 J Clin Aesthet Dermatol. 2020;13(6):11–16
J Clin Aesthet Dermatol. 2020;13(6):11–16
by Liam Mercieca, MD, MRCP; Jessica Mangion, MD, MRCP; Jessica Cefai, BSc (Hons), MSc; Tonio Bezzina, BSc; Christoph Schwaiger, BSc (Hons); Daniel Micallef, MD, MRCP, PG Dip Derm (QMUL); Roberto Corso, MD, MRCP, PG Dip Derm (QMUL); Lawrence Scerri, MD, FRCP; Michael J. Boffa, MD, MSc, FRCP; Paul Caruana, MD, MSc, FMCPath; Eileen Clark, MD, FRCP; and Susan Aquilina, MD, FRCP
Drs. Mercieca, Mangion, Micallef, Corso, Scerri, Boffa, Clark, and Aquilina are with the Department of Dermatology at Sir Paul Boffa Hospital in Floriana, Malta. Ms. Cefai, Mr. Bezzina, Mr. Schwaiger, and Dr. Caruana are with the Bacteriology Laboratory in the Pathology Department at Mater Dei Hospital in Msida, Malta.
FUNDING: Funding was provided by the Maltese Association of Dermatology and Venereology.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Antibiotics have been widely used for the management of acne vulgaris, which has led to increased resistance of Cutibacterium acnes (C. acnes).
Objectives. We sought to determine the susceptibility profile of C. acnes, isolated from patients with acne, to different prescribed antibiotics and compare our findings with global data. The relationship between antibiotic resistance and sex, age, acne severity, presence of any affected siblings, disease duration, and previous antimicrobial treatment was also investigated.
Methods. Samples were collected from randomly selected pustular acne lesions of patients attending the Dermatology Outpatients Clinic at Sir Paul Boffa Hospital in Floriana, Malta. Samples were inoculated and incubated in anaerobic conditions until 100 cultured C. acnes samples were obtained. Antibiotic susceptibility testing was then performed using azithromycin, clindamycin, doxycycline, minocycline, tetracycline, and trimethoprim/sulfamethoxazole using the agar dilution method.
Results. The highest resistance was observed to azithromycin (18%) followed by clindamycin (16%). Resistance to doxycycline and tetracycline was only found in two percent of the isolates and there was no resistance to trimethoprim/sulfamethoxazole and minocycline. Resistance to azithromycin and clindamycin was associated with acne severity (p=0.01 and p=0.03). Resistance to clindamycin was also statistically significantly higher in patients with a history of antibiotic therapy or concurrent antibiotic therapy during the study (p=0.04).
Conclusion. To our knowledge, this is the first study documenting the susceptibility of C. acnes isolates to different antibiotics in Malta. Future research is needed to determine the clinical significance of antibiotic resistance of C. acnes.
Keywords: Cutibacterium acnes, acne, antibiotics, resistance, Malta
Acne is a common dermatological disease with a prevalence of up to 57.8 percent in Europe and which causes a significant impact on quality of life.1 Cutibacterium acnes (C. acnes) is a Gram-positive anaerobe that has a well-established role in the pathogenesis of acne vulgaris. Due to their antibacterial and anti-inflammatory properties, antibiotics have been widely used for the management of acne; this has contributed to increased antibiotic resistance.2 Different studies have shown changes in the susceptibility profile of C. acnes to a variety of antibiotic therapies worldwide; however, to our knowledge, no data are available for the Maltese population. The aim of this study was to determine the antibiotic susceptibility profile of C. acnes isolated from acne patients in Malta and compare it with established data.
Methods
Subjects. This was a cross-sectional study that recruited patients with acne vulgaris who visted the Dermatology Outpatients Clinic at Sir Paul Boffa Hospital in Floriana, Malta. One hundred C. acnes isolates were cultured over a period of 21 months between December 2015 and September 2017. The study was approved by the University of Malta Research Ethics Committee and written informed consent was obtained from all participants.
Demographic data and data regarding acne duration and severity, the presence of any affected siblings, and previous and current topical and systemic acne treatments were collected.3 Out of the global assessment scales, severity was graded using the European Dermatology Forum (EDF) acne classification scheme. While it is difficult to compare classification systems, Table 1 shows how this score compares to the Investigator Global Assessment (IGA) score.

Isolation of C. acnes. Samples were collected from randomly selected pustular lesions that were cleaned with distilled water and punctured with a sterile needle. Pus was collected using a sterile swab, placed in Amies transport medium and transported to the Pathology Department of Mater Dei Hospital in Malta. The swabs were inoculated on Columbia agar plates containing 5% defibrinated horse blood (E&O Laboratories Ltd., Bonnybridge, United Kingdom), which were incubated in anaerobic conditions at 37°C for 48 to 72 hours. Gram staining was performed on each different colony grown. The colonies that showed Gram-positive bacilli were then adopted to inoculate three new plates of blood agar, which were incubated at 37°C for 48 to 72 hours—two in anaerobic conditions and one in 5 to 8 percent CO2 conditions. The colonies that did not show Gram-positive bacilli were discarded.
After three days, the three plates were observed for purity. Any colonies producing Gram-positive rods were subcultured onto three new blood agar plates and a second incubation process was repeated. However, if a pure culture was observed, a 3-McFarland suspension of the organism being tested was prepared in 3mL of 0.85% saline solution. An identification card for anaerobic organisms (ANC card) was then placed in the suspension and inserted into the VITEK system (bioMérieux, Craponne, France) to identify C. acnes and proceed with susceptibility testing.
Antibiotic susceptibility testing. When C. acnes was identified, a 1-McFarland suspension of the pure C. acnes culture was prepared in 2mL of BACTEC™ Anaerobic/F solution (Becton, Dickinson and Company, Franklin Lakes, New Jersey) containing thioglycollate broth. This was then used to inoculate the Brucella agar plate.
Minimum inhibitory concentration (MIC) determination strips (Liofilmchem, Roseto degli Abruzzi, Italy) for azithromycin, clindamycin, doxycycline, minocycline, tetracycline, and trimethoprim/sulfamethoxazole were then placed on the inoculated Brucella medium. These were incubated in anaerobic conditions at 37°C for 48 to 72 hours. The MIC of each antibiotic was determined by reading the lowest antibiotic concentration (µg/mL) that completely inhibited the growth of bacteria.
The Clinical and Laboratory Standards Institute (CLSI) resistance breakpoints were used to interpret the MIC obtained for each isolated C. acnes strain against clindamycin and tetracycline, defined as 8µg/mL or greater and 16µg/mL or greater, respectively.4
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) also provides resistance breakpoint guidelines for different antimicrobials. However, both CLSI and the EUCAST do not provide interpretive criteria for azithromycin, trimethoprim/sulfamethoxazole, doxycycline, and minocycline. For azithromycin, the EUCAST MIC resistance breakpoint established for other Gram-positive organisms was used, defined as greater than 2µg/mL.5,6 For trimethoprim/sulfamethoxazole, the EUCAST MIC resistance breakpoint against Haemophilus was used, as recommended by other authors, and defined as an MIC of 1µg/mL or higher.5,7 For doxycycline and minocycline, since both antibiotics belong to the tetracycline class, the same breakpoints as those for tetracycline were used to interpret MIC values here, given to the class effect.8 Table 2 summarizes the MIC resistance breakpoints of the antibiotics tested as recommended by CLSI and EUCAST.

Statistical analysis. Statistical analysis was carried out using the Statistical Package for the Social Sciences, version 20 (IBM Corp., Armonk, New York). Quantitative data were presented as means and standard deviations (SD), while qualitative data were presented as numbers and percentages. Differences between qualitative data were analyzed using Fisher’s exact test. Probability (p) values of less than 0.05 were considered to be statistically significant.
Results
Throughout the study period, samples were taken from 115 patients with acne vulgaris to obtain 100 cultured C. acnes isolates. Two samples cultured two distinct colonies of C. acnes; one beta-hemolytic and one nonhemolytic. The demographics and clinical details of the participants with C. acnes cultured from acne lesions are shown in Table 3. Of those who had C. acnes cultured, 50 were male and 50 were female, with a mean age of 18.4±6 years. Most patients (55%) came from the harbor area. The majority of isolates (64%) were obtained from patients with mild to moderate papulopustular acne (EDF grade 2; IGA Grades 2–3), while 31 percent were obtained from severe papulopustular acne or moderate nodular acne (EDF Grade 3; IGA grades 3–4) lesions. The mean duration of acne was 22.9 months and 42 percent reported having siblings with acne.

Previous acne treatment. Forty-one percent of the participants with cultured C. acnes isolates had received treatment for acne in the past, with systemic antibiotics and topical antibiotics being the most common types of treatment, given to 61.0 percent and 31.7 percent of patients, respectively. Topical antibiotics were prescribed in combination with benzoyl peroxide in 9.8 percent of patients. Other treatments included topical retinoids alone (17.1%) or in combination with benzoyl peroxide (4.8%), hormonal antiandrogens (12.2%), topical azelaic acid (7.3%), and systemic retinoids (2.4%). The most common topical antibiotic used was erythromycin (69.2%), while the most common systemic antibiotic used was minocycline (68.0%), followed by clindamycin (16.0%), lymecycline (8.0%), azithromycin (4.0%), and doxycycline (4.0%). The average duration of systemic antibiotics was four months.
Acne treatment at the time of sampling. Forty-one percent of the participants with cultured C. acnes isolates were on acne treatment at the time of sampling and the two most common treatments reported were topical retinoids (51.2%) and systemic antibiotics (43.9%). These were followed by topical antibiotics (24.4%), 2.4 percent of which were prescribed in combination with benzoyl peroxide, hormonal antiandrogens (17.1%), and systemic retinoids (4.9%). The most common topical antibiotic that the participants were on was erythromycin (90%), while the most common systemic antibiotic was minocycline (61.1%), followed by lymecycline (16.7%), clindamycin (11.1%), azithromycin (5.6%), and doxycycline (5.6%).
Antimicrobial susceptibility of C. acnes isolates. Of the cultured C. acnes isolates, 22 percent were resistant to at least one antibiotic tested, with 17 percent resistant to more than one antibiotic. Eighteen percent of isolates were resistant to azithromycin, 16 percent were resistant to clindamycin, two percent were resistant to tetracycline, and two percent were resistant to doxycycline. There was no resistance to trimethoprim/sulfamethoxazole or minocycline (Table 4). The majority of resistant isolates came from patients with Grade 2 (40.9%) and Grade 3 (50%) acne.

Among those who were resistant to a particular antibiotic, 54.5 percent had received previous acne treatment, of whom the majority had received previous systemic antibiotics (31.8%) or topical antibiotics (27.3%). Systemic antibiotics were given as monotherapy to 27.3 percent of patients, while all topical antibiotics were given as monotherapy. Fifty percent of the patients were receiving treatment at the time of the sampling, with the majority taking topical and systemic antibiotics as monotherapy. Table 5 summarizes the percentage of resistant isolates in patients who had received or were receiving acne treatment at the time of sampling.
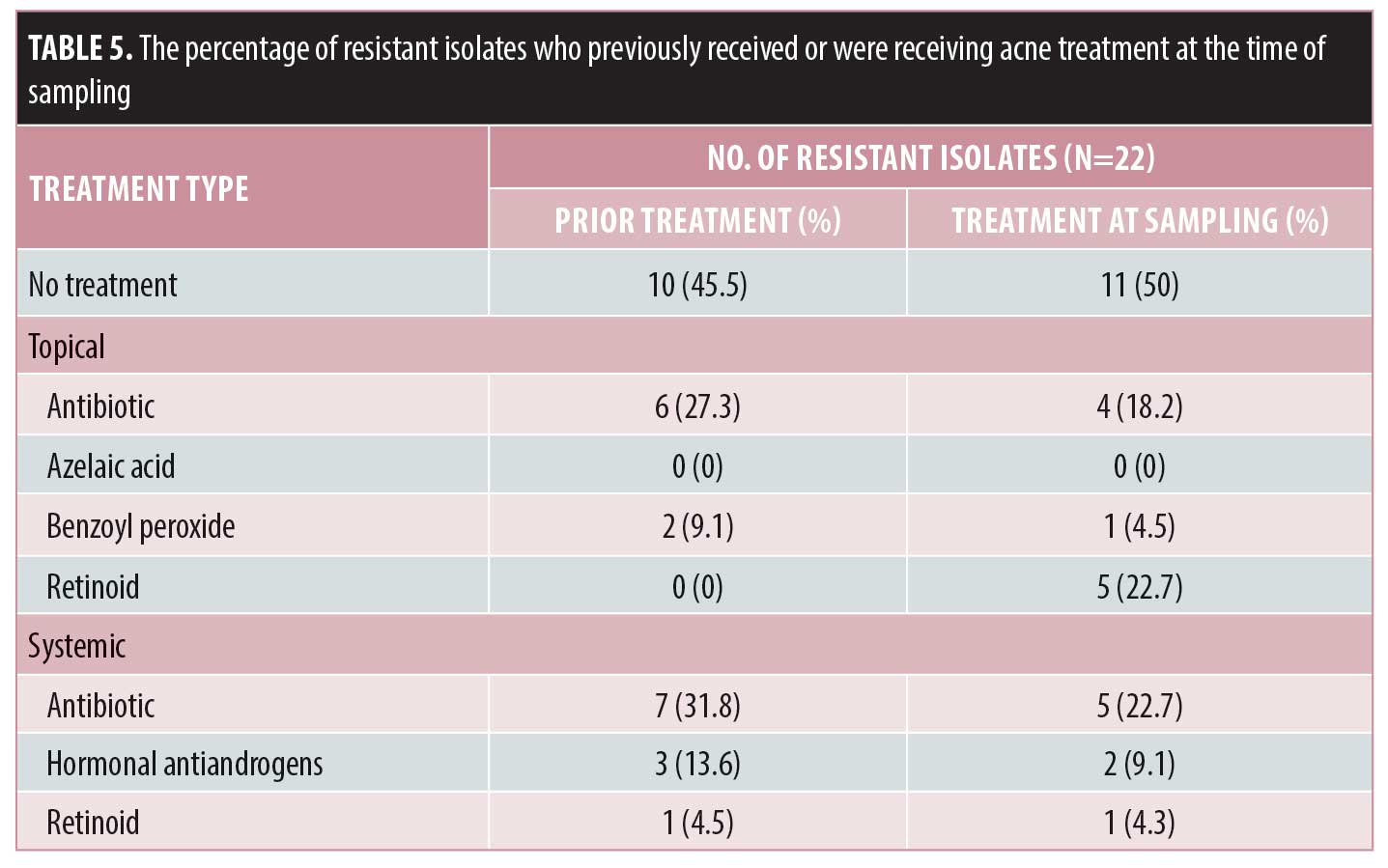
From the resistant isolates, 31.8 percent showed resistance to the same antibiotic previously or currently being used, while 45.5 percent showed cross-resistance to antibiotics different from those previously or currently being used. Almost 23 percent showed resistance to antibiotics despite not being given any previous or current antibiotic treatment for acne.
Resistance to azithromycin and clindamycin had a linear association with acne severity (p=0.01 and p=0.03, respectively) according to Fisher’s exact test. Resistance to clindamycin was also statistically significant among those who had previous or were on antibiotic therapy during the study (p=0.04). However, there was no statistically significant difference between the antibiotic susceptibility patterns of C. acnes to all tested antibiotics regarding age (younger than 18 years or 18 years and older), disease duration (less than two years or two years or more), sex, and having siblings affected by acne (Table 6).

Discussion
Resistance to C. acnes is increasing in Europe and the rest of the world. Until the late 1970s, C. acnes had no resistance to antimicrobials.9 However, with the widespread use of topical and systemic antibiotics, antibiotic resistance has become a problem. A 10-year surveillance study done in the United Kingdom between 1991 and 2000 showed that antibiotic resistance rose steadily from 34.5 percent in 1991, reaching a peak of 64 percent in 1997. In 1999, the antibiotic resistance rate dropped to 50.5 percent and then reached another peak of 55.5 percent in 2000.10
There were no previous studies determining the antibiotic susceptibility of C. acnes isolates in Malta. In our study, the proportion of resistant strains to at least one antibiotic was 22 percent, which is low when compared to in Hungary and Spain (50.8% and 93.6%, respectively).11 In another study on the antibiotic susceptibility of C. acnes across Europe, the highest resistance rate was found to be in the southeastern regions, including Greece and Italy with a resistance rate of up to 54.5 percent.12
In our sample, 47.4 percent of the resistant C. acnes isolates were resistant to azithromycin, 42.1 percent were resistant to clindamycin, and 5.3 percent were resistant to tetracycline and doxycycline. No resistance was observed to trimethoprim/sulfamethoxazole or to minocycline (despite minocycline being from the same class as tetracycline and doxycycline). The high resistance to azithromycin in Malta is most likely linked to its high usage in the Maltese population.13 Drug potency against C. acnes as well as the duration of use of the drug in the population being studied could also explain the various susceptibilities. In clinical practice, an antibiotic’s effectiveness will also be determined by its pharmacokinetics and pharmacodynamics.14
Comparing antibiotic resistance rates in Malta to those elsewhere in Europe. Resistance to clindamycin was also noted to be high in other European countries, which mirrored the rate of resistance to erythromycin. However, susceptibility of C. acnes to erythromycin was not included in our study. The low rates of tetracycline- and minocycline-resistant isolates were also similar to what was observed in most European countries. Table 7 shows the percentage of isolates resistant to different antibiotics in Malta and in other European centers.11

Comparing antibiotic resistance rates in Malta to those from the rest of the world. When comparing our results to the rest of the world, azithromycin resistance was highest in Northern Mexico (82%).7 Resistant isolates to clindamycin were also noted to be high in Egypt (66.3%), northern Mexico (36%), and Korea (26.7%).7,9,15 With regard to doxycycline, most reports show that the majority of C. acnes isolates are susceptible. Despite the susceptibility of local C. acnes isolates to trimethoprim/sulfamethoxazole, in northern Mexico, 68 percent of C. acnes isolates were resistant.7 Minocycline had the lowest resistance rate reported in literature from Europe and the rest of the world, while the highest rate was observed in Korea (10%).9 Table 8 summarizes the percentage of C. acnes isolates resistant to different antibiotics in Malta and those reported in other countries outside Europe.

Recommendations for the management of acne. In 2016, the EDF published updated guidelines on the treatment of acne according to severity.3 For comedonal acne, topical retinoid has a medium strength of recommendation. For mild to moderate papulopustular acne, the topical fixed-dose combination of benzoyl peroxide and adapalene or topical fixed-dose combination of benzoyl peroxide and clindamycin has a high strength of recommendation. For severe papulopustular and moderate to severe nodular acne and conglobate acne, isotretinoin is recommended.
In view of the increasing antibiotic resistance, the use of systemic antibiotics in combination with topical adapalene (with or without benzoyl peroxide) or azelaic acid has a medium strength of recommendation for all severities of acne except comedonal acne. The use of topical antibiotics as monotherapy is not recommended. Tetracyclines, such as lymecycline and doxycycline, are the first-line systemic antibiotics recommended in combination with topical treatment. However, they should be limited to a treatment period of three months. Lymecycline and doxycycline are preferred over minocycline in view of its potential side-effects. However, the use of doxycycline as a first-line systemic antibiotic might be unfavorable in the Maltese Islands as the sunny climate in the spring and summer months can increase the risk of photosensitivity.
Limitations. This is a study with a small sample size providing local data on resistance to C. acnes. Some participants did not remember the names of any previous acne treatment, resulting in recall bias. The fixed-dose combination product containing benzoyl peroxide and adapalene became available in Malta in 2016 and this has influenced the nature of treatment prescribed by dermatologists before and after this time.
Funding in this study was limited, restricting the number of antibiotics that could be tested, and thus, we could not provide information regarding erythromycin and lymecycline resistance. Molecular typing for the accurate identification of C. acnes strains also could not be performed given the limited funds. C. acnes was not cultured from all 115 patients. The skin sampling method used can only obtain C. acnes isolates present in the superficial stratum corneum, which could possibly explain the recovery rate of 87 percent.17 Our results on minocycline resistance might not be accurate, as studies have suggested that the drug is unstable during prolonged incubation at 37°C and thus, the estimation of the resistance to minocycline cannot be done by direct plating.10,11 However, no standardized method of performing susceptibility testing for minocycline is available.
Conclusion
This is the first study documenting the susceptibility of C. acnes isolates to different antibiotics in Malta. Antibiotic resistance is increasing worldwide; however, the rate is still low in Malta relative to in other European countries. Therefore, existing guidelines should be followed and antibiotics should not be prescribed as monotherapy. Further research on the antibiotic resistance of C. acnes in Malta is needed to determine its clinical significance and, ultimately, in formulating local guidelines to be used by dermatologists and general practitioners during their management of acne patients.
References
- Wolkenstein P, Machovcova A, Szepietowski JC, et al. Acne prevalence and associations with lifestyle: a cross-sectional online survey of adolescents/young adults in 7 European countries. J Eur Acad Dermatol Venereol. 2018;32(2):298–306.
- Schafer F, Fich F, Lam M, et al. Antimicrobial susceptibility and genetic characteristics of Propionibacterium acnes isolated from patients with acne. Int J Dermatol. 2013;52(4):418–425.
- Nast A, Dréno B, Bettoli V et al. European evidence-based (S3) guideline for the treatment of acne—update 2016—short version. J Eur Acad Dermatol Venereol. 2016;30(8):1261–1268.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 28th informational supplement. CLSI document M100-S28. Wayne, PA: Clinical and Laboratory Standards Institute, 2018.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Available at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf. Accessed May 15, 2019.
- Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27(3):419–440.
- González R, Welch O, Ocampo J et al. In vitro antimicrobial susceptibility of Propionibacterium acnes isolated from acne patients in northern Mexico. Int J Dermatol. 2010;49(9):1003–1007.
- Nakase K, Nakaminami H, Takenaka Y, et al. Propionibacterium acnes is developing gradual increase in resistance to oral tetracyclines. J Med Microbiol. 2017;66(1):8–12.
- Moon SH, Roh HS, Kim YH, et al. Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol. 2012;39(10):833–837.
- Coates P, Vyakrnam S, Eady EA, et al. Prevelance of antibiotic-resistant propionibacteria on the skin of acne patients: 10-year surveillance data and snapshot distribution study. Br J Dermatol. 2002;146(5):840–848.
- Ross JI, Snelling AM, Carnegie E, et al. Antibiotic-resistant acne: lessons from Europe. Br J Dermatol. 2003;148(3):467–478.
- Oprica C, Nord CE. European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbiol Infect 2005;11(3):204–213.
- Zarb P, Borg MA. Consumption of antibiotics within ambulatory care in Malta. Malta Med J. 2011;2:13.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamic perspectives. J Clin Aesthet Dermatol. 2011;4(2):40–47.
- Abdel Fattah NSA, Darwish YW. In vitro antibiotic susceptibility patterns of Propionibacterium acnes isolated from acne patients: an Egyptian university hospital-based study. J Eur Acad Dermatol Venereol. 2013;27(12):1546–1551.
- Mendoza N, Hernandez PO, Tyring SK, et al. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int J Dermatol. 2013;52(6):688–692.
- Omer H, McDowell A, Alexeyey OA. Understanding the role of Propionibacterium acnes in acne vulgaris: the critical importance of skin sampling methodologies. Clin Dermatol. 2017;35:118–129.
