 J Clin Aesthet Dermatol. 2022;15(7):32-37.
J Clin Aesthet Dermatol. 2022;15(7):32-37.
by Ahmed Mohammed Hamed, MD; Marwa Abdel Fatah, MGE, MSc; and Ghada Mohamed Shams, MD
All authors are with the Department of Dermatology and Andrology and Faculty of Medicine at Benha University, in Banha, Egypt.
ABSTRACT: Background. The field of research into the probable link between androgenetic alopecia (AGA) and metabolic syndrome (MetS) is rapidly expanding. The exact underlying pathogenesis yet to be identified. Alarin, a galanin neuropeptide, found to be elevated in patients with metabolic syndrome and may represent a potential link between AGA and MetS.
Objective. The aim of this study was to assess serum levels of alarin in patients with AGA and investigate its possible correlation, if any, with criteria of MetS in those patients.
Methods. The study included 50 male patients with AGA and 30 healthy controls. Weight, height, waist circumference, and body mass index (BMI) were all measured. Systolic and diastolic blood pressure readings were recorded. Serum level of lipids, fasting blood glucose (FBG) and alarin were also assessed.
Results. Anthropometric measures, serum lipids, FBG, and serum alarin were much higher in patients with AGA compared to controls (p<0.05). Forty-one patients with AGA (82%) met the criteria for diagnosis of MetS. Serum level of alarin was significantly higher in those patients and correlated positively with severity and duration of AGA.
Conclusion. Serum level of alarin might represent a potential link between AGA and MetS, opening the door for better understanding of the pathogenesis of both conditions and the possible association between them.
Keywords: Alarin, metabolic syndrome, androgenetic alopecia
The most frequent cause of progressive hair loss is androgenetic alopecia (AGA), commonly known as pattern hair loss.1 AGA is a multifactorial condition with different degrees of severity, age of presentation, and distinct pattern of scalp hair loss.2 The interplay of hormonal and genetic factors was suggested by Hamilton3 as a possible pathoetiology of AGA in 1951. Since then, research continues to elaborate different disturbances in hair cycle dynamics involved in “miniaturization” of hair follicle, which is the main event characterizing the pathology of AGA.4,5 Yet, most molecular mechanisms addressing AGA are still obscure, limiting effective long-term treatment options.
Previous studies have sought to investigate the association between AGA and systemic diseases, particularly metabolic syndrome (MetS).6 The definitions and criteria for MetS are constantly being amended and reviewed, and thresholds vary between the various criteria;7-10 however, the key components are basically the same: obesity, insulin resistance, hypertension, and dyslipidemia.
Alarin, a 25-amino acid peptide implicated in food intake and metabolism, is the newest member of the galanin neuropeptide family.11,12 Studies have shown that intracerebroventricular injection of alarin in rats can promote an increase in food intake and body weight.13-15
There is growing evidence that circulating alarin levels are much higher in newly diagnosed patients with MetS and that oral glucose challenge, acute hyperinsulinemia, and lipid infusion can impact circulating alarin level.11 We aimed to investigate whether alarin could represent a novel link between AGA and MetS.
Methods
The Research Ethics Committee of Benha Faculty of Medicine at Benha University in Egypt, authorized the protocol for this case-control study, which was carried out in compliance with the Declaration of Helsinki’s precepts. Prior to participation, every subject gave their informed consent. The study comprised 80 participants: 50 male patients with clinically diagnosed and dermoscopically confirmed AGA who attended the dermatology outpatient clinic at Benha University hospitals, and 30 age- and sex-matched controls.
Subjects with alopecia other than AGA, undetermined hair loss diagnosis, any other dermatological disease, other considerations or medical conditions that may give rise to hair loss, previous use of topical minoxidil or finasteride, type 1 diabetes, hepatic and renal failure, or congestive heart failure were excluded from the study. Patients who were taking chemotherapeutic treatments or other drugs that could affect hair development prior to their first presentation were also excluded.
All study participants were given a thorough medical history, as well as a clinical general and dermatological examination. Body mass index (BMI) and anthropometric measurements (weight, height, and waist circumference) were measured. Abdominal obesity was defined as having a waist circumference (WC) of more than 94cm. Overweight was defined as a BMI of 25.0 to 29.0 kg/m2, while obesity was defined as a BMI of 30 kg/m2 or higher. The weight (kg)/height (m) formula was used to compute BMI (m2). The Hamilton–Norwood categorization method was used to categorize male patients based on their hair loss pattern.16
A blood sample was obtained from every participant under complete aseptic conditions by sterile venipuncture and put into dry sterile plain tubes. The subjects’ fasting blood glucose (FBG), blood pressure, and lipid profile including triglycerides (TG), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) were all assessed.
The levels of serum alarin were determined using a commercial ELISA kit and the manufacturer’s procedure (Phoenix Pharmaceuticals, Inc., Belmont, California). The kit exhibited a linear range of 0.08–0.78 ng/mL and a sensitivity of 0.08ng/mL. Intra-assay and inter-assay differences were 10 percent and 15 percent, respectively.
Based on the International Diabetes Federation (IDF-2005) diagnostic criteria,10 MetS was defined as the presence of waist circumference >94cm and at least two of the following criteria: triglyceride value >150mg/dL or specific treatment for this lipid abnormality, high density lipoprotein <40mg/dL or specific treatment for this lipid abnormality, blood pressure ≥130/85mmHg or antihypertensive treatment, and either FBG ≥100mg/dL or diagnosed diabetes mellitus.
Statistical Analysis
The IBM SPSS software program version 20.0 was used to examine the data that was supplied into the computer. (IBM Corp., Armonk, New York) Qualitative data were described using percentages and numbers. To ensure that the distribution was normal, the Kolmogorov-Smirnov test was employed. Quantitative data were represented using range (minimum and maximum), mean, standard deviation, median, and interquartile range (IQR). The significance of the acquired results was assessed at a 5 percent level. The Chi-square test was performed to compare categorical variables between groups. For regularly distributed quantitative data, the student t-test was employed to compare two groups. For regularly distributed quantitative data, the Mann Whitney test was employed to compare two groups. The Spearman coefficient was utilized to find a correlation between two abnormally quantitative variables that were dispersed randomly. For erratically distributed quantitative variables, the Kruskal Wallis test was employed to compare more than two investigated groups. A p value <0.05 was set for statistical significance. Plotting sensitivity (TP) on the Y axis versus 1-specificity (FP) on the X axis at various cut off levels yielded a receiver operating characteristic curve (ROC). The diagnostic performance of a test is measured by the area under the ROC curve. A performance of more than 50 percent is acceptable, while a performance of more than 100 percent is the best for the test.
Results
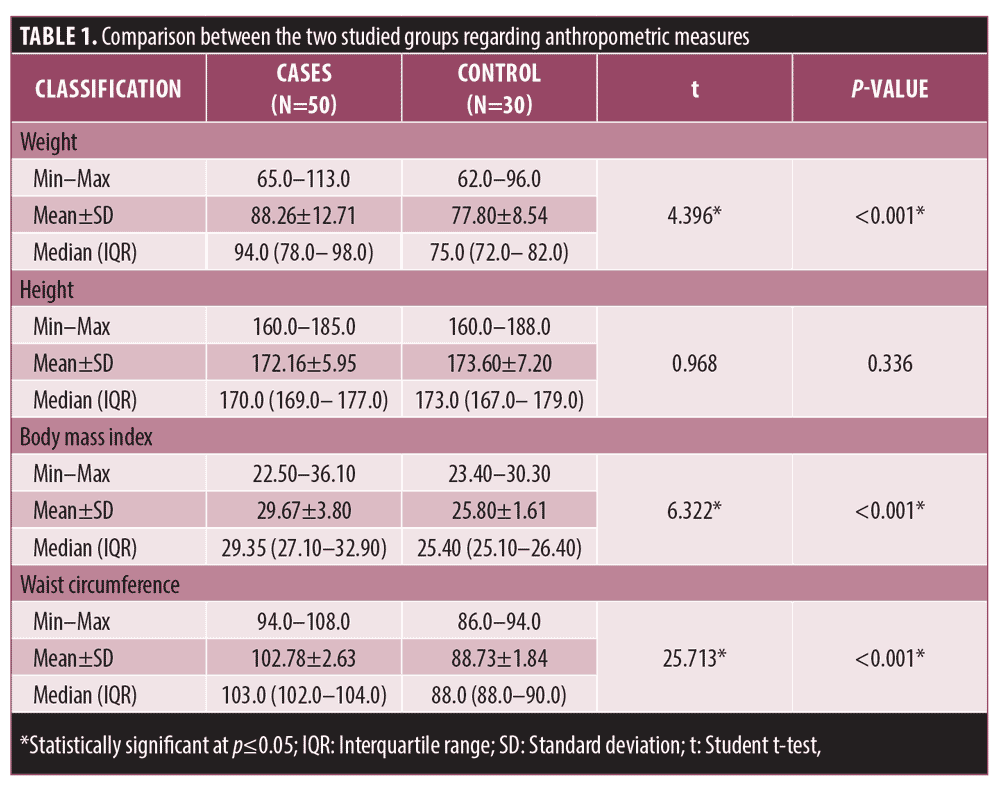
A total of 80 male subjects were enrolled in this study: 50 male patients with AGA aged 24 to 62 years old (mean 40.84±9.43), and 30 age-matched, seemingly healthy subjects as control, aged 29 to 55 years (mean 38.17±7.58). Anthropometric measures revealed significantly greater weight, WC, and BMI of patients than controls (p<0.001) (Table 1).
Mean age of onset of AGA in patients was 26.50±4.41 years, with mean duration 12.32±9.11 years. All patients reported progressive course of the disease. According to the Hamilton–Norwood classification system: 10 percent were Class I, 20 percent were Class II, 16 percent were Class IIIa, 20 percent were Class IIIv, 24 percent were Class IV, and 10 percent were Class V.
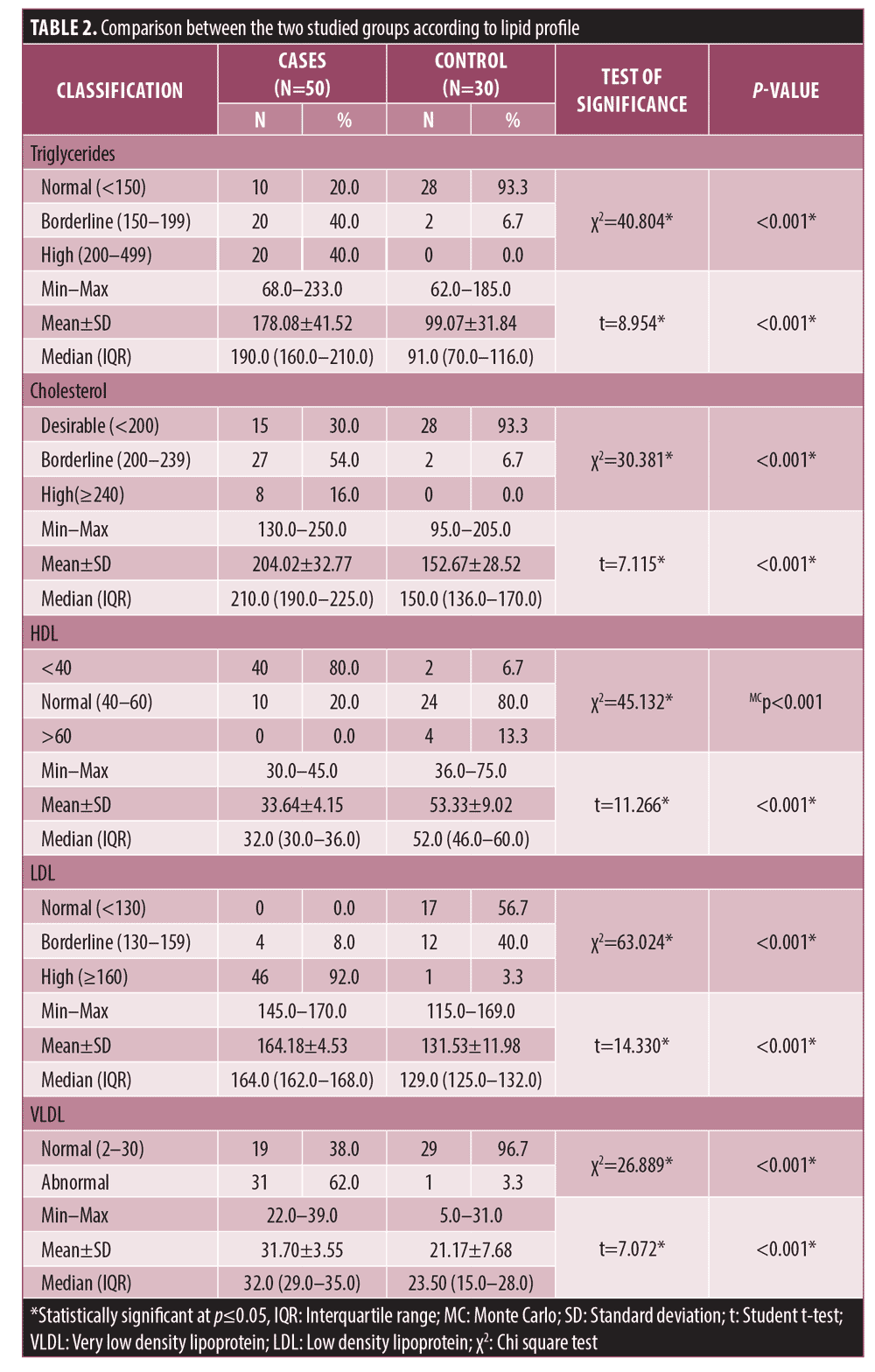
Serum TG, cholesterol, LDL, and VLDL were significantly higher in patients (n=50) than control subjects (n=30) (P<0.001) while serum HDL was significantly lower in patients (P<0.001) (Table 2).
Fasting blood glucose was also significantly higher in patients with AGA (95.32±14.32) than control subjects (78.43±13.85) (p<0.001). Recorded systolic and diastolic blood pressure was higher in patients with AGA (125.0±5.44) mmHg and (80.50±6.94) mmHg respectively, than control subjects (112.50±9.54) mmHg and (73.0±7.02) mmHg respectively (p<0.001). Accordingly, 41 patients (82%) met the IDF-2005 17 criteria for diagnosis of metabolic syndrome.
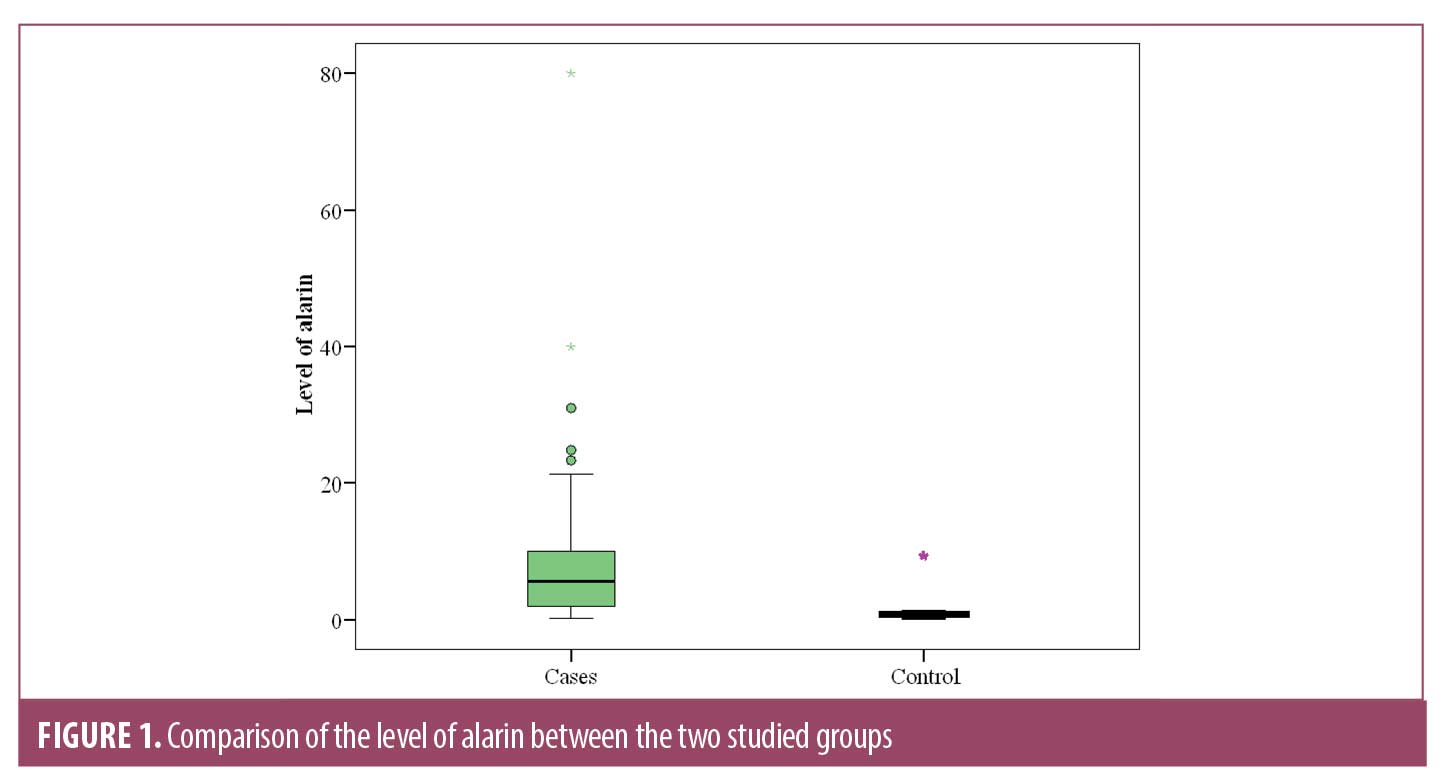
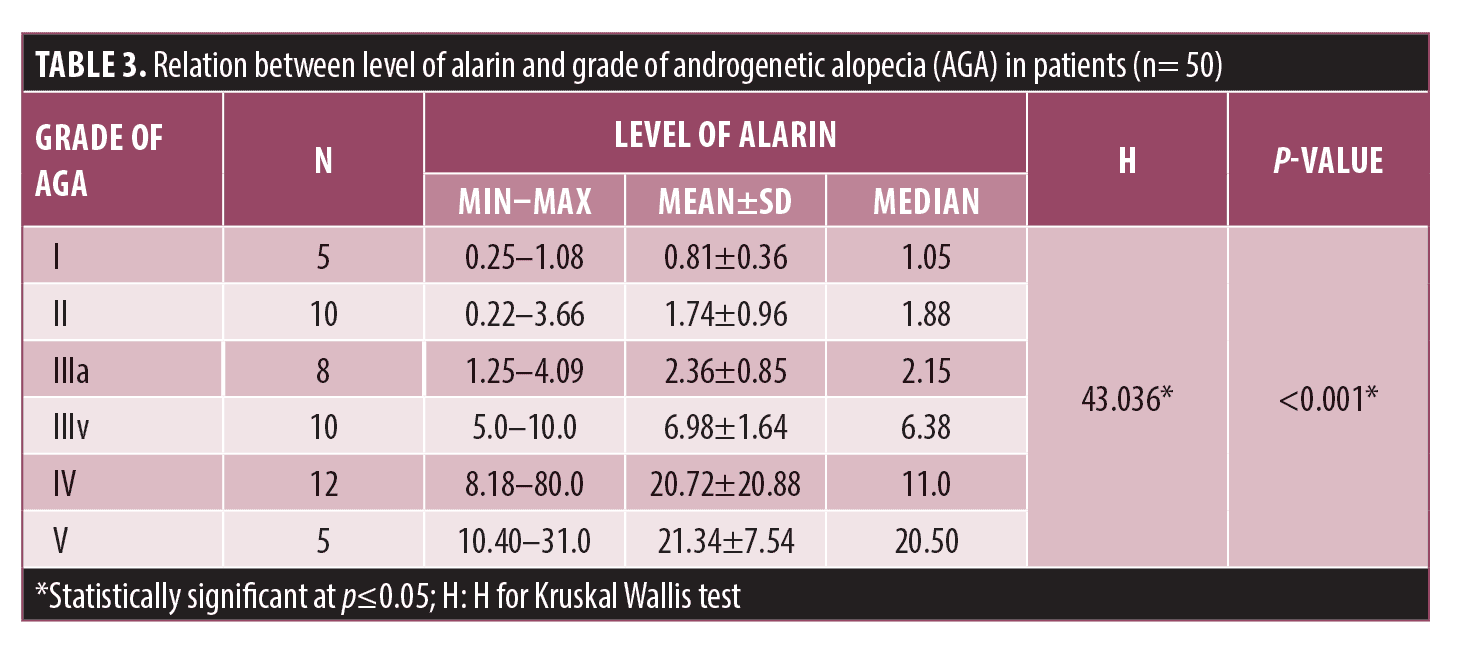
Serum alarin level was significantly higher (9.31±13.34 ng/mL) in patients than control subjects (1.09±1.60 ng/mL) (p<0.001) (Figure 1). Furthermore, a significant increase in its level was noticed with increased severity of AGA (Table 3). A positive correlation was noticed with duration of AGA in patients (Table 4).
In addition, serum alarin level was positively correlated with serum TG, cholesterol, LDL and VLDL and fasting blood glucose (p<0.001). It was also increased in relation to blood pressure readings but not to a statistically significant level. A positive correlation was also noticed between serum alarin level and BMI in patients (p<0.001) (Table 4).
Serum alarin level was accordingly significantly higher in patients with AGA who met the criteria for diagnosis of MetS (10.92± 14.21 ng/mL) versus (1.96± 2.51 ng/mL) in those who did not fulfill the MetS criteria (p<0.001).
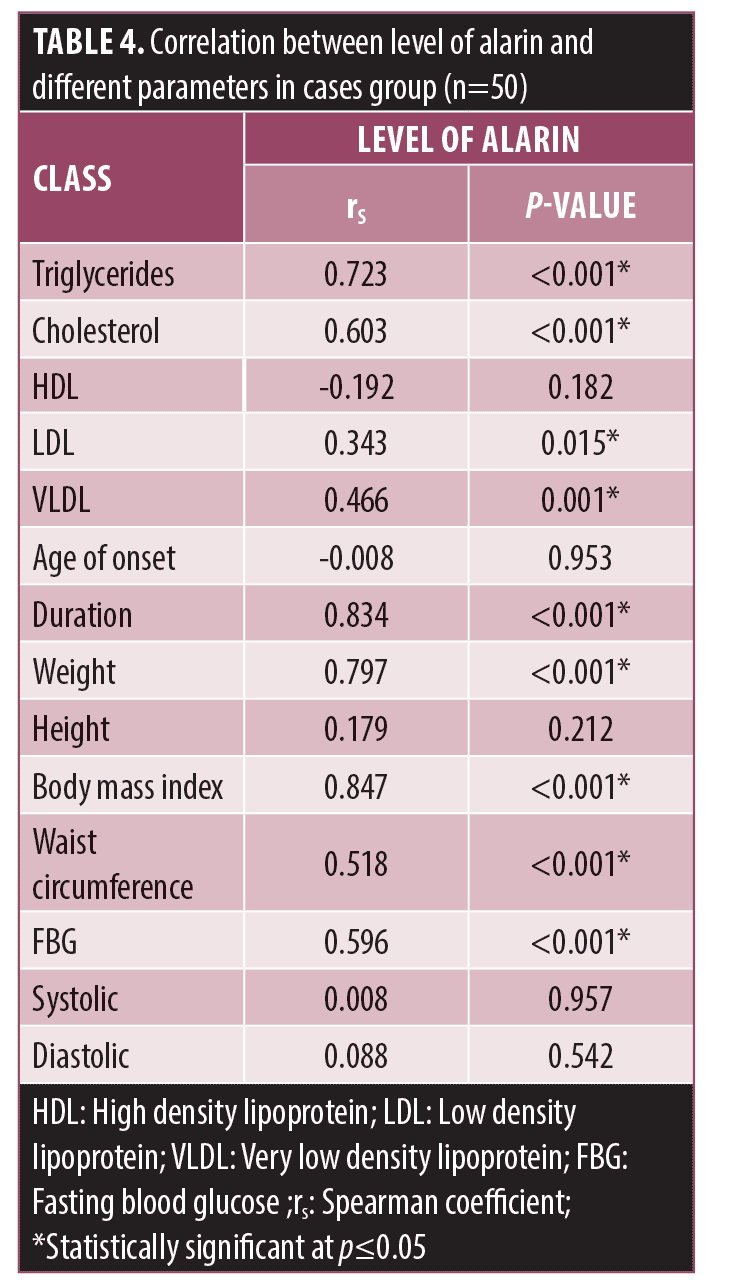
ROC curve analyses were performed. The results showed that the best cut-off value for Alarin to predict MetS was 2.16ng/mL (sensitivity 75.61%, specificity 88.89%, AUC 0.854, PPV 96.9 and NPV 44.4) (Figure 2A). While the best cut-off value for alarin to predict AGA was 1.22ng/mL (sensitivity 82%, specificity 96.67%, AUC 0.894, PPV 97.6 and NPV 76.3) (Figure 2B).
Discussion
The pathomechanism of AGA is not yet fully understood. With advances in molecular biology and genetics, a better understanding of the underlying pathologic events of pattern hair loss ensues. Emerging studies continue to link AGA with systemic morbidities, including cardiovascular risks17 and MetS6,18 extending the scope of AGA beyond cosmetic appearance.
The current study demonstrated significant hyperlipidaemia (including high serum TG, cholesterol, LDL and VLDL) (p<0.001) in male pattern hair loss patients as compared to control group. That was consistent with variable studies19-21 that also demonstrated the increased risk of cardiovascular comorbidities in such patients. A meta-analysis demonstrated more than 20 studies linking high lipid profile values with AGA22 with the underlying mechanism linking both conditions remaining unclear.23
Based on previous studies suggesting increased serum free testosterone level in patients with AGA24 and considering the anabolic effect of testosterone on lipid profile,25 some authors suggested this as a possible mechanism explaining the association between both conditions.26,27 Nowadays, it is well established that increased peripheral sensitivity to circulating androgens is the key mechanism in AGA pathogenesis rather than increased serum free testosterone level, making this hypothesis unreliable. Moreover, multiple researchers23,28 demonstrated even a negative correlation between serum testosterone and lipid profile values.
Weight, WC, and BMI were significantly higher in patients with AGA compared to control subjects, similar to results obtained by Bakry et al29 and Swaroop et al.20
In our work, fasting blood glucose was found to be significantly higher in patients with AGA than control subjects (p<0.001), that was also emphasized in studies by Acibucu et al,30 Matilainen et al,31 Pengsalae et al,32 and González-González et al.33 There was also a significant difference in systolic and diastolic blood pressure between both groups. That was contrary to Swaroop et al20 and in agreement with results concluded by Bakry et al,29 Hirsso et al,34 Matilainen et al,31 and Arias-Santiago et al.35
Insulin resistance and hence insulin excess was supposed to increase local androgen production and the peripheral conversion of testosterone to dihydrotestosterone (DHT), its more active form,36 which in turn contributes to miniaturization of hair follicle. Microvasculature changes associated with insulin resistance was another suggested possible mechanism.37
Consistent with previous data, 82 percent of AGA enrolled in this study met the criteria for diagnosis of MetS. A noticeably large number of published studies investigated the potential relationship between AGA and MetS, with the number of studies supporting the association between both conditions overwhelming the number of studies opposing it.6 This variation maybe explained by different age, sex and ethnic populations involved in those studies. The exact underlying mechanism connecting both conditions has not been yet fully clarified.
Alarin is a member of the galanin family of peptides with dose-dependent vasoactive biological activity.38 Apart from being involved in regulation of feeding behaviour, metabolism and, subsequently, body weight,39 its receptors were detected in microvasculature of dermal papillae.38 Reduction of dermal blood flow and decreased dermal vascular permeability are the main suggested mechanisms by which alarin exerts its vasoactive response in dermis.40 As compromised vasculature was detected in dermal papillae of balding scalp,41 we tried to investigate the possible role of alarin as a vasoactive peptide in AGA.
To our best knowledge, this is the first study to investigate the alarin level in sera of patients with AGA. Our results revealed a significantly higher serum Alarin level in patients with AGA versus control subjects (p< 0.001). The serum alarin level correlates positively with the duration and severity of AGA in our studied patients.
The statistical analysis of our results revealed a positive correlation between serum Alarin level and serum TG, cholesterol, LDL and VLDL, FBG, weight, WC and BMI in patients with AGA in agreement with results concluded by Zhou et al,38 Hu et al,42 and Fang et al.43 Whether elevated serum level of alarin is a cause or a result of lipid and glucose metabolism dysregulation, still a matter of debate.21
The ROC curve analysis in current work revealed that serum alarin may predict both AGA and MetS in our studied population. However, validity of alarin as a marker for MetS in AGA needs to be further investigated in a larger sample size.
Exclusive enrolment of male AGA and relatively small sample size may represent limitations to our study.
Conclusion
In our study, serum alarin levels were significantly elevated in patients with AGA and correlates positively to multiple factors including weight, waist circumference, BMI, FBG, severity and duration of AGA. Alarin may represent a potential link between AGA and MetS. This needs to be investigated on a multicentric scale.
References
- Lolli F, Pallotti F, Rossi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9–17.
- Otberg N, Finner AM, Shapiro J. Androgenetic alopecia. Endocrinol. Metab. Clin. N. Am. 2007; 36, 379–398.
- Hamilton JB. Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 1951; 53, 708–728.
- Paus R, Cotsarelis G. The biology of hair follicles. N. Engl. J. Med. 1999; 341, 491–497.
- Pierard-Franchimont C, Pierard GE. Teloptosis, a turning point in hair shedding biorhythms. Dermatology. 2001; 203, 115–117
- Lie C, Liew C, Oon H. Alopecia and the metabolic syndrome. Clin Dermatol. 2018; 36(1), 54–61.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–553.
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497.
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–2752.
- Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet 2005; 366:1059–1062.
- Fang P, Yu M, Shi M, et al. Galanin peptide family as a modulating target for contribution to metabolic syndrome. Gen Comp Endocrinol. 2012;179(1):115–120.
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115(2):177–207.
- Santic R, Fenninger K, Graf K, et al. Gangliocytes in neuroblastic tumors express alarin, a novel peptide derived by differential splicing of the galanin-like peptide gene. J Mol Neurosci. 2006;29(2):145–152.
- Van Der Kolk N, Madison FN, Mohr M, et al. Alarin stimulates food intake in male rats and LH secretion in castrated male rats. Neuropeptides. 2010;44(4):333–340.
- Boughton CK, Patterson M, Bewick GA, et al. Alarin stimulates food intake and gonadotrophin release in male rats. Br J Pharmacol. 2010;161(3):601–613.
- Norwood OT. Male pattern baldness: Classification and incidence. South Med J. 1975; 68:1359–1365.
- Vayá A, Sarnago A, Ricart JM, et al. Inflammatory markers and Lp(a) levels as cardiovascular risk factors in androgenetic alopecia. Clin Hemorheol Microcirc. 2015; 61:471–477.
- Ozbas gok S, Akin belli A, Dervis E. Is There Really Relationship between Androgenetic Alopecia and Metabolic Syndrome? Dermatol Res Pract. 2015; 2015:980310.
- Santic R, Schmidhuber S, Lang R, et al. Alarin is a vasoactive peptide. Proc Natl Acad Sci USA. 2007; 104(24),10217–10222.
- Swaroop MR, Kumar BM, Sathyanarayana BD, et al. The association of metabolic syndrome and insulin resistance in early-onset androgenetic alopecia in males: a Case-Control Study. Indian J Dermatol. 2019;64(1):23–27.
- Bakry OA, El Farargy SM, Ghanayem N, et al. Atherogenic index of plasma in non-obese women with androgenetic alopecia. Int J Dermatol. 2015;54(9):e339–e344.
- Kim M, Shin I, Yoon H, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2016; 31(6), 942–951.
- Zhang N, Zhang H, Zhang X, et al. The relationship between endogenous testosterone and lipid profile in middle-aged and elderly Chinese men. Eur J Endocrinol. 2014; 170: 487–494.
- Narad S, Pande S, Gupta M, Chari S. Hormonal profile in Indian men with premature androgenetic alopecia. Int J Trichol. 2013; 5: 69–72.
- Glazer G. Atherogenic effects of anabolic steroids on serum lipid levels. A literature review. Arch Intern Med. 1991; 151: 1925–1933.
- Mumcuoglu C, Ekmekci TR, Ucak S. The investigation of insulin resistance and metabolic syndrome in male patients with early-onset androgenetic alopecia. Eur J Dermatol. 2011; 21: 79–82.
- Choe SW, Yoon YH, Ro BI. Correlation between androgenetic alopecia and lipid parameters for risk factors of coronary artery disease. Korean J Dermatol. 2004; 42: 1277–1284.
- Jiann BP, Hsieh JT, Liu SP, et al. Associations of endogenous testosterone and lipid profiles in middle-aged to older Taiwanese men. Int J Impot Res. 2011; 23: 62–69.
- Bakry OA, Shoeib MA, El Shafiee MK, et al. Androgenetic alopecia, metabolic syndrome, and insulin resistance: Is there any association? A case-control study. Indian Dermatol Online J. 2014; 5:276–281.
- Acibucu F, Kayatas M, Candan F. The association of insulin resistance and metabolic syndrome in early androgenetic alopecia. Singapore Med J. 2010; 51(12):931–936.
- Matilainen V, Koskela P, Keinänen-Kiukaanniemi S. Early androgenetic alopecia as a marker of insulin resistance. Lancet. 2000; 356:1165–1166.
- Pengsalae N, Tanglertsampan C, Phichawong T, Lee S. Association of early-onset androgenetic alopecia and metabolic syndrome in Thai men: A case-control study. J Med Assoc Thai. 2013; 96:947–951.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol (Oxf). 2009; 71:494–499.
- Hirsso P, Laakso M, Matilainen V, et al. Association of insulin resistance linked diseases and hair loss in elderly men. Finnish population-based study. Cent Eur J Public Health. 2006; 14:78–81
- Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, et al. Male androgenetic alopecia and cardiovascular risk factors: A case-control study. Actas Dermosifiliogr. 2010; 101:248–256
- Horton R, Pasupuletti V, Antonipillai I. Androgen induction of steroid 5 alphareductase may be mediated via insulin-like growth factor-I. Endocrinology.1993;133:447–451.
- Abdel Fattah N and Darwish Y. Androgenetic alopecia and insulin resistance: are they truly associated? Int J Dermatol. 2011; 50 (4), 417–422,
- Zhou X, Luo M, Zhou S, et al. Plasma Alarin Level and Its Influencing Factors in Obese Newly Diagnosed Type 2 Diabetes Patients. Diabetes Metab Syndr Obes. 2021;14:379–385.
- Bauer JW, Lang R, Jakab M,et al. Galanin family of peptides in skin function. Exp Suppl. 2010; 102:51–59.
- Chew E, Tan J, Bahta A, et al. Differential Expression between Human Dermal Papilla Cells from Balding and Non-Balding Scalps Reveals New Candidate Genes for Androgenetic Alopecia. J Investig Dermatol. 2016; 136(8), 1559–1567.
- Vora RV, Kota R, Singhal RR, et al. Clinical profile of androgenic alopecia and its association with cardiovascular risk factors. Indian J Dermatol. 2019;64(1):19–22.
- Hu W, Fan X, Zhou B, et al. Circulating alarin concentrations are high in patients with type 2 diabetes and increased by glucagon-like peptide-1 receptor agonist treatment: a consort-compliant study. Medicine (Baltimore). 2019; 98(28):e16428.
- Fang X, Zhang T, Yang M, et al. High Circulating Alarin Levels Are Associated with Presence of Metabolic Syndrome. Cell Physiol Biochem. 2018, 51(5), 2041–2051.

