 J Clin Aesthet Dermatol. 2020;13(12):41–43.
J Clin Aesthet Dermatol. 2020;13(12):41–43.
by Tara Gallagher, BS; Mark Taliercio, BS; John K. Nia, MD; Peter W. Hashim, MD, MHS; and Joshua A. Zeichner, MD
Ms. Gallagher is with the University of Notre Dame in Notre Dame, Indiana. Mr. Taliercio and Drs. Nia, Hashim, and Zeichner are with Mount Sinai Hospital in New York, New York.
FUNDING: There was no reported funding for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective: Despite common administration of intralesional triamcinolone to acne lesions, there is little published data or consensus on best practices. This study aimed to evaluate specific characteristics of intralesional triamcinolone for acne among various dermatology healthcare professionals.
Design: One hundred participants (82 attending physicians, 9 physician assistants, 8 other healthcare professionals, and 1 unidentified) from private practices and academic centers completed a 10-question survey to assess specific characteristics of intralesional triamcinolone injections, including frequency, indication, depth of injection, concentration, volume, as development of adverse events.
Results: The most common reported concentration of intralesional triamcinolone was 2.5mg/mL (52.5%). The most frequently used volume injected was 0.05mL (42.3%). In total, 61.6 percent of those surveyed answered that they inject into the center of the lesion. Additionally, 50.5 percent of respondents counsel patients on potential adverse effects of hypopigmentation and atrophy before every injection. The majority of respondents (88.8%) reported that less than one percent of their patients returned for adverse events resulting from triamcinolone usage, and 48.4 percent reported that atrophy lasted over six months (48.4%).
Conclusion: The data collected from this study can offer guidance on best practices in administering intralesional kenalog to patients. While consistency exists for the concentration of triamcinolone used, there was significant discordance in the volumes and depth of triamcinolone injection. Observed skin atrophy rates are extremely low, but they are long lasting when it occurred. We can use these data to refine our treatment techniques as well as improve treatment outcomes and patient satisfaction.
KEYWORDS: Triamcinolone, cyst, inflammatory papule, injection, corticosteroids, acne
Corticosteroid injections are common procedures performed across a variety of medical specialties, including dermatology, rheumatology, and orthopedics. Within dermatology, there is great disparity in concentration and volume of injected triamcinolone used for different inflammatory skin diseases.1,2 In acne, different volumes of triamcinolone injections have been recommended depending upon the size, location of the lesion, and depth of the lesions.3–6 Despite common use, there are little published guidelines or consensus on best techniques.
Intralesional steroids are an effective way of reducing inflammation of individual acne lesions. Their anti-inflammatory benefits are explained through the inhibition of phagocytosis and migration of leukocytes and monocytes, by providing a direct vasoconstrictor effect to reduce oxygen and nutrient flow to the wound, and the reduction of endothelial growth factor, thereby inhibiting fibroblast growth.3,4 While effective, intralesional steroids have been associated with potential adverse events including skin atrophy, capillary dilation, hypopigmentation, and, in high doses, Cushing Syndrome.7,8 The risk of side effects is dependent on the concentration, volume, and depth of the corticosteroid injection. Skin atrophy develops as a result of thinning across all layers of the skin.9 In the dermis, fibroblast and keratinocyte growth is inhibited, extracellular matrix proteins are disrupted, and lipid production is decreased. Moreover, the size and number of fat cells are reduced with separation of fat cells by hyaline material.10,11 Side effects might be more common with the use of long-acting corticosteroids, such as triamcinolone.12
In the following, we report results of a survey designed to gain insight on real-world use of intralesional steroids in acne among dermatology heathcare professionals. Standardization of injections is important to improve patient outcomes and minimize potential side effects.
Methods
A 10-question online survey was completed by 100 dermatology healthcare professionals through the online service Survey Monkey.
Results
Of the 100 respondents, 82 were self-identified as board-certified dermatologists, nine as physician assistants, eight as other healthcare professionals, and one unidentified. Almost 88 percent of respondents have been in practice for over 10 years, 11 percent in practice from 5 to 10 years, and one percent for less than five years.
The most commonly injected lesions were cysts (94.9%), inflammatory papules (60.2%), and pustules (23.5%). No respondents reported use of intralesional triamcinolone for comedonal lesions.
A 2.5mg/mL concentration was most commonly reported (52.5%). Other concentrations included (Figure 1A) 4.0mg/mL (11.1%), 2.0mg/mL (11.1%), 1.0mg/mL (10.1%), 3.0mg/mL (7.1%), 1.5mg/mL (3.0%), 5.0mg/mL (3.0%), 3.5mg/mL (2.0%).
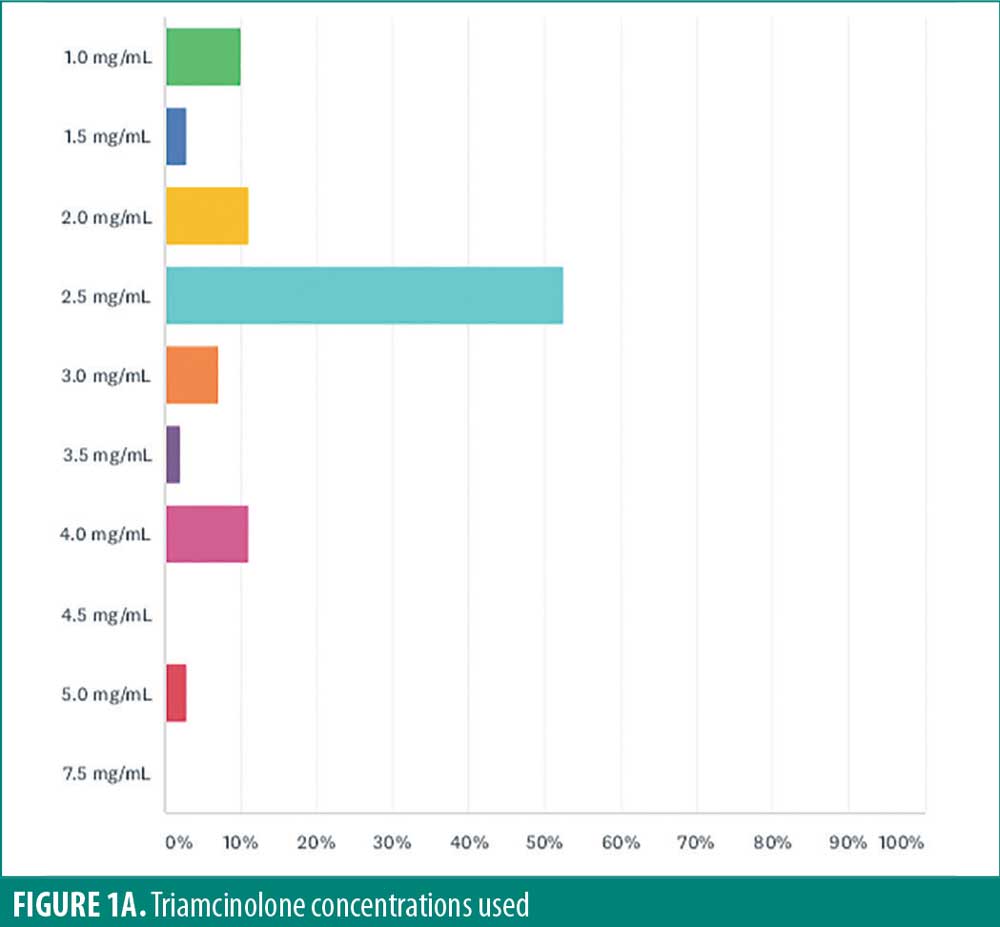
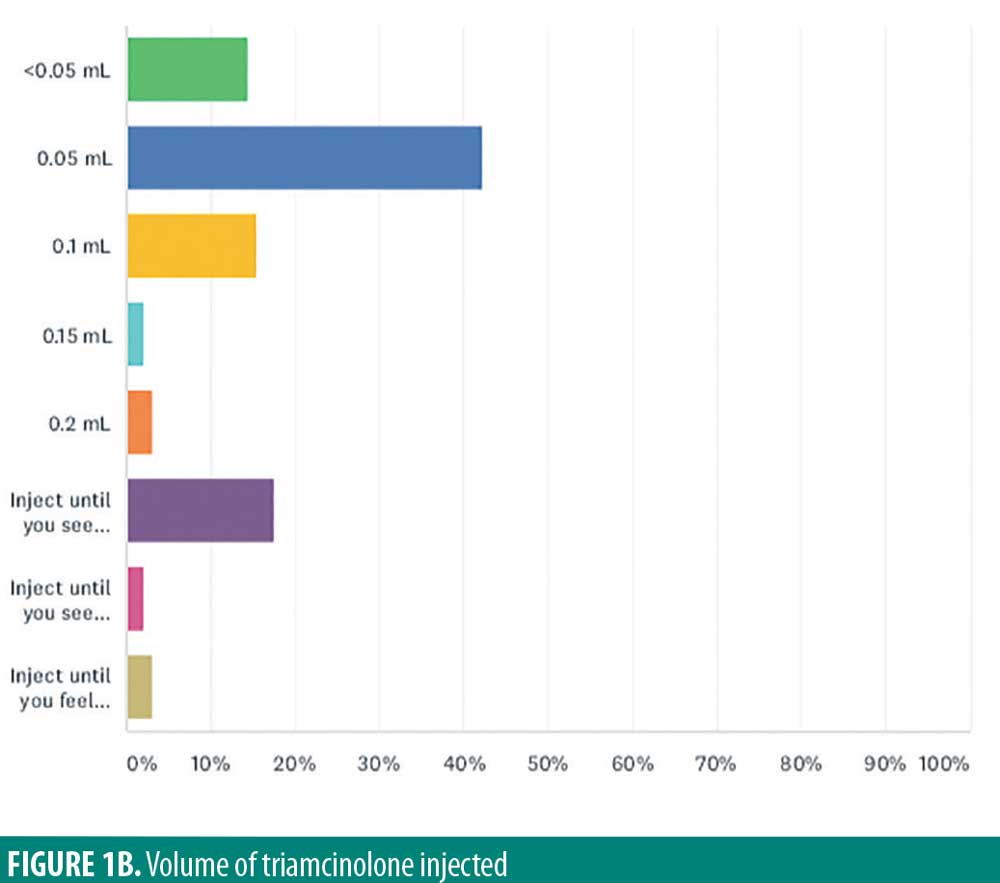
Volume of triamcinolone injected per lesion varied among respondents (Figure 1B), with the most frequent volume of 0.05mL (42.3%). Other volumes used were 0.1mL (15.5%), less than 0.05mL (14.4%), 0.2mL (3.1%), and 0.15mL (2.1%). Rather than adhering to a specific volume, 17.5 percent of respondents reported injection until blanching of the skin of the skin was observed, pressure was felt (3.1%), or induration was observed (2.1%).
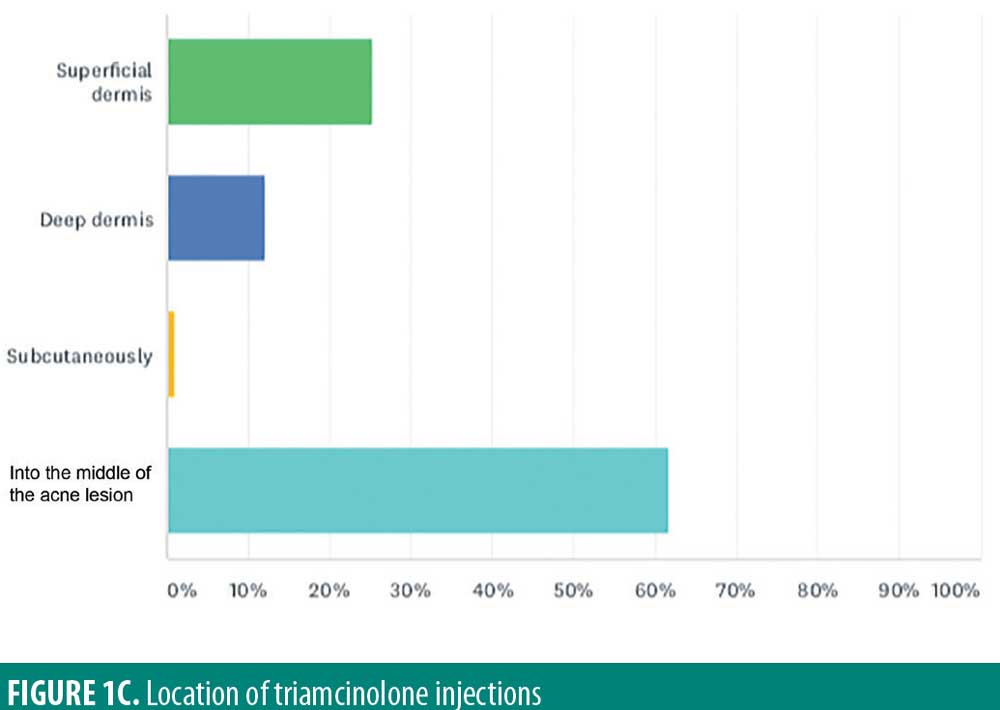
The majority of respondents reported injection into the middle of the acne lesion (61.6%). Injection at the level of superficial dermis (25.3%), deep dermis (12.1%), or subcutaneously (1%) were less frequent (Figure 1C).
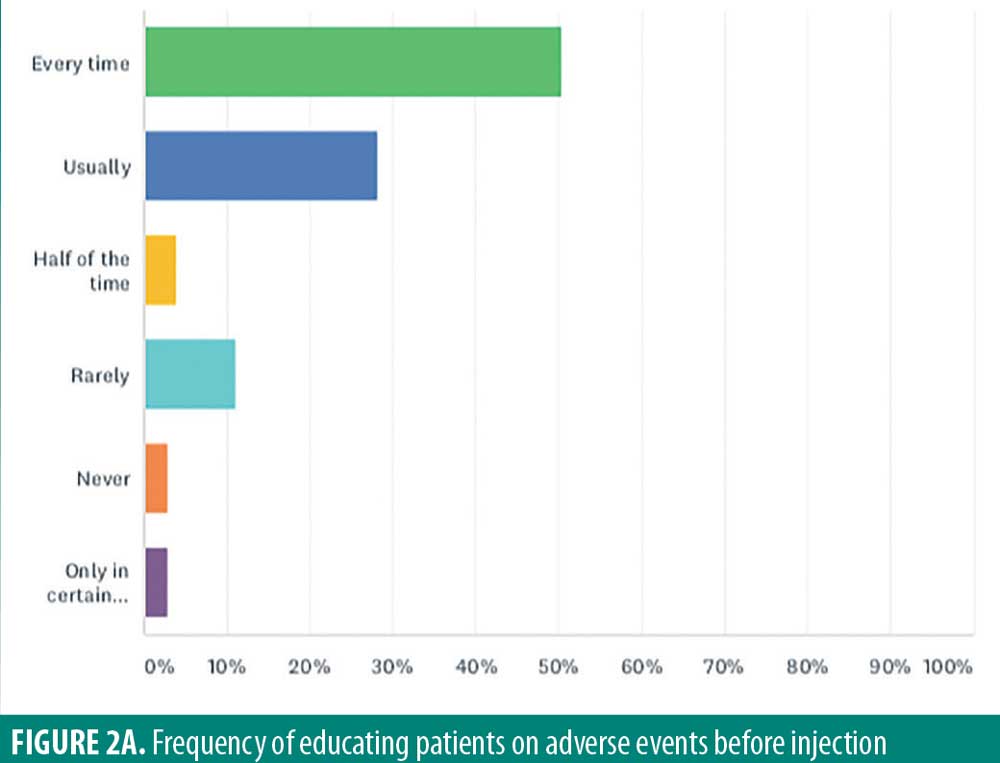
While adverse events from intralesional steroid injections might occur, only half (50.5%) of respondents warned patients about side effects such as atrophy and hypopigmentation side effects prior to injection in every patient (Figure 2A). Twenty-eight percent of respondents “usually” warned patients, and four percent warned patients “half of the time.” Eleven percent “rarely” warned patients, and three percent “never” warn patients. Finally, three percent of respondents only warn their patients about side effects “under certain circumstances.”
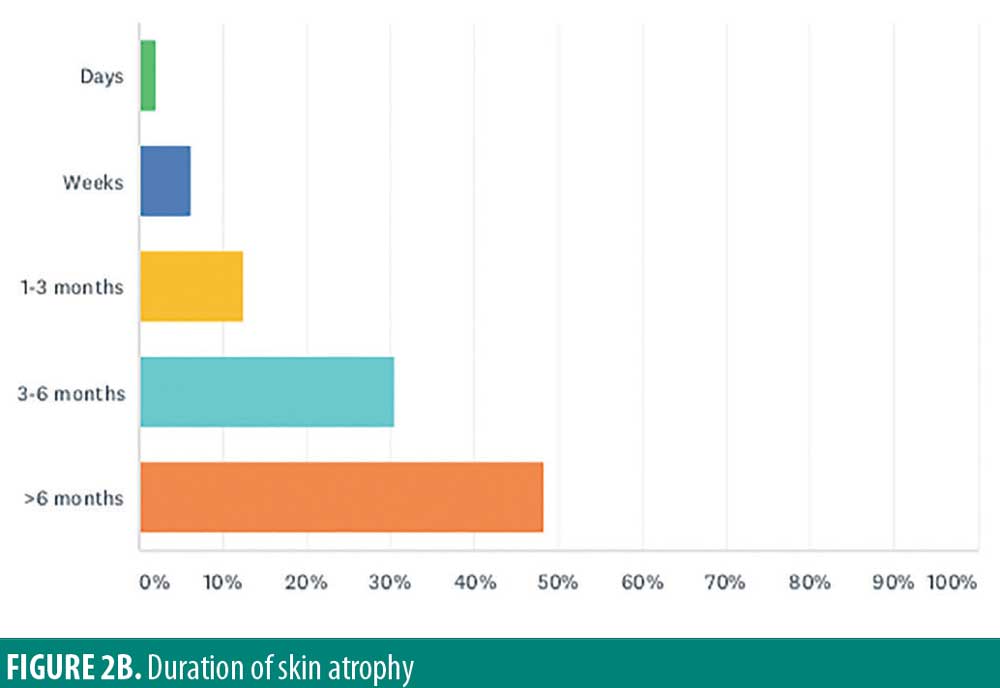
Almost half of respondents believe potential atrophy would last more than six months (48.4%). Other respondents report it lasting 3 to 6 months (30.5%), 1 to 3 months (12.6%), weeks (6.3%), or days (2.1%) (Figure 2B).
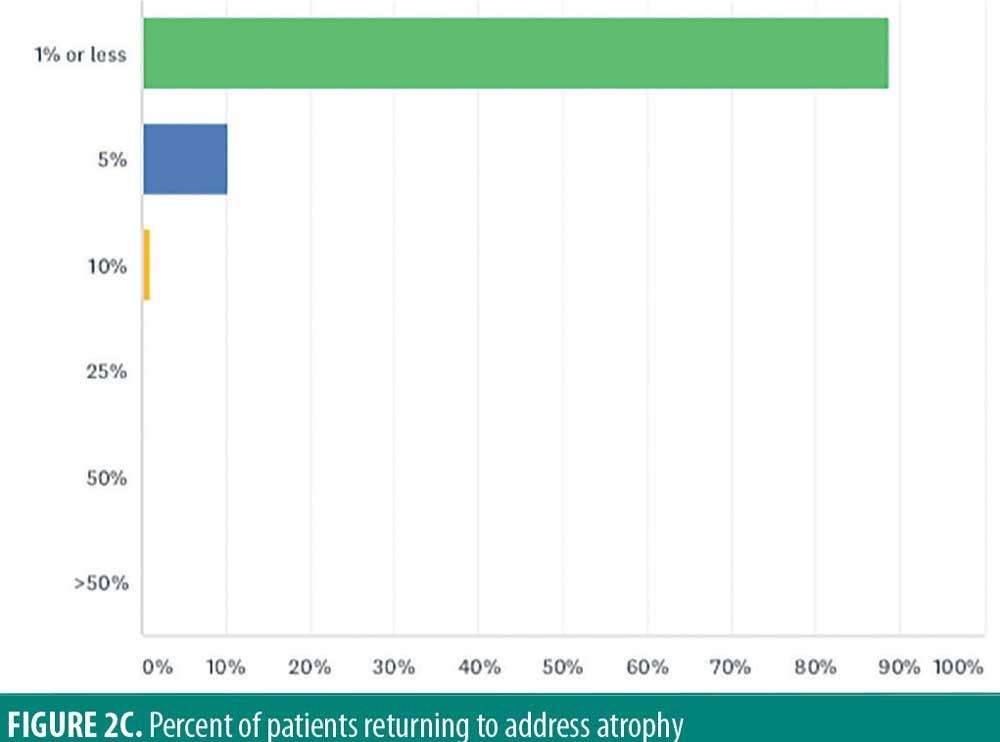
The overwhelming majority of respondents have one percent or less of patients come back for cutaneous side effects (88.8%). In total, 10.2 percent reported five percent of patients returning, and one percent reported that 10 percent of injected patients return because of atrophy (Figure 2C).
Limitations. There are several limitations to this study. The majority of respondents have been in practice for at least five years, so we do not have accurate data on how recent graduates are practicing. In addition, being self reported, there is likely a bias to minimize the incidence of experiencing adverse events. This survey offers initial guidance on best practices based on real-world opinion, but investigation, including ex-vivo testing in the lab, will be needed to validate these recommendations.
Conclusion
The results of this survey can help offer practicing dermatology healthcare professionals guidance in treating patients based on common practices. Based on these real-world results we can make the following recommendations:
In acne, intralesional steroid injections should be used to treat cysts and inflammatory papules.
A concentration of or close to 2.5mg/mL is what is commonly used. For the average lesion, a volume of around 0.05mL is what is most commonly used to inject to the center of the lesion itself.
While the reported incidence of atrophy is extremely low, the majority of respondents report it lasting at least three months.
Especially for medical–legal reasons, it is best practice to advise all patients of potential adverse events on every occasion. Since this is not currently being done by all practitioners, improvements can be made in patient care.
References
- Hayward WA, Sibbitt WL, Sibbit RR, et al. Intralesional injection of triamcinolone acetonide for subcutaneous lipoma causing musculoskeletal and neurologic symptoms. J Clin Aesthet Dermatol. 2018;11(5):38–42.
- Firooz A, Tehranchi-Nia Z, Ahmed AR. Benefits and risks of intralesional corticosteroid injection in the treatment of dermatological diseases. Clin Exp Dermatol. 1995;20(5): 363–370.
- Coppola MM, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;11:387–396.
- Perdanasari AT, Lazzeri D, Su W, et al. Recent developments in the use of intralesional injections keloid treatment. Arch Plast Surg. 2014;41(6):620–629.
- Pariser H, Murray PF. Intralesional injections of triamcinolone. Arch Dermatol. 1963;87(2):183.
- Shumaker PR, Rao J, Goldman MP. Treatment of local, persistent cutaneous atrophy following corticosteroid injection with normal saline infiltration. Dermatol Surg. 2005;31(10):1340–1343.
- Margulies SL, Morris A. Successful treatment of lipoatrophy with normal saline. JAAD Case Rep. 2015;1(6):415–417.
- Chen X, Wang G, Zeng Q, et al. Intralesional treatment with 5-Fluorouracil and steroid improves allergic contact dermatitis without causing skin atrophy and rebound lesions. Dermatitis. 2017;28(3):223–224.
- Ting P, Barankin B. Dermacase. Atrophic patches. Can Fam Physician. 2006;52(12):1547–1552.
- Dahl PR, Salla MJ, Winkelmann RK. Localized involutional lipoatrophy: a clinicopathologic study of 16 patients. J Am Acad Dermatol. 1996;35(4):523–528.
- Jang WS, Park J, Yoo KH, et al. Branch-shaped cutaneous hypopigmentation and atrophy after intralesional triamcinolone injection. Ann Dermatol. 2011;23(1):111–114.
- Skármeta NP, Hormazábal FA, Alvarado J, Rodriguez AM. Subcutaneous lipoatrophy and skin depigmentation secondary to TMJ intra-articular corticosteroid injection. J Oral Maxillofac Surg. 2017;75(12):2540.e1–2540.e5.

