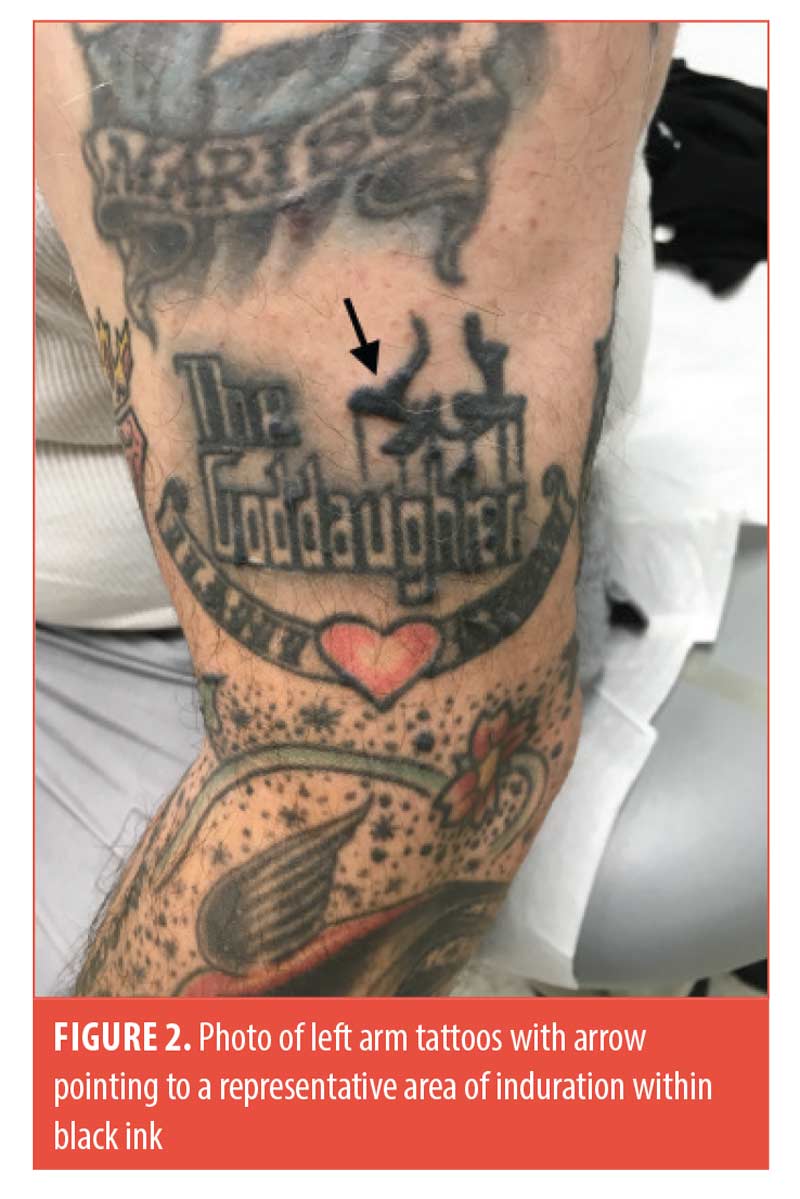
The patient was treated with a 15-day prednisone taper with incomplete resolution and flaring upon completion. He was then started on hydroxychloroquine 200mg oral twice daily, topical triamcinolone 0.1% ointment twice daily for two weeks, and an injection of 0.1mL of 5mg/mL intralesional triamcinolone to the right forehead lesion. He had initial resolution of the forehead nodule with intralesional therapy and 100-percent resolution of papules and nodules within his tattoos after three months of hydroxychloroquine therapy.
We report a novel case of a recurrent cutaneous sarcoid-like reaction within a tattoo in the setting of recent COVID-19 infection. While our patient had prior episodes with the same presentation, his disease was quiescent for two years until he recovered from a long but mild illness due to COVID-19, suggesting the immune insult from this viral infection might have triggered his recurrence. Development of a sarcoid-like cutaneous reaction has been reported previously in a patient convalescing from COVID-19 pneumonia who had no prior history of the cutaneous eruption.2 It has also been postulated that COVID-19 and sarcoidosis share certain common pathways, including angiotensin-converting enzyme-2 receptor downregulation and dysregulation of macroautophagy.2,3 As suggested by Behbahani et al,2 sarcoid-like reactions in COVID-19 patients could be a sign of disease convalescence.
Although the immunologic features of COVID-19 are not completely understood, a prominent feature of infection is transient lymphopenia, which might involve both CD4+ and CD8+ T-cell subsets.4 Several models of T-cell responses during COVID-19 progression have been described, which can differ depending on disease severity.4 Determining how lymphopenia in patients with COVID-19 impacts T-cell hyperactivation and potential immunopathology is an active area of investigation, and it is currently unknown how it may influence cutaneous sarcoidosis.4
Given the relationship between the patient’s sarcoidosis flare and COVID-19 disease remission, we postulate the granulomatous inflammation might have been incited by the recovery of CD4+ T-lymphocytes. A similar phenomenon has been demonstrated in HIV-positive patients with sarcoidosis who begin treatment with highly active anti-retroviral therapy (HAART).5 A case series by Lenner et al5 describes the recurrence of pulmonary sarcoidosis in HIV-infected patients who were successfully treated with HAART. The authors conclude that immunologic reconstitution and inhibition of viral replication might cause exacerbation of other lymphocyte-dependent disease, including sarcoid-like pulmonary granulomatous disease.5
The development of cutaneous granulomatous lesions within tattoos has been well established, but triggers for recurrence are incompletely understood. We postulate that the mechanism outlined above might have contributed to the recurrence of a sarcoid-like cutaneous eruption within a tattoo.
With regard,
Laryn Steadman, MD; Nicole Mastacouris, MS; and David Eilers, MD
Affiliations. Dr. Steadman is with Loyola University Medical Center, Division of Dermatology, Maywood, Illinois. Ms. Mastacouris is with Loyola University Chicago Stritch School of Medicine in Maywood, Illinois. Dr. Eilers is with Edward Hines Jr. VA Hospital, Dermatology Section in Hines, Illinois.
Funding. No funding was provided for this article.
Disclosures. The authors declare no conflicts of interest related to the content of this article.
References
- Freeman EE, Mcmahon DE, Lipoff JB, et al. The spectrum of COVID-19–associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118–1129.
- Behbahani S, Baltz JO, Droms R, et al. Sarcoid-like reaction in a patient recovering from coronavirus disease 19 pneumonia. JAAD Case Reports. 2020;6(9):915–917.
- Calender A, Israel-Biet D, Valeyre D, Pacheco Y. Modeling Potential Autophagy Pathways in COVID-19 and Sarcoidosis. Trends Immunol. 2020;41(10):856–859.
- Chen Z, Wherry EJ. T cell responses in patients with COVID-19. Nature Reviews Immunology. 2020;20(9):529–536.
- Lenner R, Teirstein AS, DePalo L, Bregman Z. Recurrent pulmonary sarcoidosis in HIV-infected patients receiving highly active antiretroviral therapy. Chest. 2001;119(3):978–981.

