 J Clin Aesthet Dermatol. 2020;13(6):18–21
J Clin Aesthet Dermatol. 2020;13(6):18–21
by Pezhman Mobasher, MD; Delila Pouldar Foulad, MD; Jodie Raffi, BA; Cameron Zachary, MS; Nathan Fackler, MS; Natasha Zohuri, MD; Margit Juhasz, MD; and Natasha Atanaskova Mesinkovska, MD, PhD
Drs. Mobasher, Foulad, Juhasz, Mesinkovska, Ms. Raffi, Mr. Zachary, and Mr. Fackler are with the Department of Dermatology, University of California, Irvine in Irvine, California. Mr. Zachary and Mr. Fackler are also with the Georgetown University School of Medicine in Washington, D.C. Dr. Zohuri is with the Department of Internal Medicine at UCLA-Olive View in Sylmar, California.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Changes in skin pigmentation patterns related to the fluctuation of estrogen receptors and progesterone receptors during menstruation, also known as catamenial hyperpigmentation, have been reported in several studies.
Objective. We sought to summarize the literature on catamenial skin hyperpigmentation and menses-induced exacerbations of skin pigmentation disorders.
Methods. We searched PubMed/MEDLINE and the Cochrane Skin database with the search terms menses and pigment, estrogen and pigment, progesterone and pigment, and hyperpigmentation and menses, then assessed the relevant literature on skin diseases related to nonpathological menstruation.
Results. The most commonly reported primary catamenial hyperpigmentation disorders are postinflammatory hyperpigmentation (PIH) after laser therapy and ultraviolet sensitivity (UV). The most reported chronic skin pigmentation exacerbated by menses is melasma. The literature detailing catamenial hyperpigmentation is limited to cross-sectional studies, experimental studies, surveys, review articles, case reports, and small trials, leading to a lower level of evidence.
Conclusions. Our review of the literature revealed that the most common catamenial hyperpigmentation is melasma. We also found a reported higher risk of PIH after laser therapies and UV sensitivity. Estrogen and progesterone are two of the major factors responsible for catamenial hyperpigmentation of the skin. Generally, the changes happen in the luteal phase of the menstrual cycle when the serum levels of sex hormones are at their peak. Although the exact balance of influence is controversial, most recent studies indicate that estrogen has a more prominent role than progesterone in inducing hyperpigmentation.
Keywords: Catamenial hyperpigmentation, melasma, ultraviolet sensitivity, postinflammatory hyperpigmentation
The menstrual cycle is a result of the rise and fall of estrogen and progesterone levels in the body. Changes in the balance of these hormones cause physiological changes throughout multiple organ systems. The physiology of menstruation and effect of the hypothalamic-pituitary-gonadal axis on the reproductive system organs is widely understood; however, our knowledge about the effect of these changes on the largest organ in the human body, the skin, is still limited.1,2
The skin contains both estrogen and progesterone receptors that are located in the dermis and epidermis.1,3 There is a higher number of estrogen receptors than progesterone receptors.1 As such, during the menstrual cycle, systemic changes in these hormones have a downstream effect on several characteristics of the skin. Changes in skin pigmentation patterns related to the fluctuation of these hormones during menstruation, also known as catamenial hyperpigmentation, have been reported in several studies. Understanding this condition is critical because women are menstruating now for more years of their lives compared to in the past, which might be related to puberty at younger age, proper health care during pregnancy, and increased lifespan.1 In this study, we reviewed the pathophysiology and clinical manifestations of catamenial hyperpigmentation. A thorough understanding is critical for the management of menstrual-related hyperpigmentation in women.4
Methods
A literature search was conducted in PubMed/MEDLINE and the Cochrane Skin database with the search terms menses and pigment, estrogen and pigment, progesterone and pigment, and hyperpigmentation and menses. Given the focus of this article was catamenial hyperpigmentation, only studies reporting on pigmentary changes during the menstrual period were considered for inclusion; clinically relevant reviews, clinical trials, survey studies, experimental studies, cross-sectional studies, and case reports were considered.
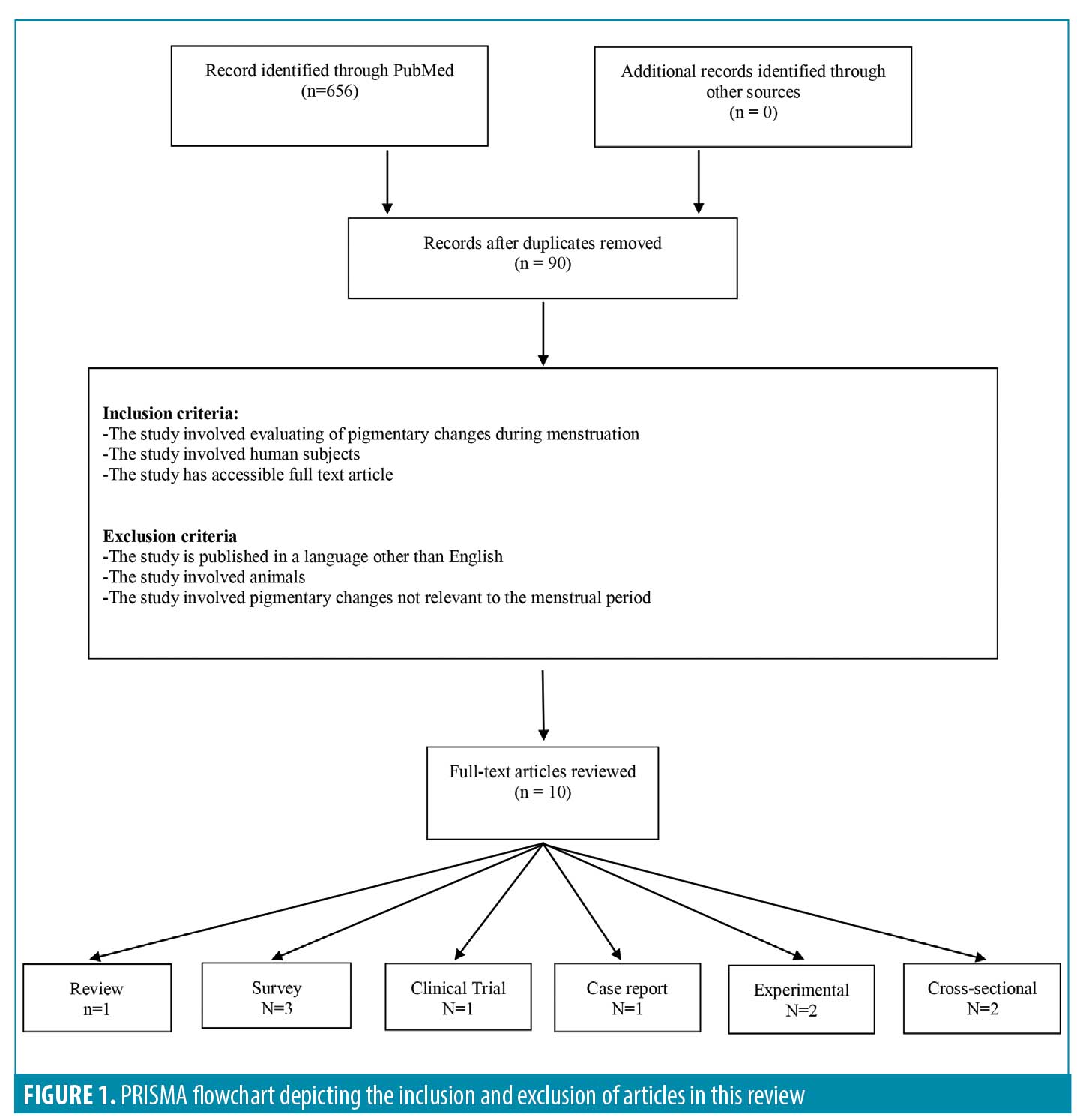
Inclusion and exclusion criteria. The inclusion criteria were studies involving evaluating of pigmentary changes during menstruation in human subjects written in the English language. Excluded studies included those that were not written in English, those that involved animals, and those that discussed any pigmentary changes not related to the menstrual period. A total of 10 full-text articles met the inclusion criteria and were included in the review (Figure 1).
Results
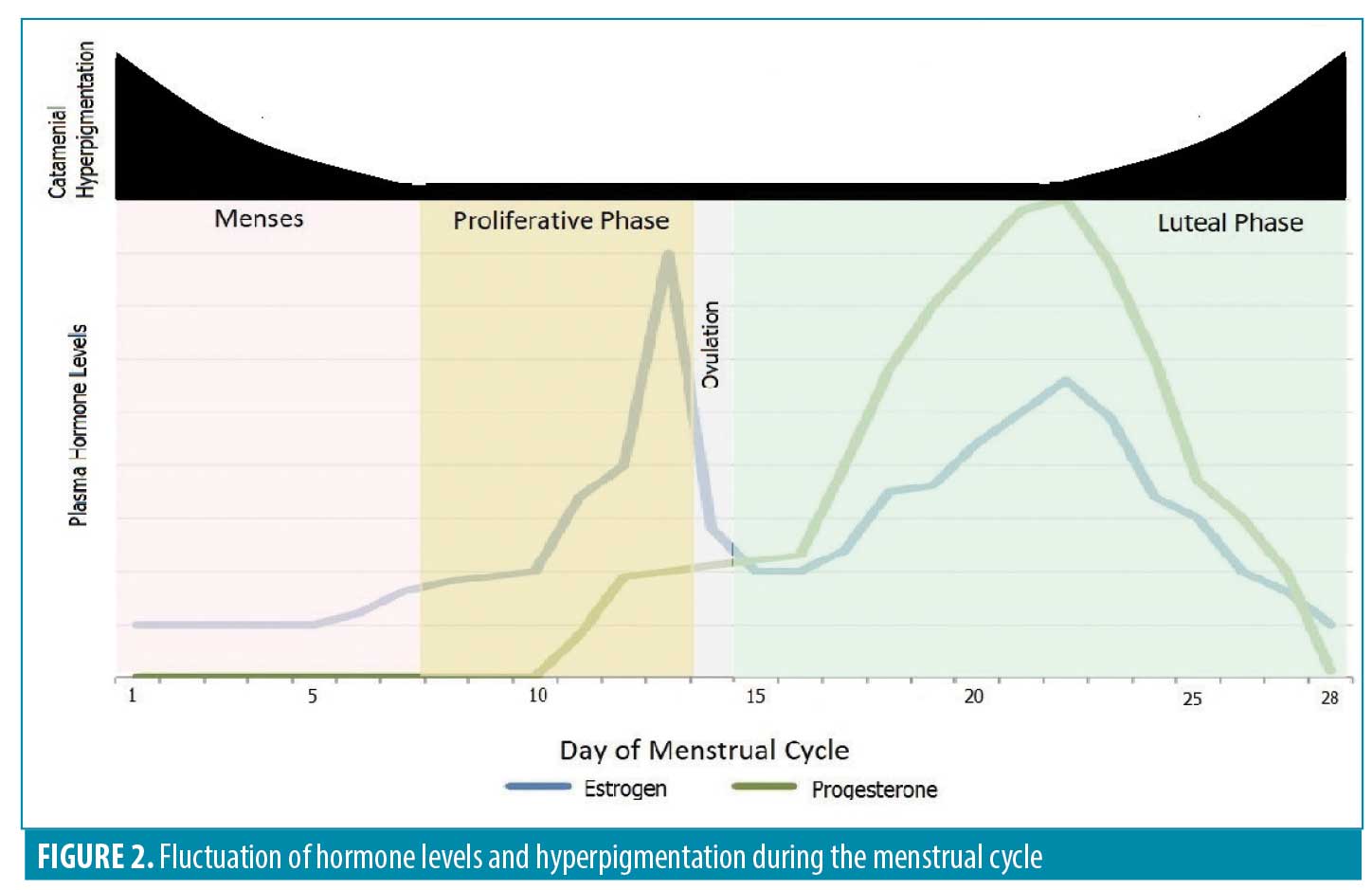
Physiology of the menstrual cycle and its affect on the skin. The onset of menstruation on Day 1 is traditionally considered the beginning of the menstrual cycle.5 The follicular phase typically occurs between Days 7 and 14, while ovulation occurs at the end of this time period. In premenopausal women, estrogen is secreted from the ovaries during the follicular phase. There are several types of estrogen in the human body, with estradiol being the strongest form. One day prior to ovulation, estradiol reaches its maximum concentration. The luteal phase then starts at Day 14 following ovulation and continues until Day 28. During ovulation, the corpus luteum develops and produces progesterone throughout the luteal phase. In the luteal phase and again five days after ovulation, serum estradiol levels rise. During the final days of the luteal phase, the amounts of estrogen and progesterone in the blood decrease, resulting in menstruation4,6 (Figure 2).
The role of estrogen and pigmentation has been studied in pregnant women with hyperpigmentation of the face, areola, perineum, and abdomen, in women taking oral contraceptives, and in children using estrogen creams.1,4,7,8 Estrogen increases tyrosinase activity, which is an important rate-limiting enzyme in the synthesis of melanin.9 Melanocytes have receptors for estrogen in their cytoplasm and nuclei; the stimulation of these receptors causes an increase in MC1R mRNA and regulates melanin synthesis.9 A recent study revealed that upregulation of a G protein-coupled estrogen receptor (GPER) by estrogen in melanocytes induces melanogenesis.10 Estrogen can also cause melanogenesis in combination with other hormones, including thyroxine and melanocyte-stimulating hormone.1,11,12
Thus, estrogen not only increases melanocyte production, but affects most other components of the skin, including the vasculature, hair follicles, and sweat glands.7,11 Melanocytes, fibroblasts, and macrophages have estrogen receptor-alpha (ER-alpha) and estrogen receptor-beta (ER-beta). Keratinocytes and hair follicles only have ER-beta.1,4,7,11,13,14
Estrogen also plays a role in collagen synthesis, barrier function, and wound healing and affects the thickness and dermal water content of the skin.6 Estrogen enhances collagen, sebum, and the production of other stratum corneum lipids.1 Estrogen also increases the water-binding capacity of the epidermis and dermis by the increment of acid mucopolysaccharides and hyaluronic acid in the dermis.1 Given the decrease of estrogen in postmenopausal women, collagen atrophy and reduced water content have been noted in this population.6
The exact role of progesterone in these pathways on the skin is still undetermined and controversial among experts. Some believe that progesterone causes an increase in the number of melanocytes and tyrosinase activity in these cells. Others point to the inhibitory role of progesterone on melanocyte proliferation.9,15 A study by Natale et al16 demonstrated that, in human models, estrogen increases pigment production and melanocytes, while progesterone decreases melanin production but does not change the number of melanocytes. Interestingly, this study revealed that estrogen and progesterone bind to a membrane-bound GPER and progestin and adipo-Q-receptor 7 (PAQR7), further supporting that these receptors, in addition to estrogen and progesterone receptors, are promising targets for future treatments of hyperpigmentation.16 It has also been hypothesized that progesterone might be responsible for the increased vascularity and sebum production seen in the second phase of the menstrual cycle.4,5 Additionally, researchers believe progesterone has immunosuppressive activity through the inhibition of monocytes.17,18
Melasma. Melasma, also known as chloasma or the “mask of pregnancy,” is a chronic hyperpigmentation disorder characterized by brown macules and patches with irregular borders involving the sun-exposed areas on the face, neck, and, sometimes the arms and the frontal chest.19,20,21 It is reported in many studies that melasma worsens during the menstrual cycle.1,11,12,22,23,24,25 Results from a questionnaire showed that 18 of 29 women (62%) showed consistent darkening of the periocular skin immediately before menstruation, and concluded that a proportion of women, especially women with dark skin and dark hair, have increased skin pigmentation of the face during the end of the luteal phase of menstruation.26 Results from another questionnaire revealed that nearly half of patients surveyed (n=63) had increased skin pigmentation during the luteal phase of menstruation and, in some cases, during menstruation.27
Several studies have demonstrated the important role that sex hormones play in the development of melasma.7,28,29,30 In melasma, estrogen and progesterone bind to ER-alpha, ER-beta, and progesterone receptors, causing downstream transcriptional changes.7,29,30
The role of progesterone in melasma is controversial. Some studies show that progesterone can worsen melasma; Chompootaweep et al31 reported that contraceptives containing the synthetic progestin levonorgestrel induces melasma and Alvarez et al32 suggested that levonorgestrel-releasing implants cause local hyperpigmentation.33 However, at a molecular level, Wiedemann et al15 showed progesterone prevents melasma by reducing the proliferation of melanocytes without any effect on tyrosinase, a melanogenic enzyme. In another study, Famenini et al35 noted that men who were on oral finasteride, a 5-alpha-reductase and progesterone inhibitor, also developed melasma.35,36
The role of estrogen in melasma is less debated than that of progesterone. There is an increase in estrogen receptors in skin affected by melasma and this increase is thought to preclude the development of melasma.29,37 The inhibition of estrogen receptors by an antagonist also leads to a decrease in melanogenesis.29,38,39 Melanocytes and keratinocytes of hyperpigmented skin also show overexpression of the protein PDZK-1, a member of the sodium-hydrogen exchanger regulatory factor (NHERF) family (NHERF-3). NHERF family proteins play a major role in most of the protein-protein interactions. Estrogen causes an increase in PDZK-1, which consequently enhances tyrosinase expression and melanosome transfer to keratinocytes. This indicates that PDZK-1 could mediate the effect of estrogen through interactions with other proteins, such as ion exchangers, resulting in melanogenesis and the transfer of melanosomes in melasma.29,38
Postinflammatory hyperpigmentation. Separately, postinflammatory hyperpigmentation (PIH) is an acquired disorder characterized by increases in pigment following an inflammatory episode or trauma to the skin, including laser treatment.40 In a study by Al Mohizea et al,41 seven patients underwent carbon dioxide (CO2) fractionated laser treatment on their right inner arms on four different days throughout one menstrual cycle. Four of the seven subjects developed hyperpigmentation, two of the seven subjects developed hypopigmentation, and one of the seven subjects did not experience hyerpigmentary changes. Patients with Fitzpatrick Skin Type III or higher who developed PIH following fractionated CO2 laser treatment showed maximal levels of hyperpigmentation just before or after the menstrual bleeding period, at the end of the luteal phase. It is unknown why this time of the menstrual cycle increases the risk of developing PIH. These results suggest hormonal influence on susceptibility to the development of PIH, but more research will need to be completed before a significant correlation can be made between the two.41
Ultraviolet sensitivity. The degree of ultraviolet (UV) sensitivity can also be affected by the menstrual cycle. This condition is defined as inflammation and erythema of the skin after exposure to UV light, which can later develop into hyperpigmentation. In a study by Muizzuddin et al,42 the susceptibility of the skin of 20 women to UVB light was evaluated; the researchers found that these women were very sensitive to UV light during Days 20 to 28 of the menstrual cycle. They concluded that a decreased estrogen-to-progesterone ratio on Days 20 to 28 results in the weakening of the skin barrier and consequent sensitization of the skin to the UV light, increasing the risk for hyperpigmentation in the future.1,42 Another study by Jemeca et al43 showed that topical estrogen can increase UV sensitivity, although it was not clear whether this reaction was attributable to the direct effect of estrogen on the skin or secondary to a phototoxic reaction.
Discussion
The hormonal changes during a woman’s menstrual cycle affect many organs, including the skin. Pigmentary changes of the skin related to the menstrual cycle are known as catamenial hyperpigmentation. Exacerbation of some dermatoses during the menstrual cycle has been observed in many studies.4 Studying the physiology of the menstrual cycle shows that cyclic changes of estrogen and progesterone are responsible for many of these conditions. One of the most common conditions resulting from this cyclic fluctuation is hyperpigmentation of the skin.
Few studies have been performed to specifically evaluate catamenial hyperpigmentation thus far. Melasma, PIH after laser treatment, and UV light sensitivity are the most studied catamenial pigmentation conditions to date. Most of the available studies emphasize the role of estrogen in inducing hyperpigmentation, whereas the role of progesterone is controversial and less understood. Some studies show that it can cause hyperpigmentation, while others demonstrate an inhibitory effect on hyperpigmentation.
Based on our review of the literature, hyperpigmentation conditions like melasma worsen during the luteal phase of the menstrual cycle, possibly due to the fact that, during this time of the menstrual cycle, the serum levels of both estrogen and progesterone rise. Even though it is widely accepted that melasma is induced by estrogen, further studies are needed to elucidate the pathophysiology of catamenial hyperpigmentary disorders.
These conditions can have a significant effect on the quality of life of patients. Utilizing the MelasQol questionnaire, Pandya et al44 reported that social life, recreation/leisure, and emotional well-being were affected the most in 102 patients with melasma. Furthermore, this effect did not correlate with the severity of disease, meaning that even a small amount of hyperpigmentation can have dramatic psychological effects. Jiang et al45 reported that melasma also had a significantly negative effect on self-esteem, with patients reporting greater self-consciousnesses and decreased freedom and frustration because of expensive and ineffective treatments.
Conclusion
Considering the negative effect on the quality of life and the fact that these conditions can occur in patients with normal physiology, physicians should be aware of the numerous types of catamenial hyperpigmentation and why they occur so as to be able to educate their patients. Further research needs to be conducted on the etiology of catamenial hyperpigmentation and the pathophysiology of sex hormones in the skin to better understand these dermatologic conditions and to develop more effective treatment strategies.
References
- Farage MA, Neill S, MacLean AB. Physiological changes associated with the menstrual cycle: a review. Obstet Gynecol Surv. 2009;64(1):58–72.
- Verdolini R, Atkar R, Clayton N, Hasan R, Stefanato CM. Catamenial dermatoses: has anyone ever considered prostaglandins? Clin Exp Dermatol. 2014;39(4):509–512.
- Jang YH, Lee JY, Kang HY, et al. Oestrogen and progesterone receptor expression in melasma: an immunohistochemical analysis. J Eur Acad Dermatol Venereol. 2010;24(11):1312–1316.
- Raghunath RS, Venables ZC, Millington GWM. The menstrual cycle and the skin. Clin Exp Dermatol. 2015;40(2):111–115.
- Stephens CJM. Perimenstrual eruptions. Clin Dermatol. 1997;15(1):31–34.
- Shah MG, Maibach HI. Estrogen and skin. Am J Clin Dermatol. 2001;2(3):143–150.
- Thornton MJ. The biological actions of estrogens on skin. Exp Dermatol. 2002;11(6):487–502.
- Verdier-Sévrain S, Bonté F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol. 2006;15(2):83–94.
- Tamega A de A, Miot HA, Moço NP, et al. Gene and protein expression of oestrogen-beta and progesterone receptors in facial melasma and adjacent healthy skin in women. Int J Cosmet Sci. 2015;37(2): 222–228.
- Sun M, Xie H, Tang Y, et al. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J Steroid Biochem Mol Biol. 2017;165:236–246.
- Videira IF dos S, Moura DFL, Magina S, et al. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88(1):76–83.
- Millington GWM. Proopiomelanocortin (POMC): the cutaneous roles of its melanocortin products and receptors. Clin Exp Dermatol. 2006;31(3):407–412.
- Conrad F, Paus R. Estrogens and the hair follicle. JDDG J Dtsch Dermatol Ges. 2004;2(6):412–423.
- Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 2005;38(1):1–7.
- Wiedemann C, Nägele U, Schramm G, Berking C. Inhibitory effects of progestogens on the estrogen stimulation of melanocytes in vitro. Contraception. 2009;80(3):292–298.
- Natale CA, Duperret EK, Zhang J, et al. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife. 2016; 5:e15104.
- Corrigan EM, Clancy RL, Dunkley ML, et al. Cellular immunity in recurrent vulvovaginal candidiasis. Clin Exp Immunol. 1998;111(3):574–578.
- Nguyen T, Razzaque Ahmed A. Autoimmune progesterone dermatitis: update and insights. Autoimmun Rev. 2016;15(2):191–197.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771–782.
- Miot LDB, Miot HA, Silva MG da, Marques MEA. Physiopathology of melasma. An Bras Dermatol. 2009;84(6):623–635.
- Tamega A de A, Miot LDB, Bonfietti C, et al. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J Eur Acad Dermatol Venereol. 2013;27(2):151–156.
- McKenzie AW. Skin disorders in pregnancy. The Practitioner. 1971;206(236):773–780.
- Wade TR, Wade SL, Jones HE. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52(2):233–242.
- Hassan I, Kaur I, Sialy R, Dash RJ. Hormonal milieu in the maintenance of melasma in fertile women. J Dermatol. 1998;25(8):510–512.
- Ingber A. Obstetric Dermatology: A Practical Guide. Berlin, Germany: Springer; 2009.
- McGuinness BW. Skin pigmentation and the menstrual cycle. Br Med J. 1961;2(5251):563–565.
- Snell RS, Turner R. Skin pigmentation in relation to the menstrual cycle. J Invest Dermatol. 1966;47(2):147–155.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7(3):305–318.
- Lee A-Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015;28(6):648–660.
- Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histol Histopathol. 2004;19(2):629–636.
- Chompootaweep S, Kochagarn E, Sirisumpan S, et al. Effectiveness of Norplant® implants among Thai women in Bangkok. Contraception. 1996;53(1): 33–36.
- Alvarez F, Brache V, Faundes A, et al. Local side effects observed among long-term users of norplant contraceptive implants. Contraception. 2003;68(2):111–115.
- Schmidt AN, Nanney LB, Boyd AS, et al. Oestrogen receptor-beta expression in melanocytic lesions. Exp Dermatol. 2006;15(12):971–980.
- Famenini S, Gharavi NM, Beynet DP. Finasteride associated melasma in a Caucasian male. J Drugs Dermatol. 2014;13(4):484–486.
- Freeman DA, Gocze PM, Porpaczy Z. Finasteride blocks progesterone synthesis in MA-10 Leydig tumor cells. Endocrinology. 1993;133(4):1915–1917.
- Vermeulen A, Giagulli VA, De Schepper P, Buntinx A. Hormonal effects of a 5 alpha-reductase inhibitor (finasteride) on hormonal levels in normal men and in patients with benign prostatic hyperplasia. Eur Urol. 1991;20 Suppl 1:82–86.
- Lieberman R, Moy L. Estrogen receptor expression in melasma: results from facial skin of affected patients. J Drugs Dermatol JDD. 2008;7(5):463–465.
- Kim N-H, Cheong KA, Lee TR, Lee A-Y. PDZK-1 upregulation in estrogen-related hyperpigmentation in melasma. J Invest Dermatol. 2012;132(11): 2622–2631.
- Jee SH, Lee SY, Chiu HC, et al. Effects of estrogen and estrogen receptor in normal human melanocytes. Biochem Biophys Res Commun. 1994;199(3): 1407–1412.
- Arora P, Sarkar R, Garg VK, Arya L. Lasers for treatment of melasma and post-inflammatory hyperpigmentation. J Cutan Aesthetic Surg. 2012;5(2):93.
- Al Mohizea S. The effect of menstrual cycle on laser induced hyperpigmentation. J Drugs Dermatol. 2013;12(12):1335–1336.
- Muizzuddin N, Marenus KD, Schnittger SF, Sullivan M. Effect of systemic hormonal cyclicity on skin. J Cosmet Sci. 2005;56(5):311–321.
- Jemec GBE, Heidenheim M. The influence of sex hormones on UVB induced erythema in man. J Dermatol Sci. 1995;9(3):221–224.
- Sheth VM, Pandya AG. Melasma: a comprehensive update: part I. J Am Acad Dermatol. 2011;65(4): 689–697.
- Jiang J, Akinseye O, Tovar-Garza A, Pandya AG. The effect of melasma on self-esteem: a pilot study. Int J Womens Dermatol. 2017;4(1):38–42.

