 J Clin Aesthet Dermatol. 2022;15(7):26-31.
J Clin Aesthet Dermatol. 2022;15(7):26-31.
by Howyda Ebrahim, MD; Amal Elardi, MD; Sayed Khater, MD; and Hala Morsi, MD
All authors are with Zagazig University Hostpital in Zagazig, Egypt
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Acne scars are a source of cosmetic concern for most of the patients.
Objective. We sought to compare the clinical efficacy and safety of topical botulinum toxin A (BTX-A) application immediately after microneedling (Mn) versus Mn with saline in the treatment of atrophic acne scars.
Methods. Forty patients with atrophic acne scars (rolling, boxcar, and mixed types) were enrolled in a split-face study; microneedling was performed on both sides of the face followed by an application of topically diluted botulinum toxin on one side (Side A) and saline on the other (Side B) for two sessions both two weeks apart. Evaluation was done at baseline, two and four weeks after the session. Follow-up was performed after six months. The assessments included blinded clinical assessment and patient’s satisfaction.
Results. After the treatment, acne scars in (Side A) showed 70 percent overall improvement versus zero percent in Side B (P<0.0001). A statistically highly significant reduction of acne scars severity occurred in (Side A) (P=0.0008). Patient’s satisfaction was higher in (Side A) (P<0.0001). No serious side effects were reported.
Conclusion. Microneedling delivery of BTX-A could be simple, safe, and innovative modality improving the appearance and decrease the depth of atrophic acne scars.
Keywords. Atrophic acne scars, botulinum toxin A, microneedling, topical botulinum toxin
Acne scars may result from degradation of collagen in addition to its aberrant production during the process of healing.1,2 Microneedles have been used for transdermal drug delivery since the 1990’s. Microneedling (Mn) facilitates the delivery of macromolecules across the skin barrier. Multiple techniques have been developed to induce skin barrier disruption, such as Mn, fractional CO2 laser, or iontophoresis. Mn has been used in dermatology either alone or in combination with other modalities for treating acne scars, alopecia, vitiligo, melasma, and facial rejuvenation.3,4 Off-label using of botulinum toxin A (BTX-A) are still active areas for research. Botulinum toxin (BTX) injections have many applications either in dermatologic diseases or in aesthetic medicine. The intradermal microbotox (i.e., highly diluted botulinum toxin) targets the superficial fibers of facial muscles, sweat, and sebaceous glands inducing pores shrinkage, decreasing the sebum and excessive sweating, in addition to face lifting.5,6 This makes the skin tighter and smoother. While the conventional BTX-A injection targets the muscles and has been used to improve the dynamic wrinkles. BTX has been successfully used in treatment of keloid or hypertrophic scars.7,8 It has been reported that intradermal microbotox improves the appearance of the scars. In the present study, we aimed to use Mn to help BTX-A penetrate into the dermis to improve the appearance of acne scars.
Methods
Study design. This was a randomized, controlled, double-blind, split-face design. It was approved by the local Ethical Committee and an informed consent was obtained from the patients before the study.
Patients. Forty patients of both sexes, aged from 19 to 55 years, with atrophic acne scars were selected from Dermatology, Venereology, and Andrology outpatient clinic at Zagazig University Hospitals during the period from July 2019 to July 2020. Before the treatment, all of the patients had nearly the same qualitative global scarring grading system on both sides of the face. Every patient received Mn on both sides of the face followed by an application of diluted BTX-A on one side (Side A) and saline on the other side (Side B). Two sessions were performed two weeks apart. Follow-up visits were performed at six months. Patients were included if they had rolling, boxcar, or mixed type atrophic acne scars and were aged from 19 to 55 years. Potential subjects were excluded due to pregnancy, lactation, allergy to BTX, neuromuscular diseases, active acne lesions, tendency for keloid formation, or bleeding. Patients who underwent other cosmetic procedures, such as laser procedures, chemical peels, or oral retinoids within six months of the study period were also excluded. The patients were subjected to dermatological examination to assess scar types and scar severity according to the qualitative global acne scarring grading system.
Assessment. Blinded clinical assessment. The clinical evaluation was completed via assessment of serial photographs using a digital camera (Sony DSC-W380, Tokyo, Japan) at baseline and two and four weeks following the treatment session. Analyses of the therapeutic effects were performed by three independent blinded investigators, who compared the photographs before and after the treatment using Goodman and Baron qualitative global scar grading system9 and quartile grading scale (QGS); the improvement was categorized into very good (improvement >75%), good (improvement ranged from 50%–74%), mild (improvement ranged from 25%–49%), and no improvement (improvement <25%).
Patient satisfaction. Patient satisfaction was categorized into very satisfied, satisfied, slightly satisfied, and unsatisfied. All of the patients graded the pain during the procedure based on the visual analog scale (VAS), ranging from 0 (no pain) and 10 (intolerable pain). Any side effects or complications were recorded either during the therapy or in the follow-up period.
Treatment technique. BTX-A 100U vial (onabotulinum toxin-A; Allergan, Dublin, Ireland) was reconstituted with 5mL saline to yield a concentration of 20U/1mL. The face was cleaned with an alcohol solution. A numbing cream (lidocaine 2.5% and prilocaine 2.5%) was applied and washed off after 40 minutes. The microneedling device (Dermapen; Bomtech Electronics, Korea) equipped with a head supplied by 36-needles, was applied on both sides of the face with a penetration depth of 3mm. Three passes were performed in three directions (transverse, vertical and diagonal) over the area with acne scars. After achieving pinpoint bleeding, the bleeding was cleaned from the skin surface using saline-soaked gauzes. Then, on one side (Side A) of the face, 1mL of the reconstituted BTX-A was dropped directly onto the skin using a 1mL, 30G insulin syringe (20U/mL of BTX-A was used on one side of the face immediately after the microneedling). While on the other side of the face (Side B), 1mL of saline was applied. Both the patients and physician were blinded to the content of the syringe. The total applied dose was 20U/mL. Two sessions only were done with two weeks apart.
Follow-up. Follow-up was performed at six months to evaluate the duration of the effect of BTX-A using this technique or the incidence of any side effects. All patients were advised to use only emollient and sunblock cream during the follow-up period.
Statistical analysis. The data were analyzed using SPSS, version 20 (Chicago, Illinois). Data were used as mean ± SD, percentages. Chi-square test (χ2) was used. P-value <0.05 was considered significant.
Results
This study included forty patients, 19 male patients (47.5%) and 21 female patients (52.5%) with different types of atrophic acne scars, (15 rolling, 15 boxcars, and 10 mixed). The age of the patients ranged from 19 to 55 years, with a mean age of 26.7±5.02 years. The scarring duration ranged from 2 to 8 years. Ten percent of the patients had Grade IV, 37.5 percent had Grade III and 37.5 percent had Grade II (Table 1). Two weeks after the first session, 12 percent of the patients showed very good improvement on Side A (Mn + BTX-A) versus zero percent of patients on Side B (Mn + saline) with statistically significant difference between both sides (P=0.02) (Table 2).
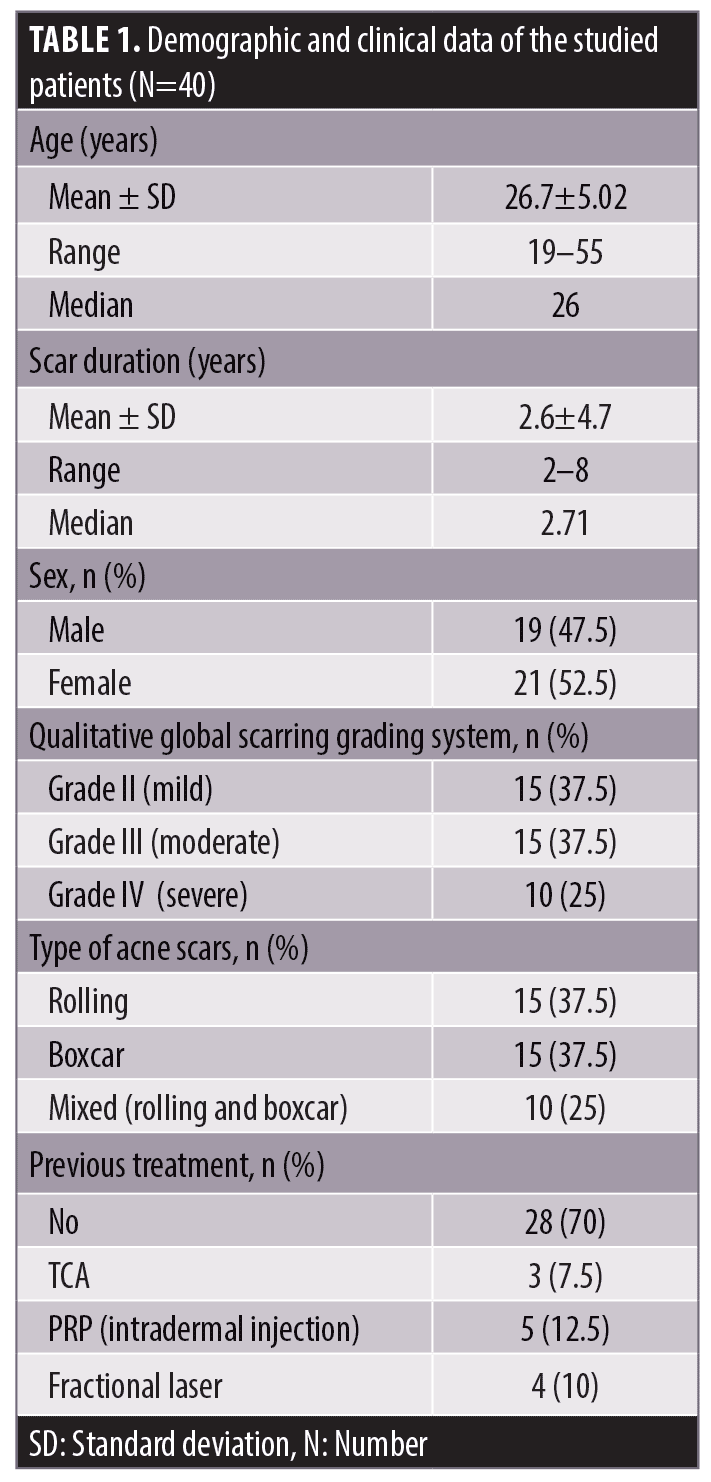
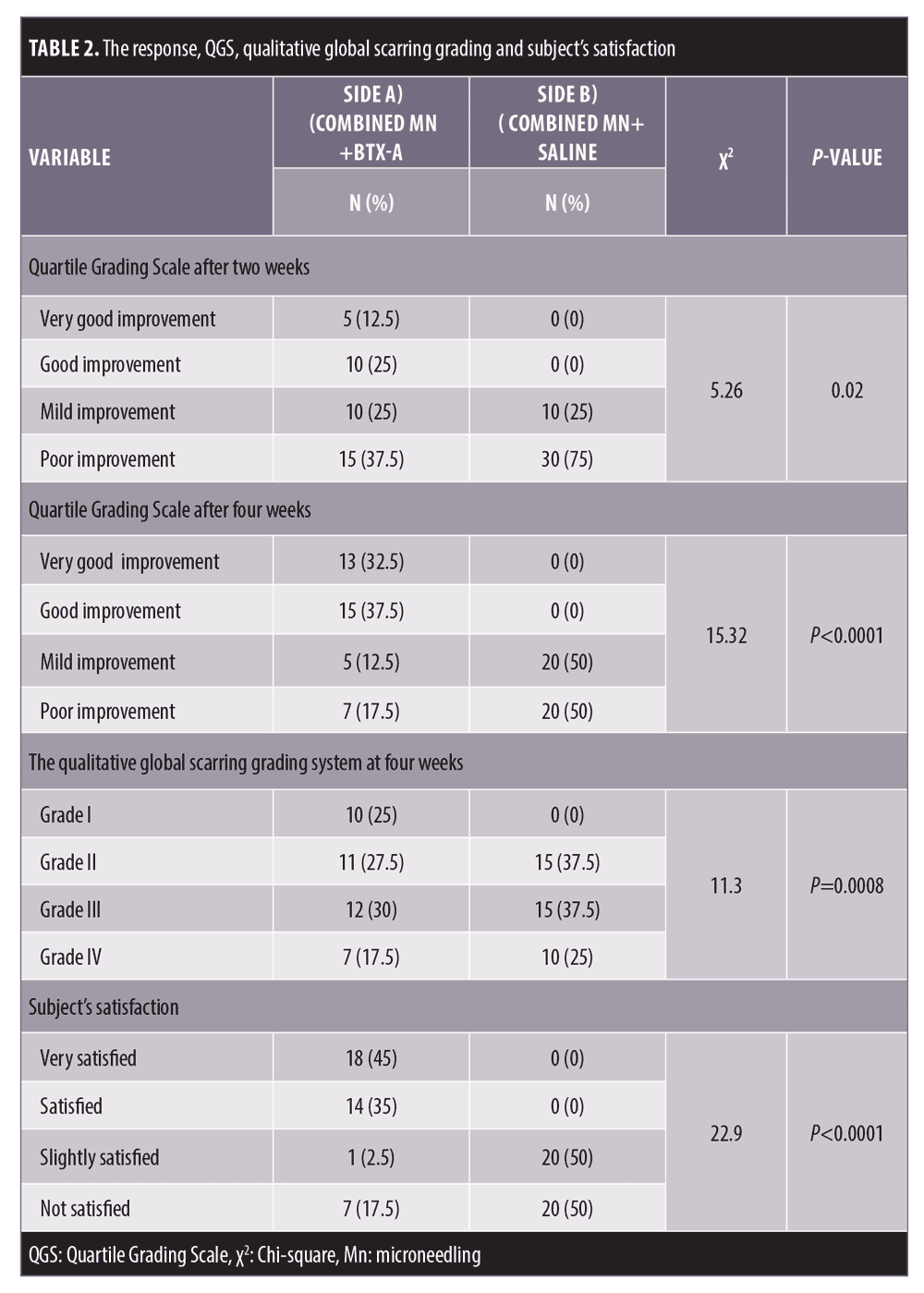
Comparing the results of both sides of the face at the fourth week after the treatment sessions. Two weeks after the second sessions, (Side A) showed statistically highly significant improvement in acne scars compared to (Side B). Side A showed very good improvement in 32.5 percent of the patients and good improvement in 37.5 percent while (Side B) revealed mild improvement in 50 percent of the patients (P<0.0001) (Table 2) (Figure 1 and 2). Regarding the improvement in acne scarring grades: in Side A, 3 out of 10 patients improved from Grade IV to Grade III, 6 out of 15 patients improved from Grade III to Grade II, 10 out of 15 patients improved from Grade II to Grade I. Side B showed no change in acne scarring grades (P=0.0008) (Table 2). Patients reported higher satisfaction in (Side A); 45 percent of the patients were very satisfied and 35 percent were satisfied (P=0.0001)(Table 2). Fifty percent of the patients in Side B were slightly satisfied. There was no statistically significant relation between the patient’s sex, age, or the scar duration, and the degree of improvement (Table 3). Concerning the scar types, the rolling scar type showed a very good response in 61.5 percent of the patients compared to 30.7 percent of the boxcar type with no significant difference (P=0.12)( Table 3). In addition, regarding to the severity of the scars, scars Grade II and III showed a better response than Grade IV with no significant difference (P=0.69) (Table 3).
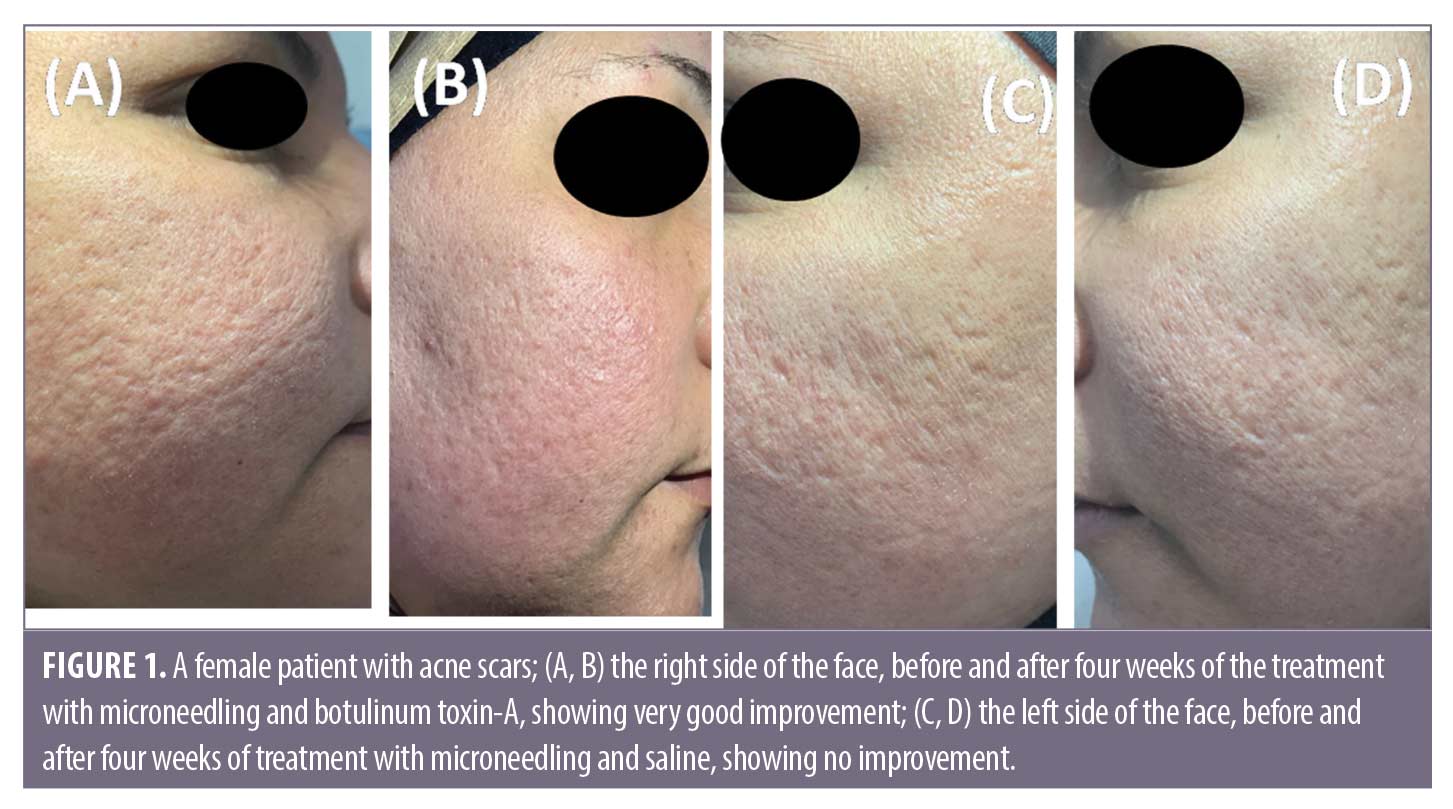
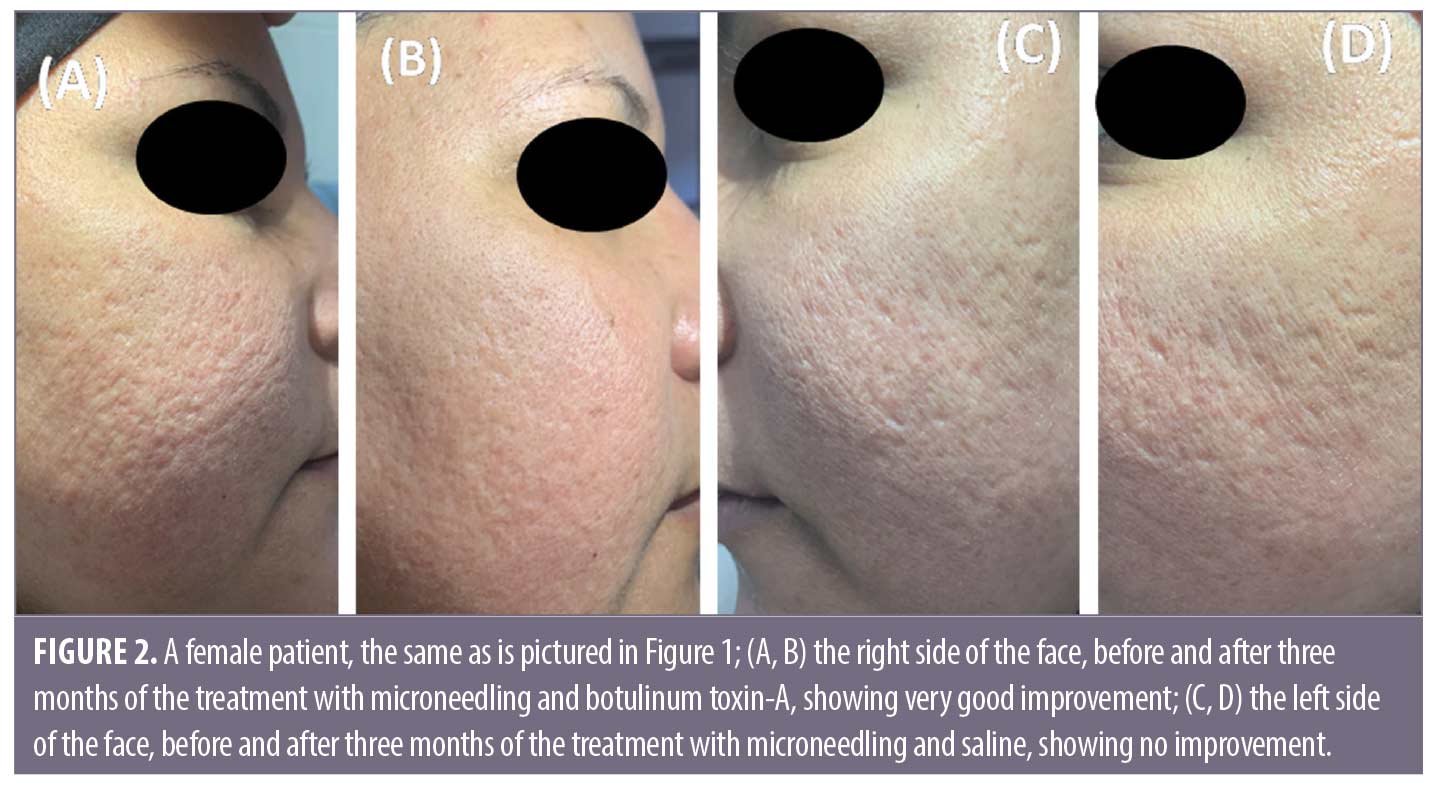
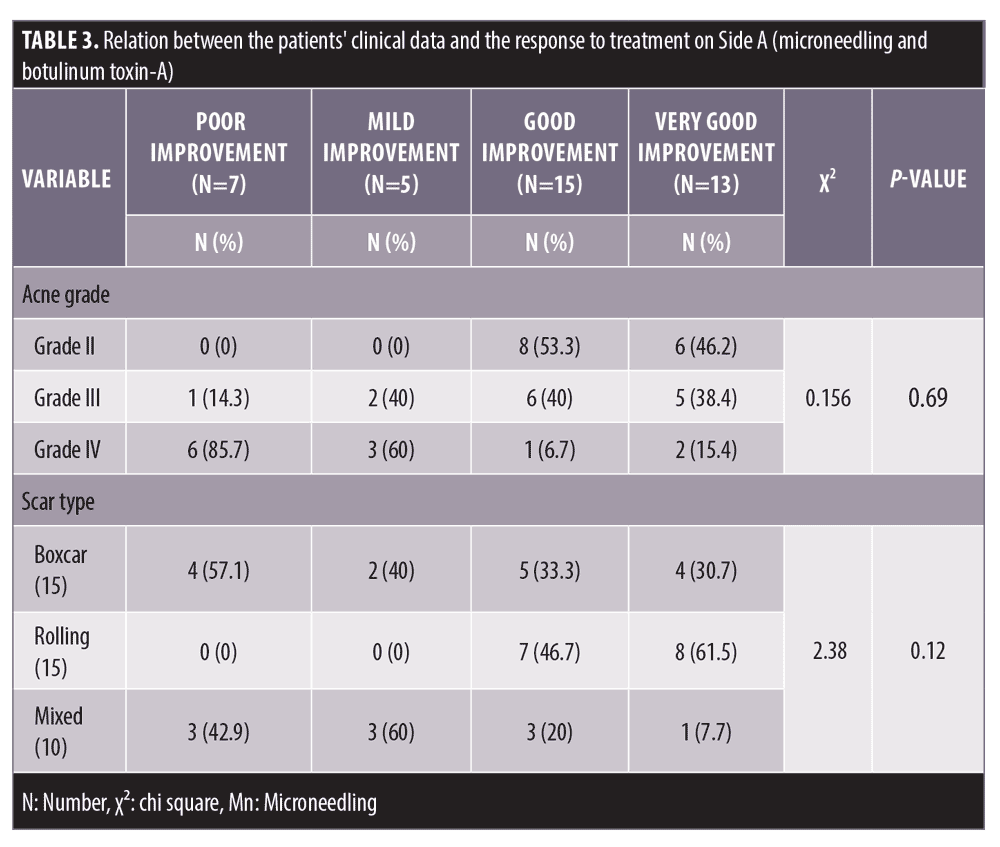
Follow-up. The follow up after the treatment session revealed that the results on Side A endured for three months.
Adverse effects. The technique was well tolerated, with only slight redness and swelling directly following the procedure; this redness and swelling faded two hours after the treatment on both sides. The mean pain during the session was 2.3± 1.2 with no significant difference between both sides.
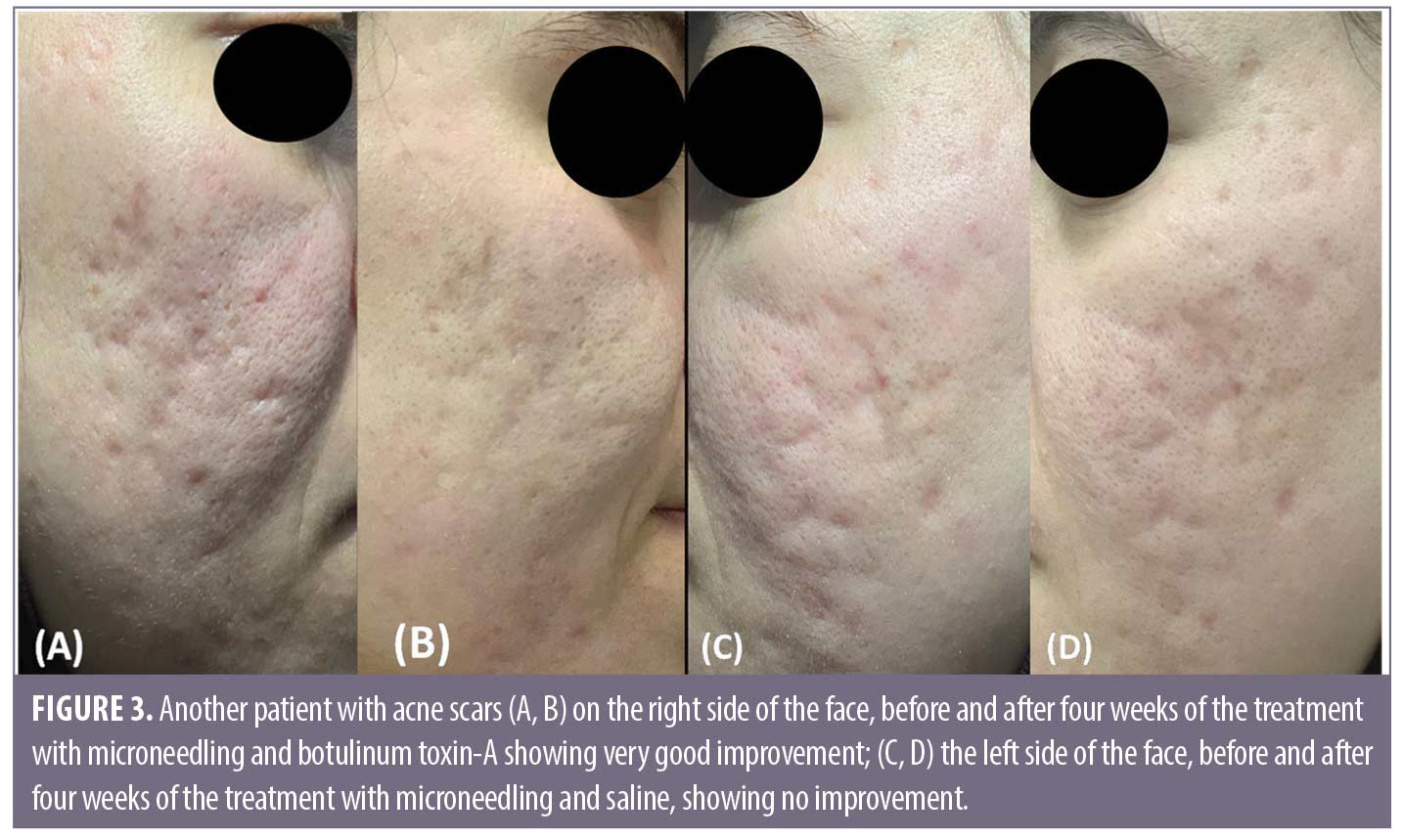
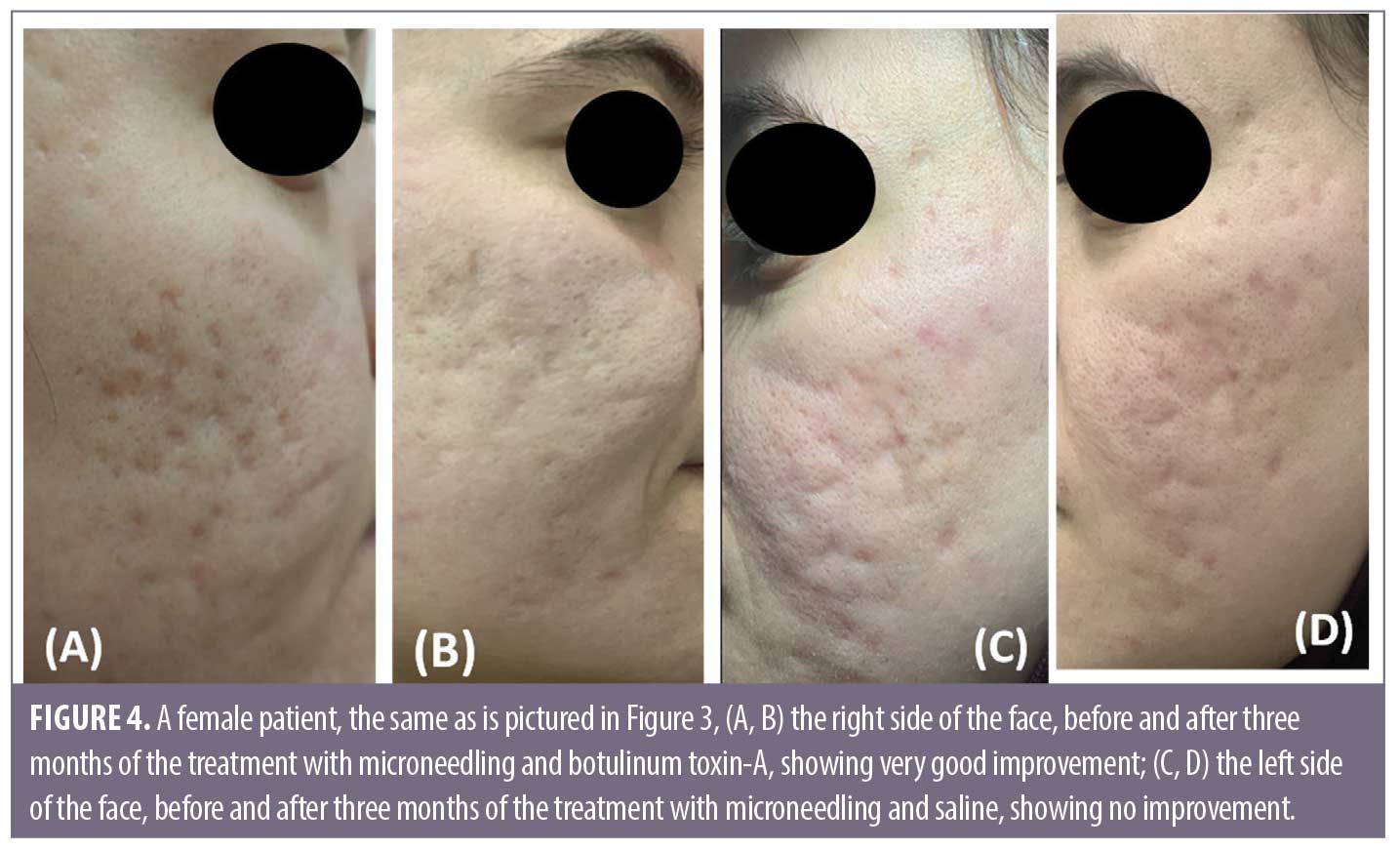
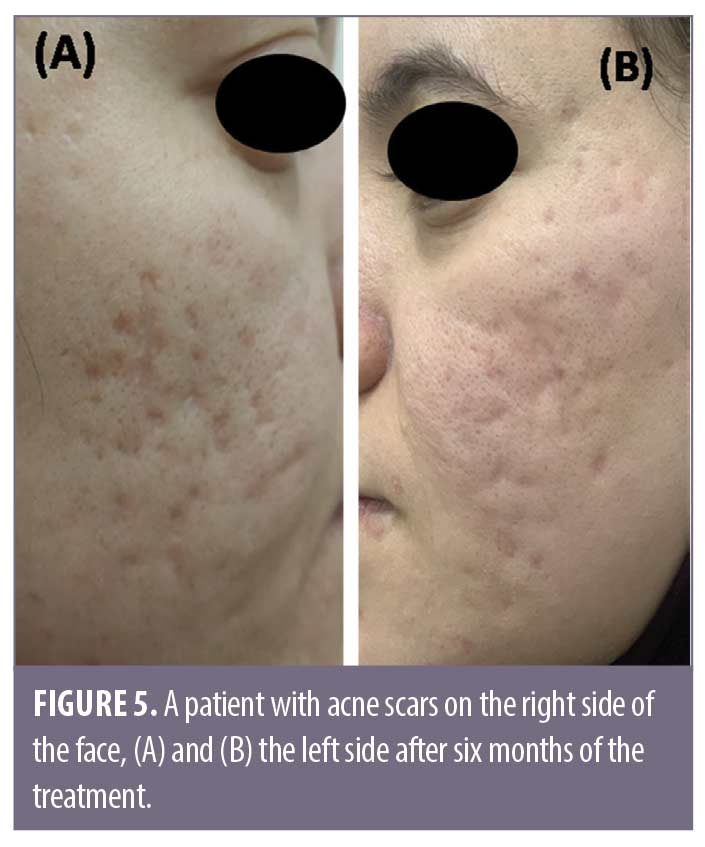
Discussion
Microneedling (Mn) is a strategy that permits macromolecules transport such as peptides and proteins across the skin barrier.10,11 To the best of our knowledge, this is the first split-face study comparing topical BTX delivery via Mn versus saline delivery via Mn for the treatment of acne scars. In the current study, two weeks from the final session, the overall improvement in QGS was 70 percent in (Side A) compared to zero percent in Side B (P<0.0001). Additionally, Side A revealed a statistically significant difference in acne severity grading score compared to Side B; 25 percent of the patients achieved improved to acne scar Grade I. Side B provided only some mild improvement in 50 percent of the patients. Using Mn and BTX provided high patient satisfaction, with 80 percent reporting overall satisfaction compared to slight satisfaction in 50 percent of patients in Side B (P<0.0001) which indicate the effectiveness of Mn delivery of BTX-A into the dermis. Previously, it was found that topical BTX-A has an absence of cutaneous bioavailability. However, inducing abruption of the barrier fractional CO2 laser has been successfully used for delivering topical BTX-A into the superficial layer of the orbicularis oculi muscles for the treatment of crow’s feet.13 Another study used fractional CO2 combined with topical BTX-A for facial rejuvenation.12–13 Mn and fractional CO2 laser share the same goal of inducing stratum corneum disruption to create channels for enhancing the drugs to pass through the stratum corneum. Morever, Mn has many advantages that can overcome the associated drawbacks of fractional laser. Mn is more convenient and cost effective than laser treatments and requires minimal training, with fewer side effects and little to no downtime period compared to the fractional laser.7,11 The intradermal injection of diluted BTX involves multiple injections of very small blebs of highly diluted BTX into the dermis for face lifting, decreasing pores size, and reducing sebum secretion.6,14 However, the intradermal injections are very painful, as the pain receptors are present in the dermis. Additionally, the intradermal injection, if inadvertently injected deep into the muscle by non-trained hands, can cause complete muscle paralysis. Mn can help to preclude the pain and bruising, which are associated with the multiple injections, and can prevent the unwanted diffusion into the muscles. Moreover, Mn allows more precise delivery of the diluted BTX-A into the dermis.11 The mechanism of action of this technique is the injection of highly diluted BTX resulting in the weakening of the superficial fibers of the muscle that have an attachment to the dermis, so it can reduce the tethering and pulling effect of the muscles surrounding the acne scars. Moreover, the micro-injuries induced by the Mn breaks away the old fibrous strands in addition to producing a state of a complex cascade of growth factors, resulting in stimulation of a new collagen formation.15 The exact mechanism of BTX in the treatment of acne scars is not clear; however, it is thought that BTX-A can limit or relieve the stress and tension exerted in the areas surrounding the scar and subsequently smooth out the scarred tissues and relax them.16,17 As a result, the scars become less visible. Some previous studies demonstrated that BTX-A can increase the production of collagen and improves its reorganization. Moreover, it stimulates vascular endothelial growth factor with subsequent increases in angiogenesis and reepithelialization of the wound that leads to an improvement of the scars18,19,20 Recently, a few clinical trials demonstrated that BTX-A injection improved wound healing and hypertrophic scarring.21 Another study confirmed the effect of the BTX-A as antiphotoaging by increasing collagen type I production, decreasing matrix metalloproteinases, and promoting scar healing.22 Botulinum toxin can also be used as prophylaxis to decrease the incidence of scarring after surgery and traumatic scars in other studies.16,19 In this study, the higher improvement was seen in the rolling type than boxcar as the rolling scars cause tethering of the dermis to the subcutaneous tissue; thereby the rolling types can benefit from botulinum toxin in reducing the pulling effect of the muscles surrounding the acne scars. To date, there has been no previous clinical studie which investigated Mn for topical BTX-A dermal delivery, and there is a lack of evidence concerning its clinical efficacy in the treatment of acne scars. Torrisia et al,23 performed an in-vitro study which used pocketed microneedles for BTX-A delivery into a human skin biopsy to be applied clinically instead of the intradermal injection of BTX-A for the treatment of palmar hyperhidrosis. The study demonstrated that microneedling delivery of the toxin was safe and effective in the dermal deposition of BTX-A.23 Another study designed a BTX-A-coated microneedle array to be utilized as a pain-free method for the treatment of plantar hyperhidrosis. They found that BTX successfully reached the dermis and penetrated the skin in a mice paw.24 A comparative study used intramuscular, intradermal injections as well as nanomicroneedle for BTX-A delivery for the treatment of crow’s feet for one session only. The authors found that the nanomicroneedles and intradermal injection of BTX both effectively treated the periorbital wrinkles.25 In this study, the physicians and patients also observed an improvement in the appearance of acne scars, and skin texture and elasticity.
Conclusion
BTX delivery via microneedling could be an effective modality and promising technology in improving the appearance of atrophic acne scars. The technique is simple, easily applied, and more suitable for patients with needle phobia who cannot tolerate pain and refuse other invasive procedures. Further large, randomized, controlled studies are required to confirm our results. Comparing the topical Mn delivery of botulinum toxin-A with the intradermal injections or with fractional CO2 laser as drug delivery is also required.
References
- Sanchez VM. Management of acne scars: fulfilling our duty of care for patients. Br J Dermatol. 2015;172(Suppl. 1):47–51.
- Fabbrocini G, Cacciapuoti S. Evaluation, Prevention, and Management of Acne Scars: Issues, Strategies, and Enhanced Outcomes.JDD. 2018;17(12):s44–48.
- Dogra S, Yadav S, Sarangal R. Microneedling for acne scars in Asian skin type: an effective low-cost treatment modality. J Cosmet Dermatol. 2014; 13:180–187.
- Fabbrocini G, De Vita V, Pastore F. The use of skin needling for eutectic mixture of local anesthetics delivery. Dermat Ther. 2011; 2:188–192.
- Campanati A, Martina E, Giuliodori K, et al. Botulinum Toxin Off-Label Use in Dermatology: A Review. Skin Appendage Disord. 2017;3:39–56.
- Sayed KS, Hegazy R, Gawdat HI, et al. The efficacy of intradermal injections of botulinum toxin in the management of enlarged facial pores and seborrhea: a split face-controlled study. Journal of Dermatological Treatment. 2020, doi.org/10.1080/09546634.2019.1708241.
- Harris A, Naidoo C, Murrell FD. Skin needling as a treatment for acne scarring: An up-to-date review of the literature. International Journal of Women’s Dermatology; 2015: 77–81.
- Goodman GJ. The Use of Botulinum Toxin as Primary or Adjunctive Treatment for Post Acne and Traumatic Scarring. J Cutanous Aesthet Surg. 2010; 3(2): 90–92.
- Goodman G, Baron J (2006) a: Post acne scarring: A qualitative global scarring grading system. Dermatol Surg; 32(12): 1458–1466.
- Freier H, Pachten A. Microneedling in the medical and cosmetic field. Prime. 2015; 5:48–54.
- Escobar-Chávez JJ, Bonilla-Martínez D, Villegas-González MA, Molina-Trinidad E, Casas N. Microneedles: A Valuable Physical Enhancer to Increase Transdermal Drug Delivery. Plastic Surgery. 2019; Vol. 27(2) 156–161.
- Mahmoud B, Burnett C, Ozog D. Prospective Randomized Controlled Study to Determine the Effect of Topical Application of Botulinum Toxin A for Crow’s Feet After Treatment With Ablative Fractional CO2 Laser, Dermatol Surg. 2014 ;41:S75–S81.
- Zhu L, Ji Z, Li,M, et al. The Efficacy and Safety of Fractional CO 2 Laser Combined with Topical Type A Botulinum Toxin for Facial Rejuvenation: A Randomized Controlled Split-Face. BioMed Research International 2016(2):1–7.
- Sapra P, Demay S, Sapra S, et al. A Single-blind, Split face, Randomized, Pilot Study Comparing the Effects of Intradermal and Intramuscular Injection of Two Commercially Available Botulinum Toxin A Formulas to Reduce Signs of Facial Aging. J Clin Aesthet Dermatol. 2017 Feb;10(2):34–44.
- Ziade M, Domergue S, Batifol D et al. “Use of botulinumtoxin type A to improve treatment of facial wounds: a prospective randomized study,” Journal of Plastic, Reconstructive and Aesthetic Surgery. 2013; 66: 209–214.
- Prodromidou A, Frountzas M, Vlachos GD, et al. Botulinum toxin for the prevention and healing of wound scars: A systematic review of the literature. Plast Surg 2015;23(4):260–264.
- Ziade M, Domergue S, Batifol D et al. Use of botulinum toxin type A to improve treatment of facial wounds: a prospective randomised study. J Plast Reconstr Aesthet Surg. 2013;66(2):209–214.
- Jablonka EM, Sherris DA, Gassner HG. Botulinum toxin to minimize facial scarring. Facial Plast Surg. 2012;28:525–535.
- SH, Lee Y, Seoetal YJ The potential effect of botulinum toxin type A on human dermal fibroblasts: an in vitro study,”Dermatologic Surgery.2012 ;38(10):1689–1694.
- Miao YY, Liu J, Zhu J, et al. “The Effect of Botulinum Toxin Type A on Expression Profiling of Long Noncoding RNAs in Human Dermal Fibroblasts”. Biomed Res Int. 2019; 26:1594837.
- Zhang DZ, Liu XY, Xiao WL, et al. Botulinum Toxin Type A and the prevention of hypertrophic scars on the maxillofacial area and neck: a meta-analysis of randomized controlled trials. PLoS One. 2016;11(3):e0151627.
- Permatasari, Y.-Y. Hu, J.-A. Zhang, et al, “Anti-photoaging potential of BotulinumToxinType A in UVB induced premature senescence of human dermal fibroblasts in vitro through decreasing senescence-related proteins,” Journal of Photochemistry and Photobiology B: Biology 2014; 133: 115–123.
- Torrisia BM, Zarnitsyn V, Prausnitz MR, et al. Pocketed microneedles for rapid delivery of a liquid-state botulinum toxin A formulation into human skin. J Control Release. 2013; 28: 146–152.
- Shim DH, Nguyen TT, Park PG, et al. Jeong HR Development of Botulinum Toxin A‑Coated Microneedles for Treating Palmar Hyperhidrosis. Mol Pharmaceutics. 2019; 12: 4913–4919.
- CaoY, Yang J, Zhu XG, et al. A Comparative In Vivo Study on Three Treatment Approaches to Applying Topical Botulinum Toxin A for Crow’s Feet. BioMed Research International. 2018:1-9.

