J Clin Aesthet Dermatol. 2023;16(10):31–38.
J Clin Aesthet Dermatol. 2023;16(10):31–38.
by Deirdre Hooper, MD; Ruth Tedaldi, MD; Sofia Iglesia, MS; Morgann B. Young, MS; Tatiana Kononov, BS, MBA; and Alisar S. Zahr, PhD
Dr. Hooper is with Audubon Dermatology in New Orleans, Louisiana. Dr. Tedaldi is with Dermatology Partner, Inc in Wellesley, Massachusetts. Drs. Iglesia, Young, Kononov, and Zahr are with Revision Skincare in Irving, Texas.
FUNDING: This study was funded by Revision Skincare.
DISCLOSURES: Dr. Tedaldi and Dr. Hooper have performed clinical trials and consulting for a variety of organizations and serve in multiple leadership capacities. Dr. Tedaldi and Dr. Hooper served as the clinical investigator for this trial and assisted in drafting the manuscript and serve on the Revision Skincare® Scientific Advisory Board. Dr. Zahr, Ms. Iglesia, and Ms. Young are employees of Revision Skincare® and analyzed clinical photography data, statistical data, and drafted the manuscript. Ms. Kononov is a consultant for Revision Skincare® and assisted in drafting the manuscript.
ABSTRACT: Objective. The objective of this study is three-fold. Firstly, to evaluate an enhanced vitamin C serum (eVCS) and its’ combination with a retinol-bakuchiol serum (RBS) on pigmentation in vitro. Secondly, to evaluate the effect of the eVCS on skin function ex vivo. Lastly, to evaluate eVCS and RSB in the treatment of facial hyperpigmentation and overall photodamage across a range of opposing environments.
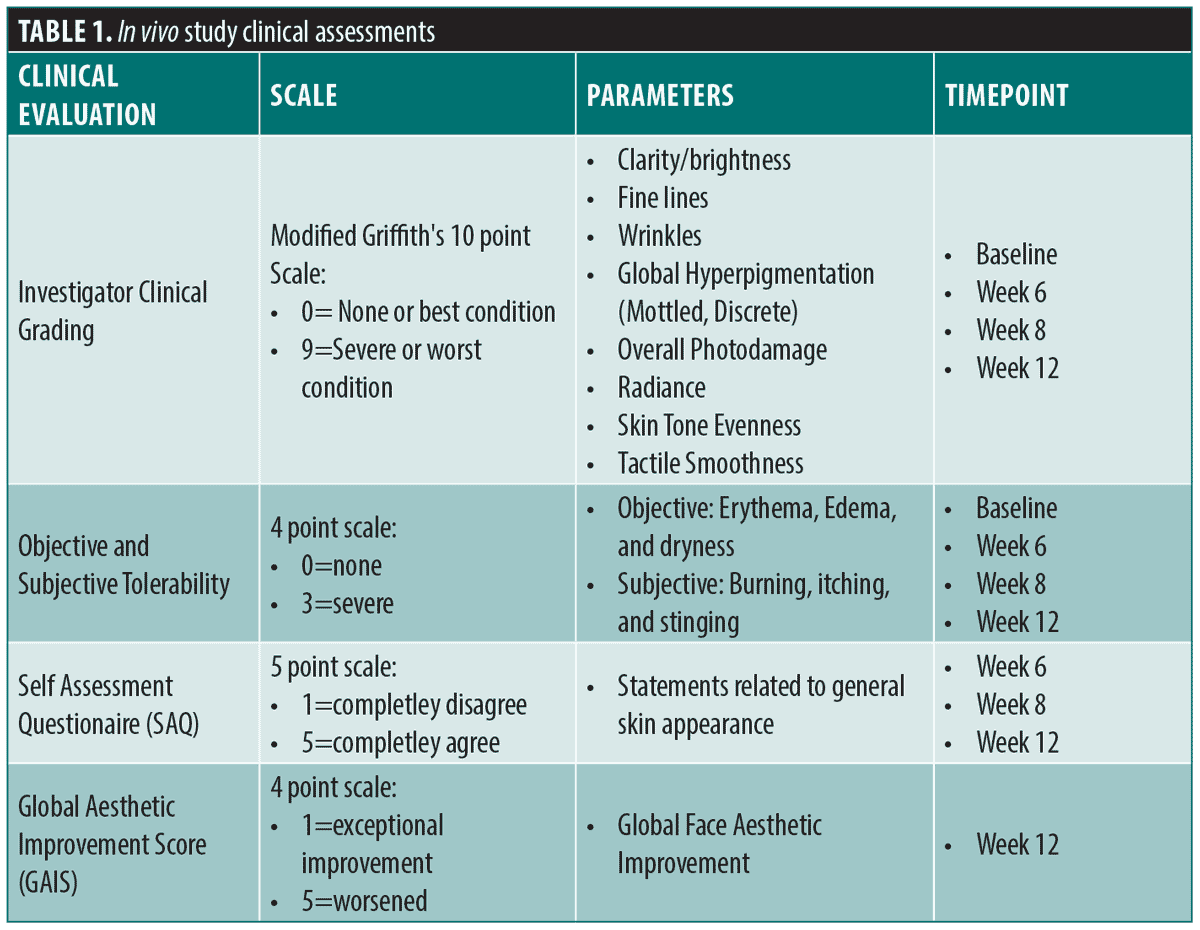
Methods. MelanoDerm™ tissues were topically treated with the eVCS, and a eVCS and RSB blend for 14 days, and then a melanin assay was performed. Surgical waste facial skin explants were incubated with the eVCS or control for five days and then fixed and stained for skin physiology and structure. A 12-week, IRB approved, study on female subjects (n=29, aged 35 to 65) with moderate global facial hyperpigmentation and overall photodamage was completed. Clinical assessment, tolerability measurements, and subject-assessments were performed baseline at Weeks 6, 8, and 12. Investigator Global Aesthetic Improvement Score was completed at Week 12.
Results. The eVCS-treated facial skin explants achieved a significant 145 percent collagen increase compared to control. The eVCS-RSB combination proved synergistic in reducing melanin compared to the eVCS alone. The eVCS-RSB combination demonstrated significant clinical improvement at all timepoints and was well tolerated. Subject responses were favorable and GAIS score of 3.0 was achieved at Week 12, indicating an improvement.
Limitations. Limitations include lack of placebo or vehicle control.
Conclusion. The product pairing, eVCS and RSB, offers patients an efficacious and well-tolerated treatment to target pigmentation and photodamage.
Clinical Trial. This study, Pro00050557, was approved by Advarra IRB (Columbia, Maryland) and submitted to ClinicalTrials.gov #: NCT05423873.
Keywords: Hyperpigmentation, photodamage, retinol, vitamin C, melanin, collagen
Repeated facial exposure to solar radiation, high-energy visible (blue) light, pollution, and other extrinsic stressors, results in hyperpigmented and photodamaged facial skin characterized by uneven skin tone, global fine lines, wrinkles, and skin roughness.1 The disruption of the skin barrier by extrinsic stressors stimulates a cutaneous inflammatory response with excess production of melanin by melanocytes, and subsequent epidermal and dermal melanin deposition.2,3 To address these skin concerns, dermatologists recommend topical vitamin C and retinoids as their gold-standard treatment.
Different forms of vitamin C are used in topical anti-aging skincare products. A water-soluble form of vitamin C, ascorbic acid (AA), is a potent antioxidant that scavenges and neutralizes free radicals, thereby protecting against ultraviolet (UV)-induced oxidative stress damage. Also, it promotes skin firmness by inducing collagen synthesis.4 Ascorbic acid is used as a treatment option for hyperpigmented skin due to its interaction with copper ions in tyrosinase, thus inhibiting melanin production.4 However, formulation and skin absorption challenges with AA exist due to its acidic, hydrophilic, and unstable nature.
A lipid-soluble form of vitamin C, tetrahexyldecyl (THD) ascorbate, overcomes the delivery and stability challenges of AA.5 THD ascorbate allows for skin-neutral pH formulation, thus reducing the undesirable skin irritation often experienced by patients treated with topical AA, which requires a low formulation pH.6–8 THD ascorbate also offers superior skin penetration compared to AA, as it penetrates the stratum corneum and is intracellularly converted to L-ascorbic acid by cytosolic esterase enzymes.9 In an in vitro study, a 30% THD ascorbate formulation penetrated a synthetic membrane (Strat-M®, EMD Millipore, Burlington, Massachusetts, USA) approximately 110 percent greater after six hours and 150 percent greater after 24 hours compared to a 20% AA formulation.10 Clinical studies demonstrated THD ascorbate to effectively improve photodamaged and hyperpigmented skin by quenching free radicals, increasing collagen production, and inhibiting melanogenesis.9,11
The combination of topical vitamin C and retinoids shows promising results in improving photodamaged and hyperpigmented skin.12,13 Retinol (vitamin A) reduces melanin production and inhibits melanosome deposition in the epidermal and dermal layers, proving to be an effective treatment option for hyperpigmented skin.14 However, skincare treatments containing retinol are unstable when exposed to light15 and irritate the skin. To overcome these drawbacks, retinol has been encapsulated to allow for a sustained release effect.16 Furthermore, encapsulated retinol paired with bakuchiol enhances retinol stability and irritation potential.17–19 Bakuchiol is a meroterpene phenol broad-spectrum antioxidant commonly found in the Psoralea corylifolia (babchi) Indian plant.14,17
In a clinical study by Herdon et al,19 female subjects with mild to moderate hyperpigmented and photodamaged skin were treated with a vitamin C serum (VCS) formulated with THD ascorbate (30% w/w) and a retinol serum (0.5% w/w) with bakuchiol (RSB). Patients applied the VCS once daily for 12 weeks, and nightly RSB application was gradually increased from every other night after two weeks from baseline. Improvements in skin tone evenness (redness), global face hyperpigmentation (mottled and discrete), overall photodamage, visual radiance, and smoothness (visual and tactile) were observed after 12 weeks. Skin dryness increased at Week 4 and Week 8 and was a result of the highly efficacious RSB. Dryness did not persist to the Week 12 timepoint, indicating skin assimilation to the RSB prior to the study’s end.
These positive results combined with innovations in plant-based antioxidants led to enhancing the VCS with a potent antioxidant blend of acetyl zingerone, hydrolyzed Eruca sativa leaf extract, and Plantago lanceolata leaf extract, as well as a prebiotic sodium carboxymethyl beta glucan. Preclinical studies demonstrated that this enhanced VCS (eVCS) protected a keratinocyte-only model of the epidermis (EpiDermTM, MatTek Corp, Ashland, Massachusetts) from high-energy visible (HEV) light and pollution-induced reactive oxygen species production and increased intracellular glutathione.10 This preclinical validation led to a clinical investigation of the efficacy and tolerability of the eVCS as a standalone treatment. A randomized, double-blinded, placebo-controlled clinical study showed that the eVCS was effective against facial wrinkles and hyperpigmentation when used twice daily for 12 weeks.10
Results of these foregoing studies led the authors to the current clinical study aimed at evaluating the efficacy and tolerability of an eVCS-RSB combination in a range of weather environments to further substantiate the superiority of the eVCS and RSB combination for a patient’s skincare routine. The authors hypothesized that, the use of the eVCS twice daily (morning and evening) and the RSB once daily (evening) would provide synergistic benefits in the treatment of moderate facial hyperpigmentation and photodamage in healthy women.
Two preclinical studies were conducted prior to the in vivo clinical study. An in vitro study was conducted to assess pigmentation changes in MelanoDerm™ (MatTek Corp, Ashland, Massachusetts) tissues treated with the eVCS alone, and the eVCS and RSB combined. An ex vivo study was conducted to examine the anti-aging effect and activity of the eVCS on facial explants. Epidermal morphology and collagen content were deemed to be appropriate markers to evaluate anti-aging effects.
The goal of the in vivo clinical study was two-fold: (1) to determine the improvement in hyperpigmentation and photodamage at Weeks 6, 8, and 12, and (2) to determine the tolerability of the skincare regimen in two cities in the United States with opposing temperatures, humidities, and sunlight hours.
Methods
In vitro. The potential of test materials to induce changes in tissue pigmentation was assessed using an in vitro tissue model of the human epidermis prepared from cultured human keratinocytes and melanocytes (MelanoDerm™, MatTek Corp, Ashland, Massachusetts). Tissues were topically treated with 50µl of the test material, including the eVCS, and a blend containing equal parts of the eVCS and RSB. A 1% (w/w) kojic acid solution was used as a positive control, and saline solution was used as a negative control (untreated tissues). Tissues were incubated at 37 +/- 2°C and 5 +/-1% CO₂. Every 24 hours, the tissues were rinsed with saline solution and fresh test material was applied. Treatments were carried out for 14 days, with each treatment performed in quadruplicate (n=4).
Melanin was extracted from the tissues and its absorbance at 405nm was determined via a 96-well plate reader. A melanin assay was then performed, where known concentrations of synthetic melanin were taken through the same extraction method, and the absorbance values were used to generate a standard curve of melanin concentrations.
Images were captured at 100x magnification using a VanGuard® Inverted Scope (VEE GEE Scientific, LLC, Vernon Hills, Illinois) with a QiClick™ LED camera connected to QCapture software (Teledyne Photometrics, Tucson, Arizona).
Ex vivo. Facial skin explants were obtained from surgical waste of a 60-year-old Asian female (Fitzpatrick Skin Type III) who had undergone blepharoplasty. Facial explants were partitioned into approximately 0.4cm by 0.4cm sections, defatted, and incubated in tissue culture medium (Dulbecco’s Modified Eagles’ Medium; Santa Cruz Biotechnology, Dallas, Texas) and 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, Missouri). Sections were placed in a six-well plate and incubated at 37°C in a 5% CO₂ humidified incubator (VWR International, LLC, Radnor, Pennsylvania). After incubation, the explants were divided into two groups: Treatment and Control. The Treatment explants were topically treated twice daily with the eVCS (2 mg/cm2), while the Control group received water. Both groups were incubated for five days and fixed in formalin. Thin (5µm) sections were processed and stained with hematoxylin and eosin (H&E) for overall morphological evaluation and with Masson’s Trichrome Stain for collagen content.
Microphotographic documentation was taken using EVOS 5000 imaging system (EVOS 5000, Thermo Fisher Scientific, Franklin, Massachusetts). Signal quantification of the blue collagen component was performed with the Celleste Image Analysis software (Thermo Fisher Scientific, Waltham, Massachusetts).
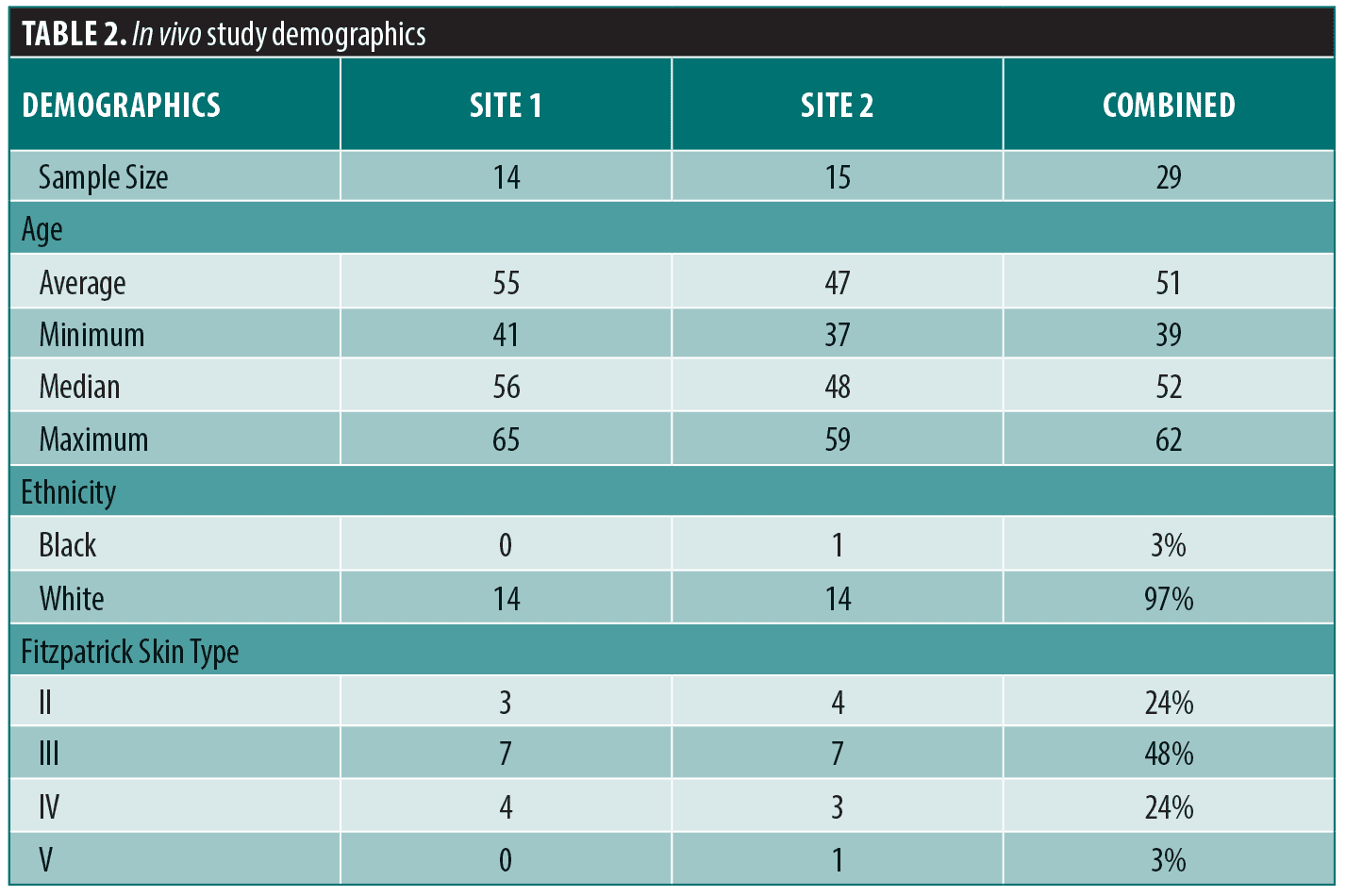
In vivo. Study design and subjects. An IRB-approved, 12-week, multi-center, open-label, cross-seasonal, cross-over clinical case study was conducted to evaluate the efficacy and tolerability of a facial skincare regimen consisting of the eVCS and RSB. Fifteen healthy female subjects aged 35 to 65, Fitzpatrick Skin Types I to VI were recruited at each clinical site, New Orleans, Louisiana (Site 1), and Boston, Massachusetts (Site 2), for a total of 30 subjects. Subjects with moderate global facial hyperpigmentation and photodamage (score 4 to 6 on a modified Griffith’s ten-point scale20) were enrolled into the study. Subjects were excluded if they were nursing, pregnant, or planning to become pregnant; had used topical retinoids within four weeks prior to baseline; had used prescription-strength skin lightening hydroquinone prior to baseline; or had undergone a cosmetic facial procedure administered by a physician or skin care professional within four months prior to baseline. Informed consent and photography release consent was obtained from all subjects prior to inclusion of the study.
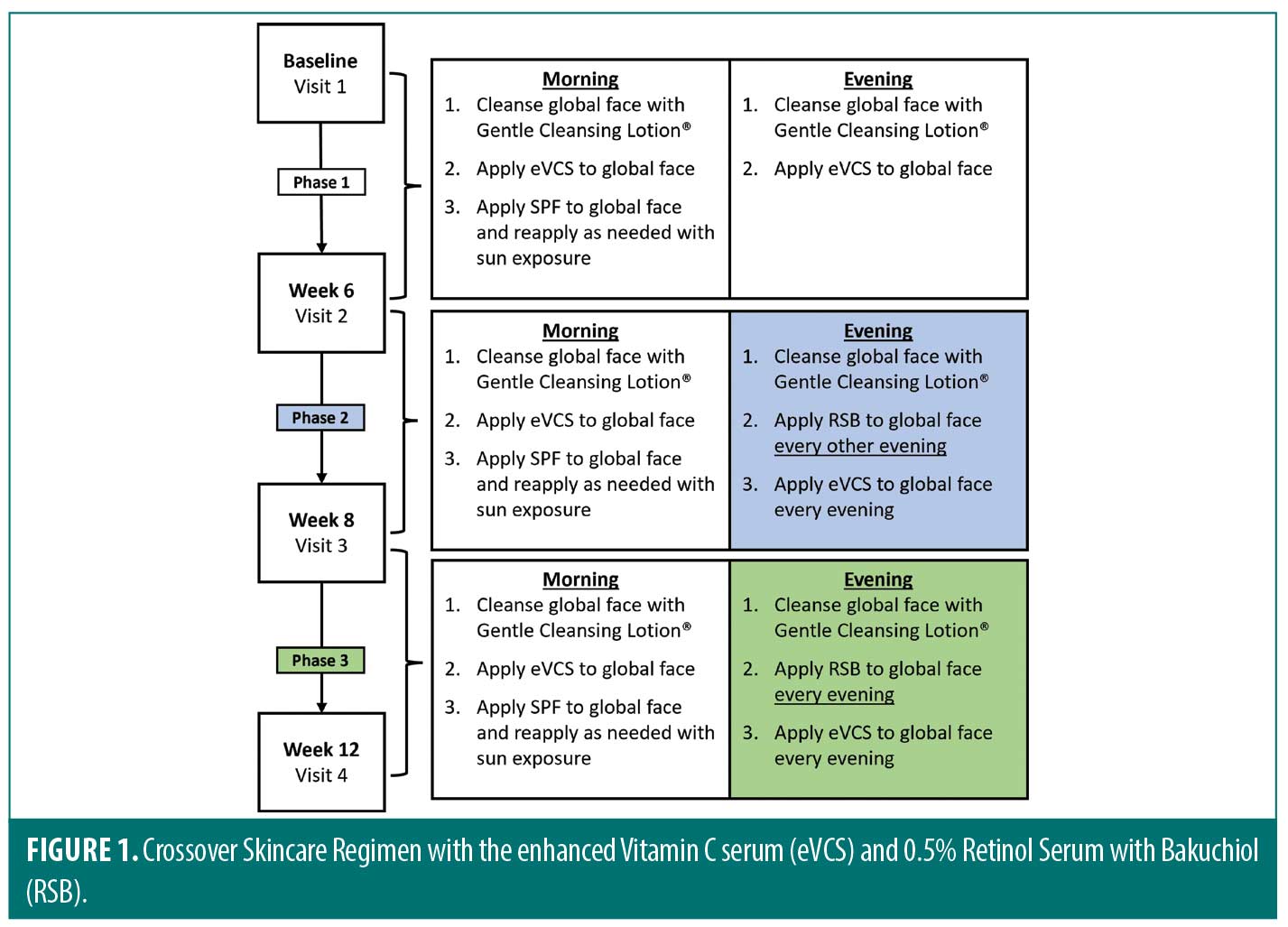
Subjects underwent a two-week washout period refraining from use of topical retinoid, vitamin C, or skin-lightening products. The cross-over skin care regimen (Figure 1) consisted of three phases. Phase 1 included the period between baseline and Week 6, Phase 2 was between Week 6 and Week 8, and Phase 3 was between Week 8 and Week 12. At baseline, subjects were given pre-weighed Gentle Cleansing Lotion (Revision Skincare®, Irving, Texas), eVCS (Revision Skincare®, Irving, Texas), and Aveeno Positively Mineral Sensitive Skin SPF 40+ (Johnson & Johnson, New Brunswick, New Jersey). During Phase 1, subjects cleansed their global face with the Gentle Cleansing Lotion morning and evening, applied the eVCS to the global face morning and evening, and applied the SPF 40 to the global face in the morning. At Week 6, the start of Phase 2, subjects were given pre-weighed RSB (Revision Skincare®, Irving, Texas) to be incorporated into their skincare regimen. During Phase 2, subjects were instructed to cleanse the entire face, apply the eVCS in the morning and evening, and apply the RSB to the global face (avoiding eye area) every other evening up to Week 8. At Week 8, Phase 3, subjects continued to follow the skincare regimen with the only modification of increased RSB application to every evening up to Week 12. Subjects were instructed to protect the treated areas from extended sun exposure during the study by reapplying sunscreen and wearing protective clothing with extended sun exposure.
Clinical evaluations and assessments. Results were evaluated by four methods (Table 1). Board-certified dermatologists performed live clinical grading at all timepoints using the modified Griffith’s 10-point scale.20 Objective and subjective tolerability evaluations were performed at all timepoints, self-assessment questionnaires (SAQs) were performed at all post baseline timepoints, and a Global Aesthetic Improvement Score (GAIS)21 was performed by the investigators at Week 12.
Facial clinical photography (central, left, right views) was obtained at baseline and at Weeks 6, 8 and 12, under standard, cross-polarized, and parallel-polarized lighting (VISIA®-CR; Canfield Imaging Systems, Parsippany, New Jersey). Lastly, subjects were given a diary to document product usage during the study. Diaries were reviewed at each study visit to ensure compliance and proper application of products. Investigational products were also weighed at each study visit to ensure compliance per product usage as outlined in the protocol.
Statistical Analysis. Ex vivo study. Signal quantification of collagen component, expressed in integrated optical density, was background adjusted and converted to percent of control. A two tailed t-test statistical analysis was performed to compare treated and control groups with statistical significance interpreted with *p≤0.05 as the cutoff value.
In vitro study. Mean values for each treatment were then compared using ANOVA. A standard curve was generated by plotting the absorbance values for the synthetic melanin in their respective concentrations. The equation that best fits this curve was determined via regression analysis and used to calculate the melanin in the MelanoDerm™ tissues. The melanin concentration in the tissues is expressed as µg melanin per tissue. Statistical significance was set with *p≤0.05 was set as the cutoff value.
In vivo study. Statistical analysis included subjects who completed the study. Evaluator scores of each clinical and tolerability parameter were used to calculate the percent improvement from baseline at Weeks 6, 8, and 12 for each subject.
The percent improvement from Week 6 (when the RSB was added to the regimen) was calculated for each subject at Weeks 8 and 12. The mean evaluator scores at each time point were tested for significant differences from baseline (and from Week 6) using a paired t-test with *p<0.05 as the cutoff level.
In addition, percent subject agreement was determined for each parameter in the Self-assessment questionnaire. Subject satisfaction was considered when subjects scored parameters from 4 (slightly agree) to 5 (completely agree).
Results
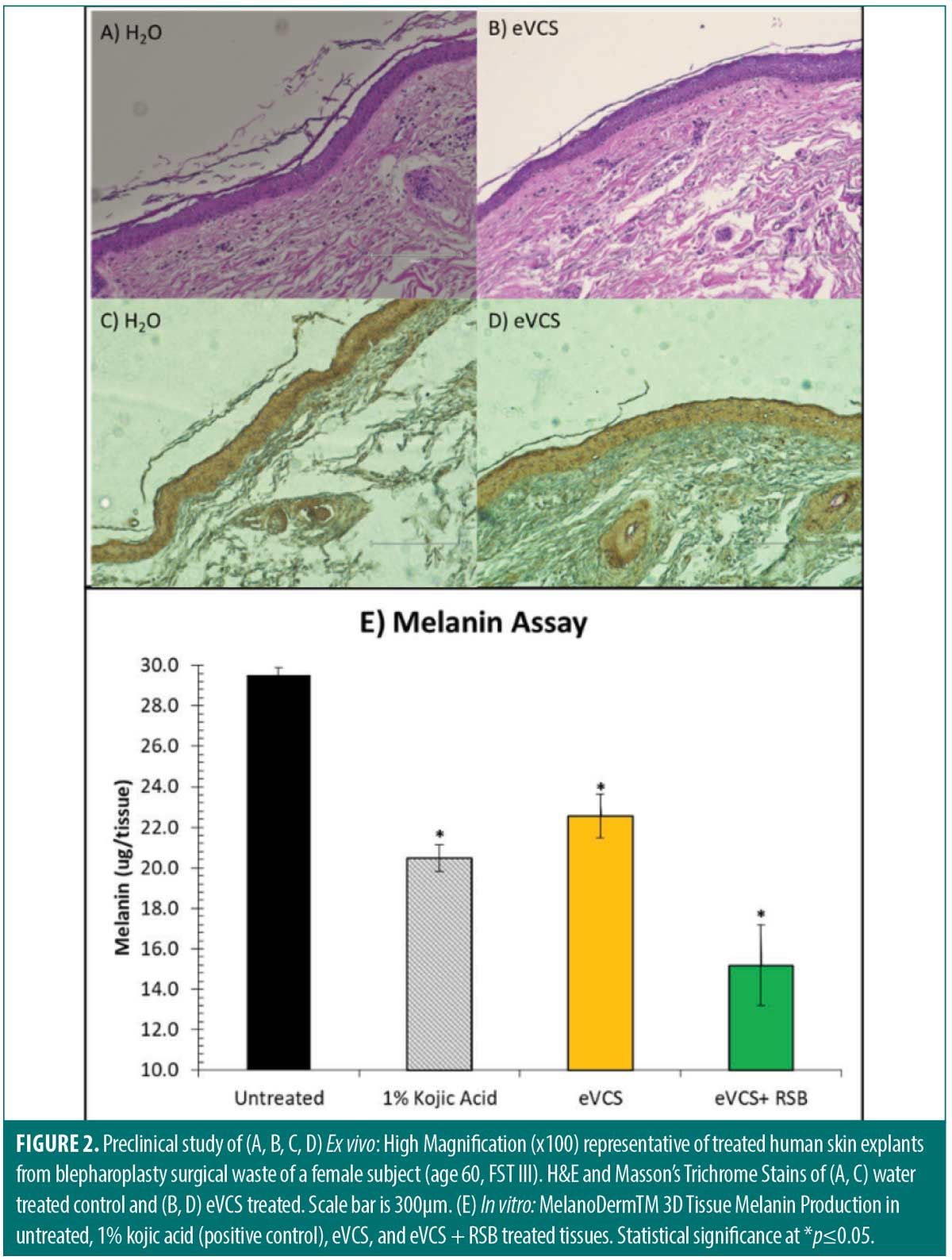
In vitro. The efficacy in the eVCS and eVCS and RSB blend to reduce melanin content on MatTek MelanoDerm™ tissues were determined. While the eVCS significantly reduced melanin content when compared to the untreated tissues (*p≤0.05), the eVCS + RSB blend provided further melanin content reduction, indicating a synergistic effect associated among eVCS and RSB combination (*p≤0.05) (Figure 2E).
Ex vivo. The efficacy of the eVCS in improving skin structure versus water control was determined. H&E staining of the water-treated controls revealed a normal stratified epithelial architecture with the columnar layer of keratinocytes superposed by supra-basal differentiating cells and a terminally differentiated nuclear stratum corneum. The keratinocytes of the stratum basale were layered on abnormally loose papillary dermis (Figure 2A). In contrast, H&E stain of the eVCS-treated tissue revealed dense proteaceous fibers that provided strong structural and mechanical continuity to the tissue, highlighting a robust dermal morphology (Figure 2B). Microscopic observation of Masson’s Trichrome Stain water-treated control tissues revealed normal epithelium with degraded dermal morphology, while the eVCS-treated tissues revealed collagen-enriched extracellular matrix (Figure 2C and 2D). Signal quantification revealed eVCS-treated facial skin explants achieved a 145-percent increase in collagen content compared to the control (*p≤0.05).
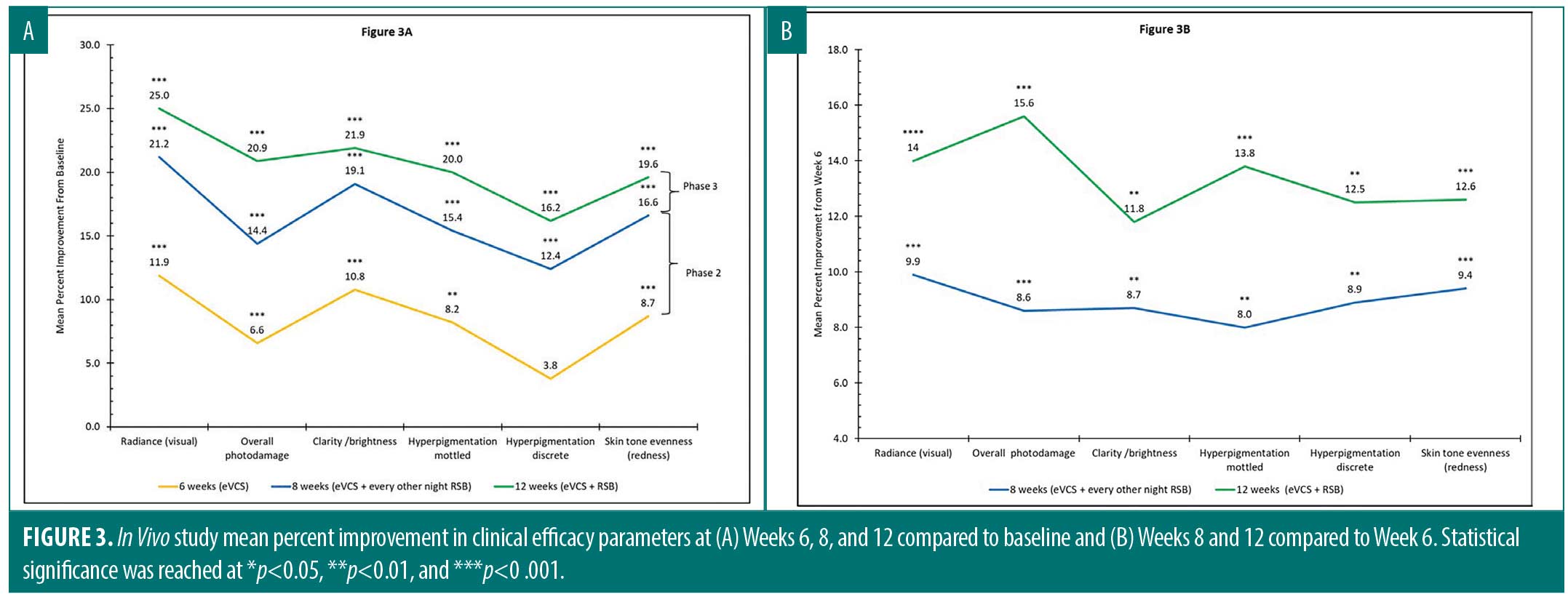
In vivo. A total of 29 female subjects (mean age=51) across the two sites completed the study. Table 2 details the study subject demographics. A progressive mean percent improvement in clinical efficacy parameters was observed over the course of the study when data was compared to baseline (Figure 3A) and to Week 6 (Figure 3B). As shown in Figure 3A, progressive and statistically significant improvement in each parameter were observed from baseline to Week 6, when subjects applied eVCS twice daily; from baseline to Week 8, when subjects added RSB every other evening; and from baseline to Week 12, when subjects increased the RSB application to every night. For example, a highly statistically significant mean percent improvement of 8.2 percent in mottled hyperpigmentation (**p< 0.01) in Phase 1 increased to 15.4 percent in Phase 2 (***p< 0.001) and then further improved to 20.0 percent (***p<0.001) in Phase 3.
As demonstrated in Figure 3B, progressive and statistically significant improvements in clinical parameters were observed from Week 6 to Week 8, when subjects applied VCS twice daily and RSB every other evening, and from Week 8 to Week 12, when subjects increased the RSB application to every night. The inclusion of RSB into the skincare regimen improved clinical efficacy parameters at Weeks 8 and 12 when compared to the eVCS alone (Week 6) (Figure 3B).
Objective tolerability evaluations of erythema, edema, and dryness were graded by the investigators at all timepoints. Facial erythema did not significantly worsen over time as expected with the irritancy potential of retinol, and as the frequency of retinol application was increased from Week 8 to Week 12 (p=1.00 at Week 8, p=0.16 at Week 12). Edema was not observed throughout the study. A statistically significant decrease in dryness was noted at Week 8 and Week 12 compared to baseline (*p<0.05), indicating improved skin moisturization in both metropolitan cities.
Among subjects who applied the eVCS twice daily, 76 percent of subjects experienced no change in erythema, 14 percent showed improved (reduced) erythema, and 10 percent showed increased erythema at Week 6 compared to baseline. When the RSB was added every other evening, 83 percent of subjects experienced no change in erythema, 10 perent showed improvement (reduction), and 7 percent showed increased erythema at Week 8 compared to baseline. When the RSB was applied nightly from Week 8 to Week 12, 83 percent of subjects experienced no change in erythema, 14 percent showed improvement (reduction), and only 3 percent of subjects experienced increased erythema at Week 12 compared to baseline.
Regarding subjective tolerability, there were no statistically significant changes in burning, stinging, and/or itching at post-baseline timepoints versus baseline. At Week 6, after twice-daily use of the eVCS, there were no reports of burning compared to baseline. When RSB was added, subjective burning increased slightly. At Weeks 8 and 12, 3 percent of subjects experienced burning compared to none at baseline. When comparing Week 8 and Week 12 to Week 6, we found that those that experienced burning at Weeks 8 and 12 when compared to baseline, resolved by Week 12 when compared to Week 6. At Week 12, 97 percent of subjects reported no changes in stinging, although in 3 percent of subjects stinging worsened upon nightly application of the RSB when compared to baseline. Itching was not reported throughout the study. Although these sensations are expected to occur with increased retinol use (due to its irritancy potential), only a few subjects experienced burning and stinging, none dropped out of the study, and serious adverse events were not reported during the 12-week study.
The efficacy and tolerability of the skincare regimen for 12 weeks was subjectively evaluated by an SAQ answered by each subject. The eVCS and the RSB were well perceived and tolerated by the subjects at both sites. Each site demonstrated 93 percent of subjects’ facial appearance was more radiant and brighter at Week 12 compared to baseline. Specifically, at Site 2, 100 percent of subjects skin texture and overall skin appearance improved at Week 12 compared to baseline, and at Site 1, 93 percent of subjects experienced little to no redness and irritation at Week 12 compared to baseline.
The GAIS score was assessed by the board-certified dermatologists at Week 12. The GAIS score indicates how much global aesthetic improvement is observed at Week 12 compared to baseline. A mean GAIS score of 3.0 was achieved at Week 12, indicating an improvement. Additionally, 17.2 percent of subjects had a score of 2 (very much improved), 72.4 percent had a score of 3 (improved), and 10.3 percent of subjects had a score of 4 (unaltered improvement). Furthermore, none of the subjects worsened after 12 weeks with the skincare regimen.
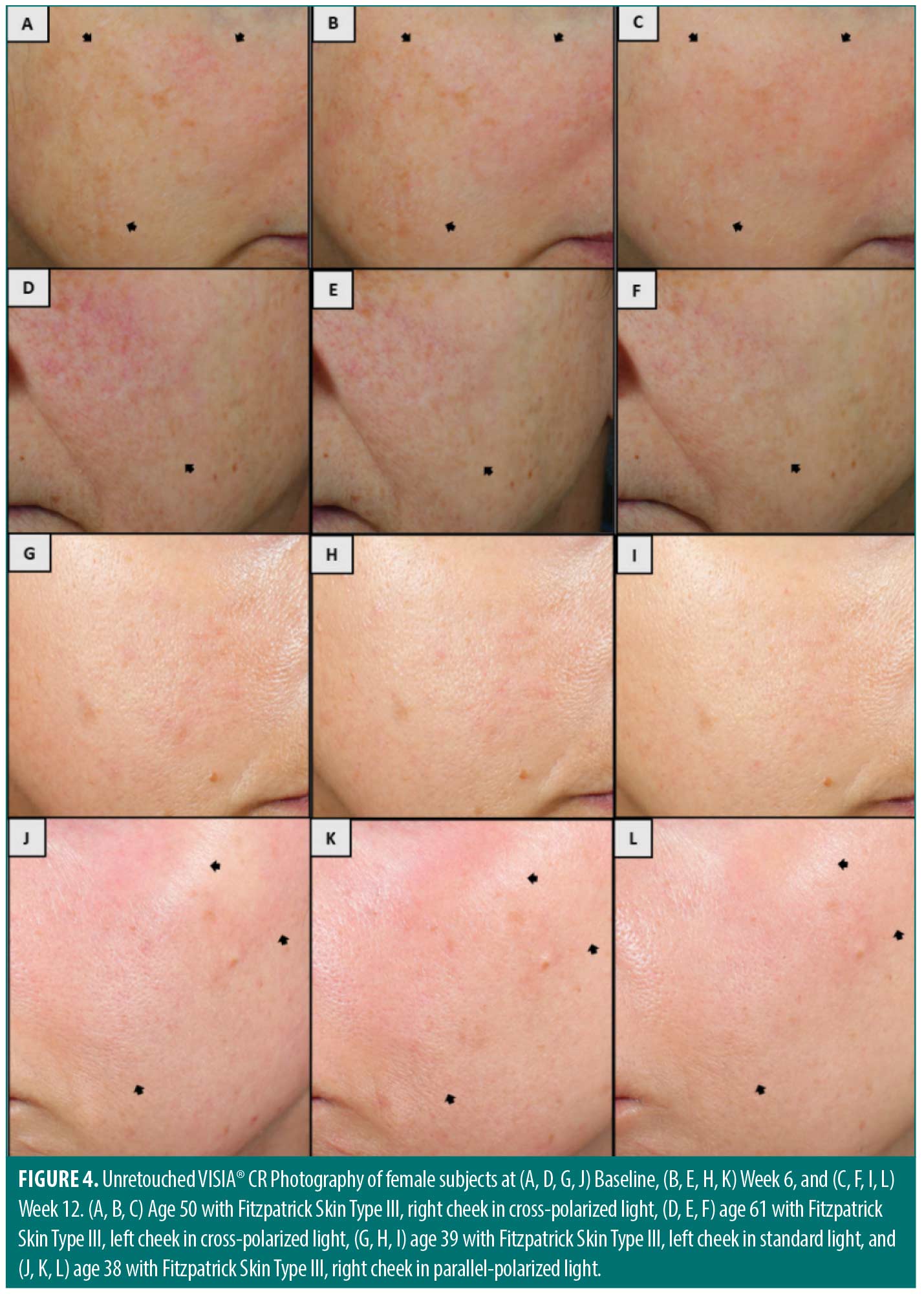
VISIA®-CR clinical photography performed at both clinical sites corroborate clinical grading assessments (Figure 4). Unretouched, cross-polarized photographs reveal improvements in hyperpigmentation in a 50-year-old female subject with Fitzpatrick Skin Type III (Figure 4A–4C) and in skin tone evenness (redness) in a 61-year-old female subject with Fitzpatrick Skin Type III (Figure 4D–4F) at baseline, Week 6, and Week 12. Standard lighting photographs at baseline, Week 6, and Week 12 demonstrate improvements in visual radiance, skin clarity, and brightness under standard light in a 39-year-old female subject with Fitzpatrick Skin Type III (Figure 4G–4I) and skin tone evenness (redness) under parallel-polarized light in a 38-year-old female subject with Fitzpatrick Skin Type III (Figure 4J–4L).
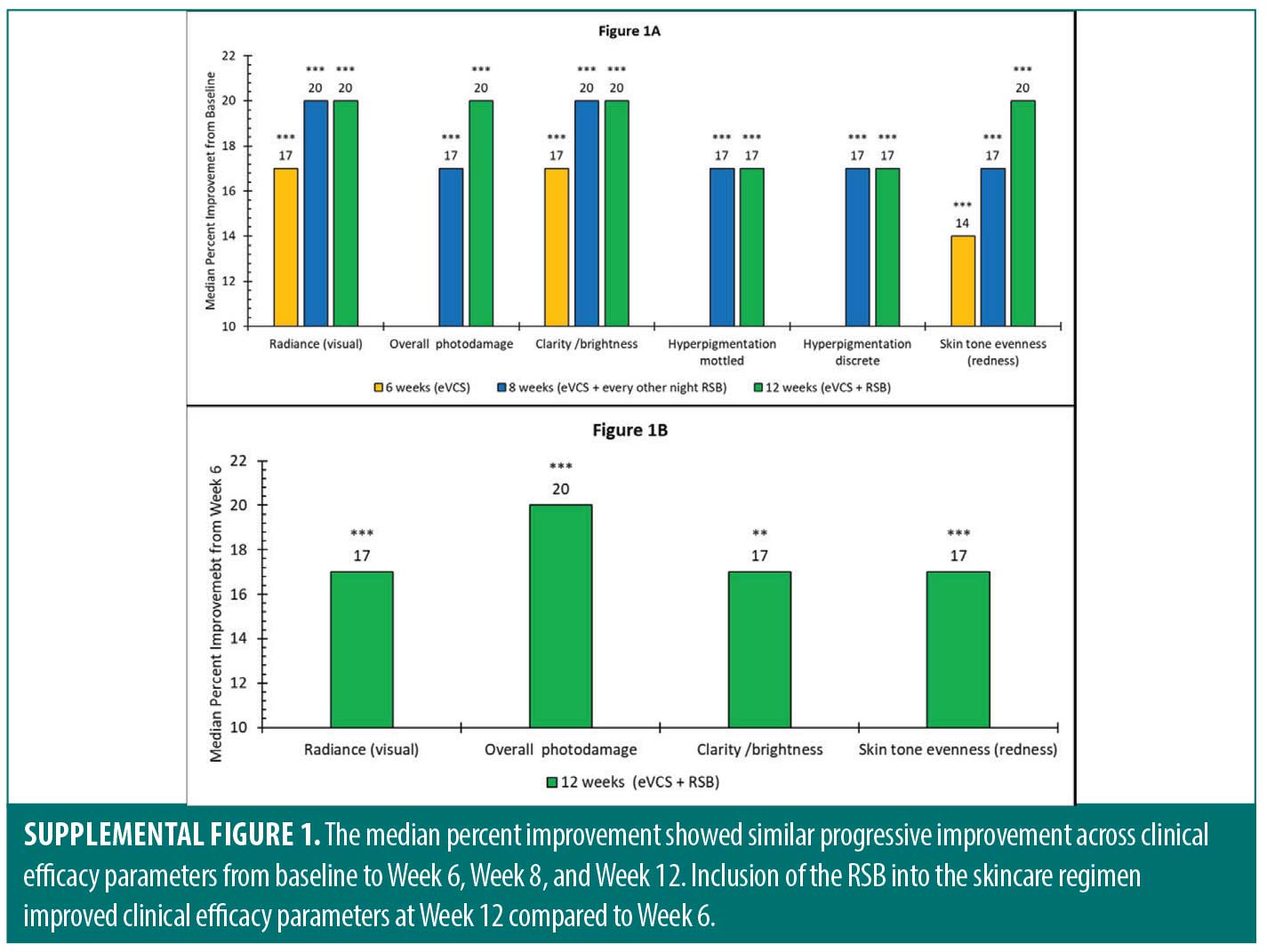
Supplementary material. Improvements in clinical efficacy parameters were also evaluated by nonparametric statistics in which the median rather than the mean percent improvement was calculated. The results are shown in Supplementary Figure 1. The median percent improvement showed similar progressive improvement across clinical efficacy parameters from baseline to Week 6, Week 8, and Week 12. Inclusion of the RSB into the skincare regimen improved clinical efficacy parameters at Week 12 compared to Week 6.
Discussion
Vitamin C and retinoid-based skincare treatments remain the gold-standard solutions for Dermatologists in treating hyperpigmentation and photodamaged facial skin. Vitamin C mitigates hyperpigmentation and photodamage by interacting with copper ions associated with tyrosinase, thereby disrupting melanogenesis and increasing collagen at the dermal-epidermal junction and in the dermis.4 In comparison, retinol (vitamin A) decreases melanosome deposition in the skin, increases cellular desquamation, and upregulates collagen production.14 When paired together in a skincare regimen, these two compounds provide strong antioxidant properties and target multiple pathways involved in hyperpigmentation and photodamage.
The present in vivo study was conducted at two locations with intense and opposing seasons. The Site 1 study was conducted during the summer in New Orleans, Louisiana, whereas the Site 2 study was conducted during the fall and winter in Boston, Massachusetts. These two locations and seasons were chosen to evaluate efficacy and tolerability in opposing environmental conditions. In New Orleans, temperatures average 89˚F in June and 92˚F in July and August, while relative humidity ranges from 75 to 98 percent.22 In Boston, temperatures reach 77°F in the fall and decrease to 36°F in the winter, with relative humidity reaching only 20 percent during these months. Daylight hours are a third factor, up to 14 hours during the summer in New Orleans compared to nine hours during the winter in Boston.23
The described environmental conditions in New Orleans and Boston may exacerbate facial skin aging; they may also impact product efficacy and tolerability, especially skincare products that include retinol. Patients with hyperpigmentation and photodamage require a skincare treatment regimen that produces clinical improvement and is tolerable. The eVCS and RSB regimen in the present study includes potent antioxidants that target multiple pathways for improving overall skin health and appearance.
The preclinical and clinical results suggest that the eVCS, formulated with 30% (w/w) THD ascorbate, enhances the structural integrity of extrinsically aged skin. Specifically, the improved dermal morphology and increased levels of collagen seen ex vivo and the reduced melanin content seen in vitro correspond to the highly statistically significant improvements in overall photodamage shown in the clinical study after six weeks of twice-daily use with the eVCS. These effects could be attributed to the ability of THD ascorbate to penetrate the skin and target the DEJ and dermis.10 Technology within the eVCS consisting of THD ascorbate (30% w/w) combined with acetyl zingerone, hydrolyzed Eruca sativa leaf extract, and Plantago lanceolata leaf extract, supports overall skin health by not only providing antioxidant protection, but also by inhibiting melanogenesis to effectively address photodamage. These results are visually manifested as improvements in fine lines and wrinkles and an improvement in skin tone evenness.
The addition of the RSB to the evening skincare regimen boosts the clinical benefits of the eVCS alone. The RSB combines 0.5% (w/w) retinol with bakuchiol to provide synergistic antioxidant benefits. Retinol and bakuchiol antioxidants work best in combination and with night-time only use, as retinol photodegrades.16 Therefore, the eVCS was used in the morning and evening, while the RSB was positioned for evening-use only. Given that skin permeability may be higher in the evening compared to the morning,24 application of an antioxidant treatment pairing at night could be particularly helpful for achieving clinical results.
Notably, all products in the skincare regimen were well-tolerated throughout the study based on both investigator and subjective assessments. When used in conjunction with a gentle cleansing lotion and sunscreen, the eVCS alone (Week 6) and the eVCS paired with the RSB (Week 8 and Week 12) were non-irritating based on statistical significance. These are important findings given that both vitamin C and retinol treatments have the potential to cause skin irritation. In fact, dryness progressively decreased over the course of the study, with statistically significant reductions in dryness seen at Week 8 and Week 12 compared to baseline. Therefore, the skincare regimen provided significant moisturization benefits despite the inclusion of retinol. The positive efficacy and tolerability results from the clinical study are further strengthened given that subjects across both sites used the eVCS and RSB in intensely different weather conditions.
Lastly, the current in vivo clinical case study utilized a crossover study design in which a retinol-based treatment was slowly added into the skincare regimen. Based on a literature review and to the best of the authors’ knowledge, this approach is unique compared to other physician-dispensed skincare companies. For example, this clinical study included a greater number of subjects compared to similar clinical studies investigating the pairing of a brightening product applied twice daily with a retinol-based product applied in the evening.25 Furthermore, sunscreen is a critical component for addressing hyperpigmentation and photodamage, and the inclusion of a basic, commercially available sunscreen in the current study design also differs from physician-dispensed skincare companies that may test numerous products from their company in a single study, thereby preventing assessments of any individual products or product pairings.26 In contrast, the stepwise approach used in the current study (Figure 1), allows for greater precision in product evaluation and comparison.
The findings in this unique cross-seasonal clinical case study demonstrate a highly efficacious and tolerable skincare treatment regimen for patients living in high humidity, high UV exposure, extreme cold, low humidity, and fluctuating sunlight hours. The eVCS paired with the RSB produced clinically meaningful results in women with moderate hyperpigmentation and photodamage over 12 weeks compared to baseline. This product pairing offers physicians a highly efficacious, well-tolerated, long-term, and non-prescription option for patients concerned with hyperpigmented and photodamaged skin.
Limitations. Limitations of this study include a lack of randomization, blinding, and placebo or vehicle control. This study included a small number of subjects at each clinical study site, and subjects were predominately White with Fitzpatrick Skin Type III. Future studies incorporating a larger and more diverse population with a randomized, vehicle control study design will be insightful in understanding the efficacy and tolerability of this dual skincare regimen across a broad-patient population.
Acknowledgements
The present study was funded by Revision Skincare®. We would like to thank Fred Wilson (Camillus, NY) for editorial assistance and performing the statistical analyses. We would like to thank the clinical team at Audubon Dermatology in New Orleans, Louisiana, and at Dermatology Partners in Wellesley, Massachusetts, for their assistance in the clinical study.
References
- Chen X, Yang C and Jiang G. Research progress on skin photoaging and oxidative stress. Postepy Dermatol Alergol. 2021; 38(6), 931–936.
- Desai, SR Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014; 7(8), 13–17.
- Vashi NA and Kundu RV. Facial hyperpigmentation: causes and treatment. Br J Dermatol. 2013; 169 Suppl 3, 41–56.
- Sanadi, RM and Deshmukh, RS The effect of vitamin C on melanin pigmentation – A systematic review. J Oral Maxillofac Pathol. 2020; 24(2), 374–382.
- Maia Campos PM, Gianeti MD, Camargo FB Jr. et al. Application of tetra-isopalmitoyl ascorbic acid in cosmetic formulations: stability studies and in vivo efficacy. Eur J Pharm Biopharm. 2012; 82(3), 580–586.
- Al-Niaimi F and Chiang NYZ. Topical vitamin C and the skin: mechanisms of action and clinical applications. J Clin Aesthet Dermatol. 2017; 10(7),14–17.
- Pinnell SR, Yang H, Omar M, et al. Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 2001; 27(2), 137–142.
- Lukić M, Pantelić I, and Savić SD. Towards optimal ph of the skin and topical formulations: from the current state of the art to tailored products. Cosmetics. 2021; 8(3) 69.
- Ochiai Y, Kaburagi S, Obayashi K, et al. A new lipophilic pro-vitamin C, tetra-isopalmitoyl ascorbic acid (VC-IP), prevents UV-induced skin pigmentation through its anti-oxidative properties. J Dermatol Sci. 2006; 44(1),37–44.
- Zahr A and Kononov T. Efficacy and tolerability of a novel antioxidant formulation containing 30 (w/w)% of tetrahexyldecyl ascorbate, a lipid-soluble form of vitamin C. Poster presented at: American Academy of Dermatology Annual Meeting. Washington, DC, USA. 2019.
- Xiao L, Kaneyasu K, and Saitoh Y. Cytoprotective effects of the lipoidic-liquiform pro-vitamin C tetra-isopalmitoyl-ascorbate (VC-IP) against ultraviolet-A ray-induced injuries in human skin cells together with collagen retention, MMP inhibition and p53 gene repression. J Cell Biochem. 2009; 106(4),589–598.
- Seité S, Bredoux C, Compan D, et al. Histological evaluation of a topically applied retinol-vitamin C combination. Skin Pharmacol Physiol. 2005; 18(2), 81–87.
- Charoo, NA Hyperpigmentation: looking beyond hydroquinone. J Cosmet Dermatol. 2022; 21(10), 4133–4145.
- Zasada M and Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019; 36(4), 392–397.
- Antoniou C, Kosmadaki MG, and Stratigos AJ. Photoaging: prevention and topical treatments. Am J Clin Dermatol. 2010; 11(2), 95–102.
- Spindler R, Luteri G, and Cureton K. The Free Library. Poly-Pore, a microparticle delivery system: this material offers sustained release, protects sensitive materials and provides multifunctional benefits in personal care formulations. (Accessed 29 Mar. 2022).
- Dhaliwal S, Rybak I, Ellis SR, et al. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br J Dermatol. 2019; 180(2), 289–296.
- Froix M, Pukshansky M and Nacht S. Retinoid formulations in porous microspheres for reduced irritation and enhanced stability. US patent 5851538. Advanced Polymer Systems, Inc. California. 1998.
- Herndon JH Jr, Jiang LI, Kononov T, et al. An open label clinical trial to evaluate the efficacy and tolerance of a retinol and vitamin C facial regimen in women with mild-to-moderate hyperpigmentation and photodamaged facial skin. J Drugs Dermatol. 2106; 15(4), 476–482.
- Griffiths CE, Wang TS, Hamilton TA, et al. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992; 128, 347–351.
- Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003; 29(6):588–595.
- Weatherspark.com [Internet]. New Orleans Summer Weather, Average Temperature (Louisiana, United States) – Weather Spark. Available from: https://weatherspark.com/s/11799/1/Average-Summer-Weather-in-New-Orleans-Louisiana-United-States#Figures-WindSpeedHeatMap
- Weatherspark.com [Internet]. Boston Spring Weather, Average Temperature (Massachusetts, United States) – Weather Spark. Available from: https://weatherspark.com/s/26197/0/Average-Spring-Weather-in-Boston-Massachusetts-United-States#Figures-Humidity
- Yosipovitch G, Xiong GL, Haus E, et al. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J Invest Dermatol. 1998; 110(1), 20–23.
- Grimes PE, McDaniel DH, Wortzman M et al. In vitro and in vivo efficacy and tolerability of a non-hydroquinone, multi-action skin tone correcting cream. J Drugs Dermatol. 2019; 18(7), 642–648.
- Herndon, JH, Makino, ET, Jiang, LI, Stephens, TJ and Mehta, RC. Long-term multi-product facial regimen in subjects with moderate-to-severe photodamage and hyperpigmentation. J Clin Aesthet Dermatol. 2015; 8(8), 16-21.