 J Clin Aesthet Dermatol. 2020;13(12):44–48.
J Clin Aesthet Dermatol. 2020;13(12):44–48.
by Ryan Rivera-Oyola, MS; Roselyn Stanger, MD; Graham H Litchman DO, MS; Quinn Thibodeaux, MD; John Koo, MD; RIchard Fried, MD, PhD; Gary Goldenberg, MD; George Han, MD; Sylvia Hsu, MD; Leon Kircik, MD; Melissa Knuckles, MD; Andrea Murina, MD; Jeffrey Weinberg, MD; Jashin J. Wu, MD; and Mark Lebwohl, MD
Mr. Rivera-Oyola and Drs. Stanger, Goldenberg, Han, Kircik, Weinberg, and Lebwohl are with the Icahn School of Medicine at Mt. Sinai Hospital in the Department of Dermatology in New York, New York. Dr. Litchman is with the National Society for Cutaneous Medicine in New York, New York. Drs. Thibodeaux and Koo are with the University of California San Francisco in their Department of Dermatology in San Francisco, California. Dr. Fried is with Yardley Dermatology Associates and Yardley Clinical Research Associates in Yardley, Pennsylvania. Dr. Hsu is with Temple University’s Lewis Katz School of Medicine in their Department of Dermatology in Philadelphia, Pennsylvania. Dr. Knuckles is with M. L. F. Knuckles Dermatology in Corbin and Richmond, Kentucky and AdventHealth Hospital in Manchester, Kentucky. Dr. Murina is with Tulane University’s School of Medicine in their Department of Dermatology in New Orleans, Louisiana. Dr. Wu is with the Dermatology Research and Education Foundation in Irvine, California. Dr. Goldenberg is also with Goldenberg Dermatology in New York, New York. Dr. Kircik is also with Indiana University Medical Center in Indianapolis, Indiana.
FUNDING: No funding was provided for this study.
DISCLOSURES: Mr. Rivera-Oyola and Drs. Stanger, Litchman, Thibodeaux, Fried, and Han have no relevant conflicts of interest. Drs. Koo, Goldenberg, Hsu, Kircik, Knuckles, Murina, Weinberg, Wu, and Lebwohl have been speakers, advisors, consultants, and/or investigators for Ortho Dermatologics or Valeant.
ABSTRACT: Brodalumab, a first-in-class interleukin-17 (IL-17) receptor blocker, carries a black box warning for suicidal ideation and behavior, yet it is also one of the most powerful biologic agents in our armamentarium. We wish to highlight three patients with moderate-to-severe psoriasis and comorbid depression who were successfully treated with brodalumab. The patients were chosen by an expert panel comprising dermatologists, psychiatrists, and psychologists. Psoriasis disease severity was measured using the Psoriasis Area and Severity Index (PASI) score. All three patients experienced PASI 100 after treatment with brodalumab (N=3). Importantly, depressive symptoms improved or resolved in two out of three patients. One patient, who had a history of psychiatric hospitalizations, required in-patient psychiatric treatment during treatment. The use of brodalumab in patients with psoriasis can provide rapid-onset improvement in both skin and depressive symptoms.
KEYWORDS: Psoriasis, depression, suicidality, brodalumab
Psoriasis is a chronic, inflammatory skin disorder known to affect 2 to 4 percent of adults in the United States.1 Recent studies have suggested that there are more than 125 million individuals affected by this disease worldwide.2 There is no predilection for affecting one sex over the other, and psoriasis is seen more frequently with advancing age.3 Even with mild disease, quality of life can be negatively affected.4 Recent studies have reported that individuals with psoriasis are 2 to 3 times more likely to develop depression compared to the general population.5,6 In addition, studies have shown that if left untreated, depression in patients with psoriasis or psoriatic arthritis can lead to higher rates of suicidality and self-harm.7,8
Brodalumab, a human anti-interleukin-17 receptor A (IL-17RA) monoclonal antibody that inhibits the biological activity of IL-17A, IL-17F, IL-17A/F, and IL-17E, is one of the most effective biologic agents currently available for psoriasis.9 Its efficacy has been shown to be superior to ustekinumab in two Phase III clinical trials, with Psoriasis Area and Severity Index (PASI) 100 response rates at Week 12 being significantly higher with brodalumab than with ustekinumab (AMAGINE-2: 44% vs. 22%; AMAGINE-3: 37% vs. 19%; p<0.001).10 Although brodalumab was well tolerated in clinical trials, suicidal ideation and behaviors (SIB) emerged as a potential risk.10,11 Analysis of the SIB events that occurred has demonstrated no evidence of causality between brodalumab and SIB.12 Unfortunately, many dermatologists are still hesitant to prescribe brodalumab.
Recently, an expert panel comprised of dermatologists, psychiatrists, and psychologists met to identify three cases that highlight the efficacy and safety of brodalumab in patients with psychiatric comorbidities who failed to respond to other systemic therapies. Our hope is to illustrate that psychiatric illnesses might not always be a non-negotiable reason to exclude the use of brodalumab, one of the most powerful biologic agents on the market, especially when depression is linked to the severity of psoriatic disease.
Results
Table 1 summarizes the clinical characteristics of the three patients in this case report. There were two men and one woman, with ages ranging from 28 to 63 years. Patients had failed an average of 2.6 systemic therapies before starting brodalumab.
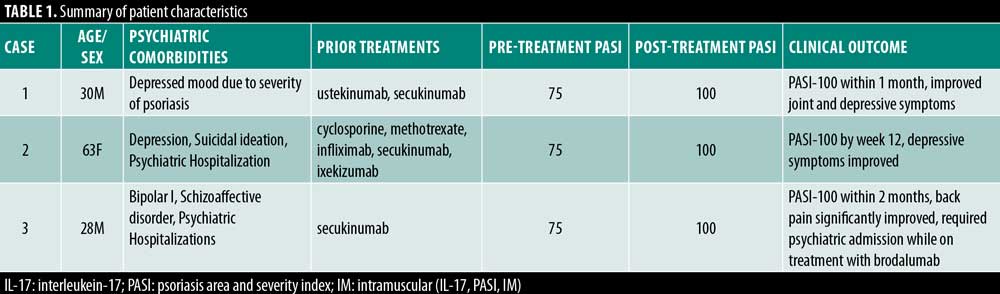
Case 1. A 30-year-old man had been suffering from severe psoriasis and psoriatic arthritis, which led him to develop a persistently depressed mood due to the severity of his disease. Notably, he never carried an official diagnosis of depression. Prior treatment with ustekinumab and secukinumab initially led to PASI 75 improvement, but then he experienced loss of efficacy. Unsurprisingly, the patient experienced worsening depressive symptoms during this time due to the treatment failure. Of note, his psoriatic lesions involved the genital area, significantly adding to his psychological distress. The decision was made to discontinue secukinumab and initiate brodalumab. Within one month, he achieved PASI 100 as well as improvement in joint pain. Importantly, he also reported decreased feelings of depression.
Case 2. Case 2 was a 63 year-old woman with a history of depression diagnosed by a psychiatrist, as well as a prior hospitalization 15 years prior for suicidal ideation. At the time of her psoriasis flare, her depression was in remission, and she was not on any antidepressive or other psychiatric medications. She had previously failed cyclosporine, methotrexate, and infliximab. She was initially placed on adalimumab with minor improvement in her psoriasis but subsequently developed paradoxical pulmonary sarcoidosis. After discussion with her psychiatrist to have close clinical followup, the patient was enrolled in the Phase III brodalumab trials. She achieved PASI 100 by Week 12, which was maintained over nine months of follow-up. After completion of the brodalumab trial, she was treated with secukinumab, achieving only PASI 50, followed by ixekizumab, achieving less than PASI 75. Brodalumab was restarted, and once again she achieved PASI 100. Importantly, she also reported improvement in her depressive symptoms following the clearance of her psoriasis.
Case 3. Case 3 was a 28-year-old Caucasian male patient with a nine-year history of plaque psoriasis who presented to our clinic for a flare of his psoriasis. The patient suffered from both bipolar I and schizoaffective disorder and had a history of four psychiatric hospitalizations for both manic and psychotic symptoms. Upon clinical examination, he was found to have moderate back pain localized to the lumbar spine. The reason for his current flare was attributed to recently being started on lithium. He had significant improved in his mental wellbeing, yet, unsurprisingly, his psoriasis flared with a body surface area (BSA) greater than 50 percent. Secukinumab was initiated with more than 80 percent improvement in skin symptoms and decreased back pain, but at, six months followup, he still had psoriatic lesions on his face, trunk, and arms. After a discussion with his psychiatrist to have close clinical follow-up, the decision was made to discontinue secukinumab and initiate brodalumab. Within two months, he achieved PASI-100. Two months after initiating treatment with brodalumab, he was hospitalized with mixed symptoms of mania, psychosis, and suicidal ideation. During the hospitalization, brodalumab was inaccessible leading to a lapse in treatment (despite the authors not recommending cessation of treatment with brodalumab). He returned to our clinic four months later with a severe flare of psoriasis with significant facial involvement and BSA greater than 15 percent. Both the dermatologist and psychiatrist agreed on restarting brodalumab.
Table 1 highlights the clinical characteristics of three patients with psychiatric comorbidities who were successfully treated with brodalumab. All three patients achieved PASI 100 clearance, including the patient who was simultaneously taking lithium. Importantly, all three patients reported improvement in their psychiatric symptoms. Case 3 did require a psychiatric admission while on treatment with brodalumab, but he had a history of four psychiatric hospitalizations before starting treatment and the authors did not recommend cessation of treatment with brodalumab during the admission; rather, there was a lapse in treatment for logistical reasons.
Discussion
With the discovery of newer classes of IL-23 and IL-17 inhibitors for the treatment of psoriasis, unprecedented rates of symptom resolution are being observed.13 Early aggressive therapy with effective biologic agents has the potential to slow disease progression, reduce inflammation, and improve long-term patient outcomes.14 Fortunately, we have many treatment options from which to choose. When selecting a systemic therapy for patients with severe psoriasis, there are several considerations: safety, efficacy, and how quickly the patient desires improvement. Safety should always be our top priority for patients, and we wish to demonstrate that brodalumab can safely be used in patients with known psychiatric comorbidities.
It is well documented in the literature that patients with psoriasis and psoriatic arthritis are already at increased risk for developing depression and SIB. Patients have been known to feel stigmatized by the psoriasis, often reporting rejection, guilt, and shame.15 Isolation, visibility of symptoms, and feelings of hopelessness have been found to have the strongest burden on affected individuals.16 In addition, patients with psoriasis and psoriatic arthritis have a higher prevalence of depression and SIB. Koo et al17 demonstrated this increased prevalence to be as high as 39.2 percent and 37.4 percent, respectively, in some study populations. A population-based cohort study analyzed the annual incidence rates of depression, suicidal ideation, and suicide attempts in 36,214 patients with psoriasis and 5,138 patients with psoriatic arthritis.6 There was an increased incidence of depression in both groups compared to the general population (incidence rate ratio [IRR] psoriasis 1.14, 95% confidence interval [CI] 1.11–1.17, P<0.0001). However, no increased risk for suicidal ideation or attempts was found (IRR psoriasis 1.19, 95% CI 0.87–1.63, P=0.2771; IRR psoriasis 1.01, 95% CI 0.85–1.20, P=0.91).6 In a Danish registry-based cohort study of 247,755 patients treated with topical, systemic nonbiological, or biologic therapies, patients with psoriasis were found to have an increased risk for developing depression (hazard ratio [HR] 1.19, 95% CI 1.17–1.20; 1.50, 95% CI 1.23–1.8; 1.50, 95% CI 1.23–1.84, respectively).5 Furthermore, the authors found that patients with more severe psoriasis and those 40 to 50 years of age had a higher risk of depression (HR 2.25, 95% CI 1.57–3.22).5 Interestingly, a recent cohort study comprised of 56,961 patients with psoriasis found that patients with psoriasis had a decreased risk for committing suicide compared to the general population (1.1 per 10,000 person years vs. 1.5 per 10,000 person years, respectively).18 One possible explanation of this finding is that patients with psoriasis are monitored far more closely for psychiatric symptoms, leading to better control and monitoring of their suicide risk. Yet, this study also found that there was a small but statistically significant increased risk of self-harm among patients with psoriasis (HR 1.15, 95% CI 1.04–1.27, P<0.006).18
The safety of brodalumab has been a controversial topic due to the SIB events observed in the initial brodalumab trials. In-depth analysis of these trials has revealed no causal association between brodalumab and SIB events.12 As previously discussed, the prevalence and risk of developing depression and SIB is increased in patients with psoriasis and might be increased in patients with simultaneous psoriatic arthritis.5,6,17,18 Importantly, the brodalumab clinical trials did not exclude patients with a prior history of psychiatric comorbidities, unlike clinical trials of other biologic agents.19,20 Although four completed suicides occurred during the studies and long-term follow-up, there was no significant increase in the rates of suicidal behavior or completed suicides in the brodalumab treatment group.12 In addition, the rate of psychiatric events was similar to that reported in other trials.21 We wish to highlight that patient selection is of the utmost importance when considering brodalumab for a patient that has both psoriasis and psychiatric comorbidities. Patients must have enough insight to be able to report a change in their mood or behavior. Patients must be trusted to have close follow-up with their psychiatrist. Furthermore, the dermatologist prescribing brodalumab should contact the psychiatrist or psychologist and should trust this provider as well. But, if this can be achieved, brodalumab can be safely used in such patients.
The efficacy of brodalumab has been well-documented in several clinical trial studies.11,22-24 At Week 12, 57.1 percent and 70 percent of patients with psoriasis treated with secukinumab or brodalumab, respectively, achieved PASI 90 (P<0.001). PASI 100 was achieved at Week 12 in 31 percent and 41 percent of patients, respectively (P<0.001).25Although brodalumab demonstrated higher rates of both PASI 75 and PASI 90 in patients without obesity in a Phase III trial (AMAGINE 1), Kaushick and Lebwohl26 found that brodalumab as well as the IL-17 class as a whole were highly efficacious drugs regardless of a patient’s weight. While PASI-75 is a significant achievement, patients can still be unhappy about their appearance and suffer from impaired quality of life if the remaining skin lesions are in sensitive body areas. For example, although Case 1 had initially achieved greater than a 75-percent improvement in his PASI score with secukinumab, his unresolved penile psoriasis greatly contributed to his depression and overall psychological burden. With the initiation of brodalumab, his penile psoriasis completely cleared, which led to resolution of his depression. Therefore, the ability to choose biologic agents that can achieve PASI 100 is substantial. This case series also demonstrates brodalumab’s efficacy in a patient simultaneously taking lithium, which is known to exacerbate psoriasis.27
When we analyze the efficacy of a biologic agent, we must also take into consideration whether a patient is biologically naïve. Failure to respond to a biologic agent can be defined as primary, in which no clinical improvement is observed from the onset of therapy, or secondary, characterized by initial therapeutic response followed by decreased efficacy and eventually relapse. One possible theory for secondary failure is that biologic agents can induce antibody formation against themselves, leading to a neutralizing effect and/or other adverse effects.28 Unfortunately, secondary failure is commonly observed, highlighting the need for additional treatment options. Here, we have demonstrated three patients with primary or secondary failure to prior biologic agents, who were able to achieve PASI 100 with brodalumab. Furthermore, the patient in Case 2, who had initially achieved PASI 100 with brodalumab during its clinical trials, was still able to achieve PASI 100 when she was restarted on brodalumab after failing two other biologic agents.
In addition to efficacy, timing is also a major factor in deciding which biologic agent is most appropriate for a patient. The patient in Case 2 achieved PASI 100 within one month, and the patient in Case 3 within two months. During the Phase III trials for secukinumab, only 57.1 percent of patients achieved PASI 90 by Week 12.25 In comparison, 70 percent of patients treated with brodalumab achieved PASI 90 by Week 12.25 For patients who need rapid-acting skin resolution, the use of IL-17 blocker should be considered strongly over other agents.
A study that surveyed both patients with psoriasis as well as healthcare providers found that the main reasons cited for not initiating or continuing biologic therapy were related to concerns about long-term safety and tolerability issues.29 Aside from the psychiatric safety profile, the use of IL-17 inhibitors as a class have proven to be safe in a variety of chronic infections. In Kaushick and Lebwohl’s study,30 the use of certain biologic agents appears to be safe in patients with chronic hepatitis B and C infections, if used after antiviral prophylaxis and with close monitoring of liver function tests and viral titers. They also concluded it was safe to use these agents in patients with latent tuberculosis.30 Even though there is no data yet on the use of IL-17 inhibitors in patients with human immunodeficiency virus (HIV), the authors found that the use of these agents is less commonly complicated with opportunistic infections compared to other treatment modalities.30
Limitations. Undoubtedly, there are several limitations to our study, most notably the small sample size. It is our hope that additional case reports or case series might be published in the future, hopefully with longer follow-up intervals. In addition, the conclusions are those of a few providers at their respective centers. Finally, prospective studies would provide useful information regarding the risk of psychiatric complications. For example, analysis of the Corrona Psoriatic Arthritis/Spondyloarthritis Registry and company data/reporting for outcomes should start yielding more information.
Conclusion
In conclusion, we hope to shed light on not only the efficacy, but the safety of brodalumab in patients with psoriasis who have a known history of psychiatric comorbidities. There are numerous articles in the literature detailing the association between psoriasis, depression, and SIB. There is also evidence that these symptoms improve once psoriasis resolves. Furthermore, careful examination of the original brodalumab clinical trials did not reveal evidence of a causal relationship between brodalumab and suicidality. In fact, failure of multiple systemic medications might itself be associated with increased psychological distress and/or worsening of a patient’s already poor psychological state. Of course, it is important to select patients who will adhere to follow-up and close monitoring. Ultimately, in a patient with depression that is exacerbated by psoriasis, we should use highly effective biologic agents that can provide quick results. Clinicians should not shy away from the use of brodalumab or other IL- 17 blockers, as they are safe, efficacious, and have rapid-onset improvement.
References
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212.
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139.
- Egeberg A, Thyssen JP, Wu JJ, Skov L. Risk of first-time and recurrent depression in patients with psoriasis: a population-based cohort study. Br J Dermatol. 2019;180(1):116–121.
- Wu JJ, Penfold RB, Primatesta P, et al. The risk of depression, suicidal ideation and suicide attempt in patients with psoriasis, psoriatic arthritis or ankylosing spondylitis. J Eur Acad Dermatol Venereol. 2017;31(7):1168–1175.
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895.
- Pompili M, Innamorati M, Trovarelli S, et al. Suicide risk and psychiatric comorbidity in patients with psoriasis. J Int Med Res. 2016;44(1 suppl):61–66.
- Papp KA, Reid C, Foley P, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132(10):2466–2469.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78(1):81–89 e5.
- Dong J, Goldenberg G. New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors. Cutis. 2017;99(2):123–127.
- Lonnberg AS, Skov L. Co-morbidity in psoriasis: mechanisms and implications for treatment. Expert Rev Clin Immunol. 2017;13(1):27–34.
- Hrehorow E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92(1):67–72.
- Bewley A, Burrage DM, Ersser SJ, et al. Identifying individual psychosocial and adherence support needs in patients with psoriasis: a multinational two-stage qualitative and quantitative study. J Eur Acad Dermatol Venereol. 2014;28(6):763–770.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31(12):1999–2009.
- Parisi R, Webb RT, Kleyn CE, et al. Psychiatric morbidity and suicidal behaviour in psoriasis: a primary care cohort study. Br J Dermatol. 2019;180(1):108–115.
- Gottlieb A, Menter A, Armstrong A, et al. Adalimumab treatment in women with moderate-to-severe hidradenitis suppurativa from the placebo-controlled portion of a Phase 2, randomized, double-blind study. J Drugs Dermatol. 2016;15(10):1192–1196.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–854.
- Gooderham M, Gavino-Velasco J, Clifford C, et al. A review of psoriasis, therapies, and suicide. J Cutan Med Surg. 2016;20(4):293–303.
- Attia A, Abushouk AI, Ahmed H, et al. Safety and efficacy of brodalumab for moderate-to-severe plaque psoriasis: a systematic review and meta-analysis. Clin Drug Investig. 2017;37(5):439–451.
- Blauvelt A, Papp KA, Lebwohl MG, et al. Rapid onset of action in patients with moderate-to-severe psoriasis treated with brodalumab: A pooled analysis of data from two Phase 3 randomized clinical trials (AMAGINE-2 and AMAGINE-3). J Am Acad Dermatol. 2017;77(2):372-4.
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled Phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020; 82(2):352–359.
- Farahnik B, Beroukhim K, Nakamura M, et al. Anti-IL-17 agents for psoriasis: a review of Phase III data. J Drugs Dermatol. 2016;15(3):311–316.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25(6):606–615.
- Atzeni F, Talotta R, Salaffi F, et al. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev. 2013;12(7):703–708.
- Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87-97.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80(1):43–53.

