 by Dana S. Saade, MD; Mayra B.C. Maymone, MD, DSc; Eric A. Secemsky, MD, MSc; Kevin F. Kennedy, MSc; and Neelam A. Vashi, MD
by Dana S. Saade, MD; Mayra B.C. Maymone, MD, DSc; Eric A. Secemsky, MD, MSc; Kevin F. Kennedy, MSc; and Neelam A. Vashi, MD
Drs. Saade, Maymone, and Vashi are with the Department of Dermatology at Boston University School of Medicine in Boston, Massachusetts. Dr. Secemsky is with the Department of Medicine at Massachusetts General Hospital and Harvard Medical School in Boston, Massachusetts. Mr. Kennedy is with Saint-Luke’s Mid America Heart Institute and University of Missouri-Kansas City School of Medicine in Kansas City, Missouri.
Funding: This study was supported in part by the Boston University Department of Dermatology.
Disclosures: The authors have no conflict of interest relevant to the content of this article.
Abstract: Background.Over-the-counter (OTC) lightening agents are commonly used to treat hyperpigmentation disorders. Objective.We sought to determine the characteristics, trends, and preferences of patients with hyperpigmentation disorders seeking OTC agents in the United States. Design. The study was a cross-sectional study of consecutive patients with a disorder of hyperpigmentation seen in a United States-based outpatient dermatology clinic. Multivariable logistic regression models were used to identify factors associated with the use of OTC lightening agents. Setting.The study setting was an outpatient US-based dermatology clinic in Boston, Massachusetts. Results.Of the 406 patients studied, the majority were women (88.9%) with Fitzpatrick Skin Types IV to VI (64.5%). The most frequent diagnoses were melasma (42.9%) and post-inflammatory hyperpigmentation (PIH, 33.9%). Of our responders, 51.0 percent reported use of OTC agents and 44.9 percent reported use of prescription lightening products. Hydroquinone was the most commonly used cream (59.1%), followed by triple combination cream (fluocinolone acetonide, hydroquinone, and tretinoin, 16.3%). Of the cohort, 28.9 percent felt that the greater expense of the product correlated with greater efficacy. After multivariable adjustment, factors associated with a greater odds of using an OTC lightening agent included having a diagnosis of melasma (odds ratio [OR] 5.36; 95% CI: 2.98, 9.63; P<0.01) or PIH (OR 2.38; 95% CI: 1.25, 4.53; P is less than or equal to 0.01). Conclusion. The use of OTC lightening agents is widespread among those patients with hyperpigmentation disorders who reside in the United States. Those with melasma and PIH were more likely to use an OTC lightening cream. The majority of patients believed that OTC creams were safe to use without physician supervision. In those who had also tried prescription products, triple combination was deemed most effective compared to other lightening agents.
Keywords: Lightening creams, melasma, postinflammatory hyperpigmentation, dyschromia, bleaching, hydroquinone, OTC creams, prescription creams
J Clin Aesthet Dermatol. 2018;11(7):26–30
Introduction
Skin homogeneity refers to the even distribution of skin color and texture, and research supports theories that visible skin condition and coloration can independently signal attractiveness, youth, and health.1 The desire for unblemished, clear skin permeates all cultures and societies, making the practice of skin lightening quite common worldwide.2 In addition, prevalent in many cultures is the belief that lighter skin equates to beauty and a higher social status.3 In fact, many studies have shown that the use and overuse of lightening creams stems from this belief that having a lighter skin tone is more beautiful and more attractive than darker skin.4–7 With the cosmetic industry endorsing and making accessible various “lightening” creams, patients with skin of color attempt to “brighten” their complexion or to treat their facial hyperpigmentation with prescription and over-the-counter (OTC) creams. The latter’s use is unsupervised, and patients are frequently unaware of the potential side effects.5–9 In this study, we sought to determine characteristics associated with the use of lightening products and to determine participants’ beliefs, attitudes, and practices toward OTC lightening agents among people in the United States.
Methods
This cross-sectional study included 406 consecutive adults with cutaneous hyperpigmentation seen in a United States-based dermatology clinic from February 2015 through July 2016. Those included in the study were 18 years of age or older with a clinic referral for cosmetically burdensome hyperpigmentation. This study was approved by the Boston University Institutional Review Board, and verbal consent was obtained from all participants.
There were two parts of the survey. The first portion included questions pertaining to patients’ demographics (e.g., age, sex, skin type, marital status, education); whether the patient had used OTC or prescription lightening products, including hydroquinone, triple combination cream, kojic acid, azelaic acid, and/or topical steroids, and their perceived benefit; monthly expenditures; and accessibility to lightening products. This portion of the survey was available in English, Spanish, and Portuguese. The second portion was completed by a board-certified dermatologist and included documentation of the hyperpigmentation disorder and skin type.
Data analysis. Data are shown as mean with standard deviation for continuous variables, and the number and frequency for categorical variables. Students t-test was used to compare continuous data and chi square test was used for categorical data. In order to determine significant predictors of use of OTC skin whitening products, a multivariable logistic regression model was developed using the following candidate variables: sex, age, skin type, marital status, education, and diagnosis type (melasma, postinflammatory hyperpigmentation [PIH], and other). Candidate variables were selected based on review of the literature and clinical knowledge. All data analysis was performed with Statistical Analysis System (SAS) version 9.4 (Cary, North Carolina). A p value <0.05 was considered statistically significant.
Results
Of the 406 participants, the mean age was 41.1±12.5 years, 88.9 percent were women, 72.3 percent were employed, 37.0 percent were married, and 45.0 percent had a college education or greater (Table 1). Most participants were Fitzpatrick Skin Types IV to VI (64.5%), and 70.2 percent reported being born outside the United States. The most frequent hyperpigmentation diagnoses were melasma (42.9%) and PIH (33.9%).
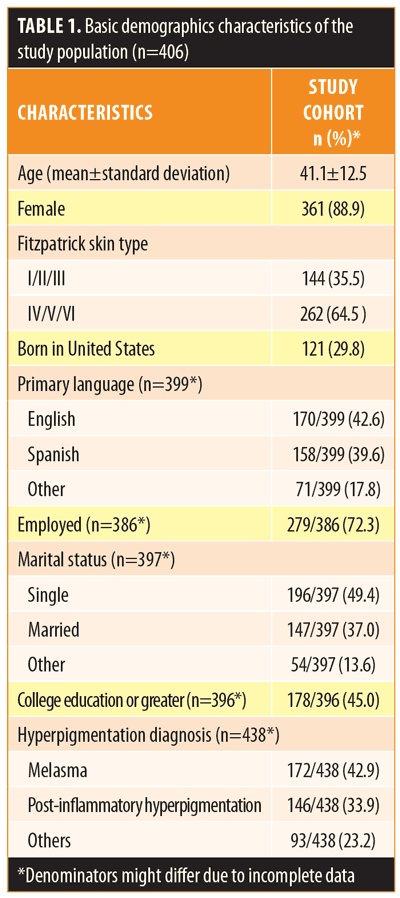
Of the participants, 51.0 percent reported use of OTC lightening products. Compared to those who were not using OTC agents, those indicating usage were of similar age, sex, and ethnicity (Table 2). Patients with a diagnosis of melasma were most likely to use an OTC whitening agent (54.1%). Overall, 26.5 percent of patients reported some improvement using an OTC product. Of those using OTC products, 56.1 percent had also tried a prescription product and 84.1 percent had consulted with a physician at some time during his/her treatment (Table 3). Products were obtained at United States pharmacies (57.5%), abroad (12.1%), from community stores (10.6%), and from friends (7.7%) (Table 3).
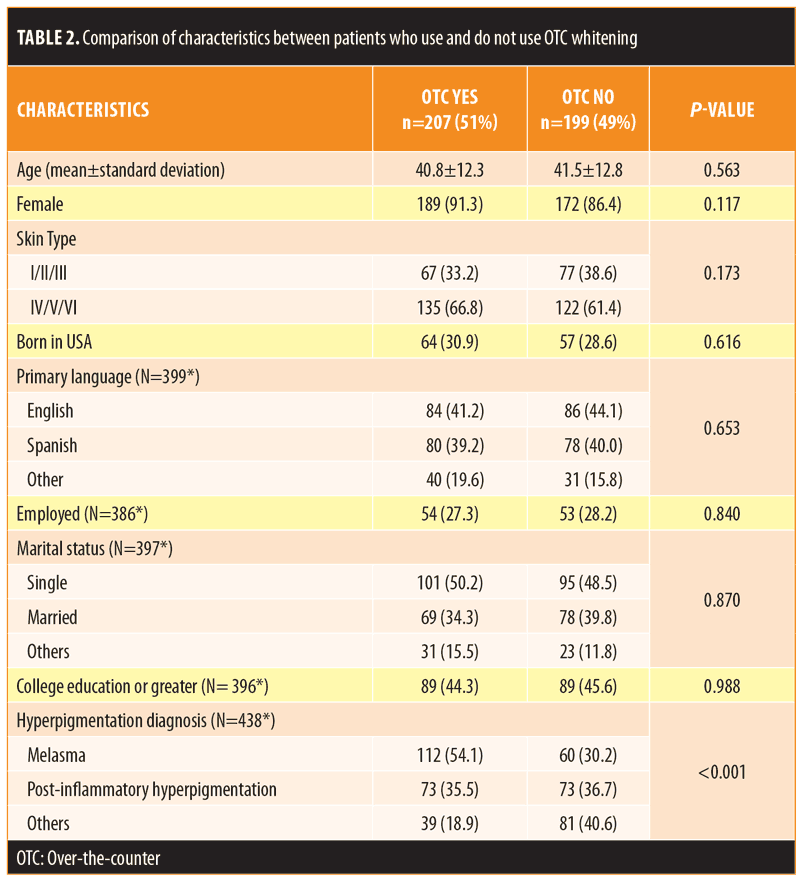
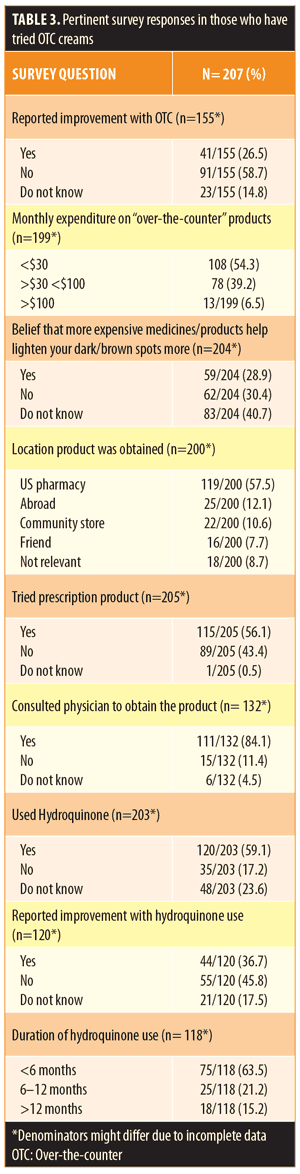
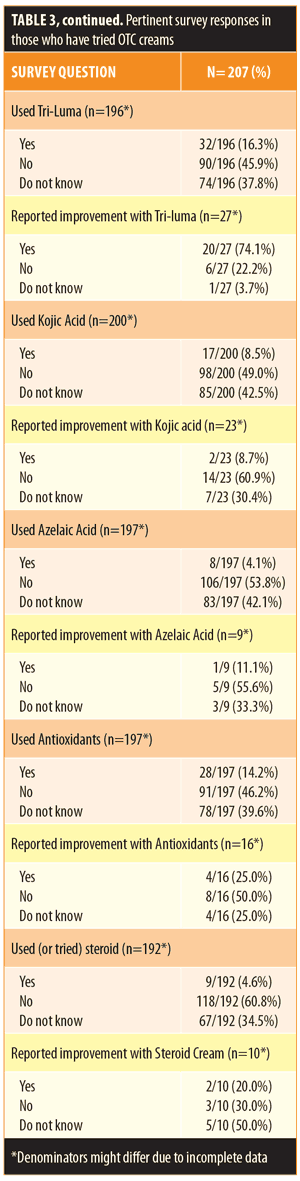
Regarding the components of the lightening cream, hydroquinone was the most commonly used ingredient (59.1%), which among users, was felt to be effective in 36.7 percent of cases (Table 3). A majority (63.5%) of patients used hydroquinone for less than six months, and 15.2 percent of participants reported using hydroquinone for more than one year. When looking at both OTC and prescription products, triple combination cream (Tri-luma®-fluocinolone acetonide, hydroquinone, and tretinoin) was the second most commonly used product (16.3%), which among users, was associated with a higher rate of improvement (74.1%). Other lightening products, including those that contained kojic acid, azelaic acid, steroids, or antioxidants, were each used by less than 15 percent of patients and were associated with overall low rates of improvement (<25% for each). Of those using lightening products, 45.7 percent were paying more than $30 monthly for their cream, and 28.9 percent believed that a more expensive agent would be more effective (Table 3). Among those using lightening creams, 78.7 percent also reported regular use of sunscreen.
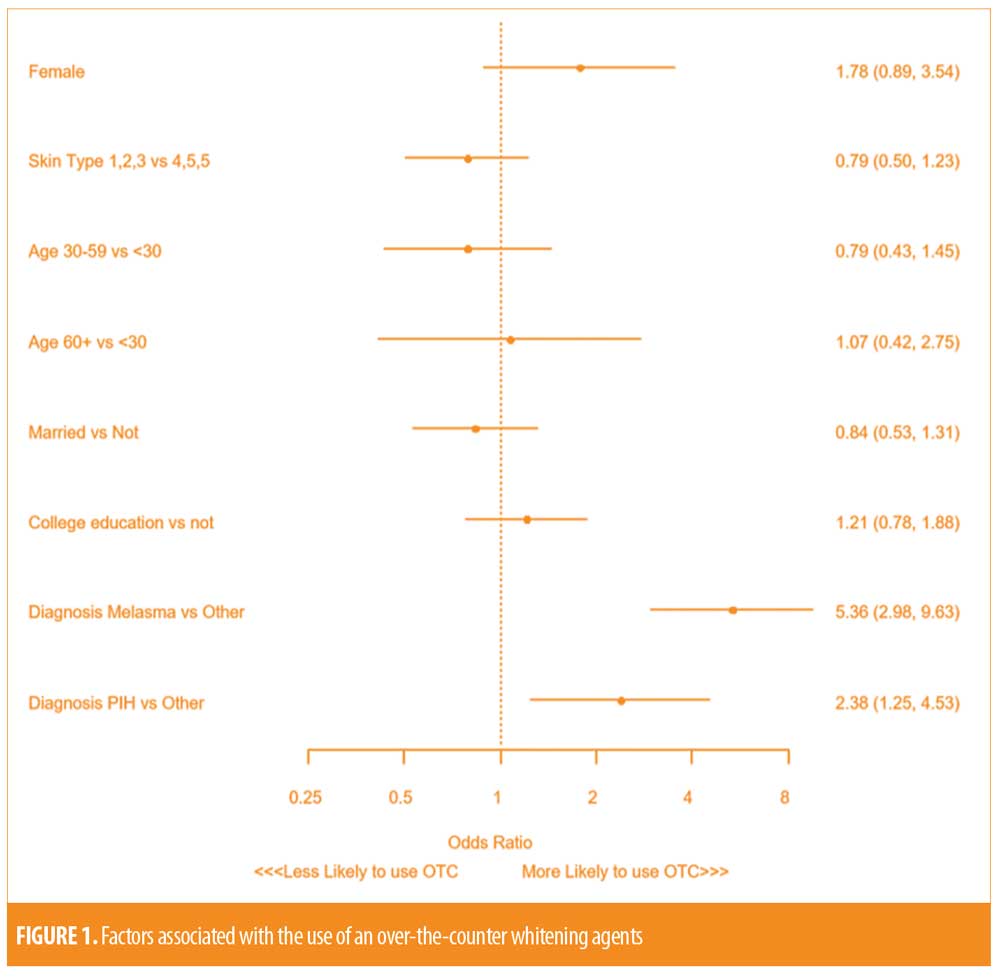
After multivariable regression, the type of hyperpigmentation disorder was the only factor independently associated with use of an OTC product. Compared to those with alternative hyperpigmentation disorders (i.e. “other”), a diagnosis of melasma (Odds ratio [OR] 5.36; 95% Confidence interval [CI]: 2.98, 9.63; p<0.001) and PIH (OR 2.38; 95% CI: 1.25, 4.53; p=0.008) was strongly associated with an increased odds of using an OTC whitening agent. Skin type, age, sex, marital status and education level did not demonstrate independent associations with OTC medication use (Figure 1).
Discussion
This novel cross-sectional study explores OTC skin lightening practices in a patient population based in the United States. The reasons behind such practices are multifold, not only inclusive of the desire to obtain an even skin tone but also due to the perception that a lighter skin tone could reflect a better socioeconomic status, better job opportunities, and more attractiveness with marital prospects.4,8,10–13 Our sample included a darkly pigmented patient population in which melasma was significantly associated with the use of lightening agents, particularly OTC agents. PIH was the second most common disorder in our population associated with the use of these agents. Dlova et al6 conducted a similar survey in Indian and African communities and found comparable results. The main purpose of these creams was to treat facial hyperpigmentation in 67 percent of their responders followed by overall skin lightening in 33 percent. However, many studies originating from countries outside of the United States describe the use of such creams as bleach.4–6,11,13,14 This is illustrated in a study from Jordan where a darker skin tone was associated with more unindicated usage of these agents, particularly for bleaching rather than treating a particular hyperchromic or hyperpigmented disorder.4 In a study performed in Sweden, where the skin type is much lighter, looking at the trends of lightening agents, it was noticed that their use is low, with only 2.6 percent of responders ever using lightening agents. And from these users, only 12 percent originated from Sweden, the majority being immigrants from Asia, Africa, and the Middle East.12
According to our survey, the purchase of the creams was mostly from pharmacies in the United States or community stores, while a small number obtained creams from abroad or friends. With OTC products easily accessible and appealing, patients assume these products are safe to use and can be used without any supervision.4,14,15 Many studies have shown that the choice of a lightening product relies primarily on the media effect with words such as “bright,” “beautiful,” “rejuvenate,” and “lovely.”8,14,16 In addition, brand names have been shown to be sought after and trusted in numerous studies.6,8,11,14 It has also been shown that patients believe more expensive products equate to improved safety and effectiveness.12 The majority of our study cohort expressed this sentiment, with approximately one third not believing that a more expensive cream would help their brown spots more, and approximately half of the responders paying more than $30 a month for their product. Put into another context, a “low price/relative affordability” is a major factor affecting the choice of Filipino, Indian, Arab, and African consumers with monthly expenditure on such products often not exceeding $20.4,6,14
Although the use of OTC lightening agents is vast, their overall effectiveness has yet to be established. In our study, more than half of our responders were not satisfied with their OTC creams, reporting that the creams did not improve hyperpigmentation. This could be due to the weaker composition of OTC products. For example, the gold standard for skin lightening, hydroquinone, is highly regulated under the United States Food and Drug Administration (FDA) Over-the-counter Miscellaneous Panel, with higher percentages needing a prescription and physician supervision.17 Although the majority of our study cohort was disappointed in the effectiveness of their OTC products, hydroquinone and triple combination cream did show efficacy that is comparable to current literature. Other studies have shown superiority of the triple combination cream in treating hyperpigmentation. When compared head to head with hydroquinone alone, 64.2 percent of cases improved with it, reducing their global melasma scale to “none” or “mild” compared to 39.4 percent of the hydroquinone cases.18 Like other studies, a large proportion of our patient population reported not knowing the composition of their creams.11 The small proportion that was aware of the composition of their creams rarely thought OTC creams were effective. Azelaic acid, antioxidants, kojic acid, and steroid-containing products were noted to lead to improvement in hyperpigmentation in less than 25 percent of the respondents.
Limitations. Limitations include the single-center study design with the majority of the responders being female. Another limitation is the cross-sectional design, with the drawbacks of recall bias, misinterpretation of questions, and reporter bias. Also, the survey questions were focused ones, looking at the trends of skin lightening for cases of dyschromia, and not inquiring about bleaching practices, commonly mentioned in other studies.
Conclusion
To the best of our knowledge, this study is among the first to report on the use of OTC lightening products in a large, United States-based population. This primarily descriptive study highlights important aspects of those who use lightening products. Women and those with a diagnosis of melasma and PIH were more likely to have tried an OTC lightening cream. More than half of our responders were not satisfied with their OTC creams, reporting that the creams did not improve their hyperpigmentation. In addition, highest efficacy was reported with prescription, triple combination cream. Although other characteristics of patients who were more bothered by hyperpigmentation tended to be of darker skin types, working, less-educated, and born outside of the United States, there was no statistical difference in these parameters in those who had actually tried OTC lightening agents or not.
The use of OTC lightening agents is widespread. Interestingly, only about half of our sample population, all of which had cosmetically bothersome hyperpigmentation, had tried OTC lightening agents, with the vast majority consulting a clinician during the course of their treatment. This indicates a large group of patients that dermatologists have the opportunity to educate and advise on proper application of lightening products as well as inform on the side effects of inappropriate use of lightening agents.
References
- Samson N, Fink B, Matts PJ. Visible skin condition and perception of human facial appearance. Int J Cosmet Sci. 2010;32(3):167–184.
- Sagoe D, Pallesen S, Dlova NC, et al. The global prevalence and correlates of skin bleaching: a meta-analysis and meta-regression analysis. Int J Dermatol. 2018 Jun 11. doi: 10.1111/ijd.14052. [Epub ahead of print].
- Shroff H, Diedrichs PC, Craddock N. Skin color, cultural capital, and beauty products: an investigation of the use of skin fairness products in Mumbai, India. Front Public Health. 2018 Jan 23;5:365. doi: 10.3389/fpubh.2017.00365. eCollection 2017.
- Hamed SH, Tayyem R, Nimer N, Alkhatib HS. Skin-lightening practice among women living in Jordan: prevalence, determinants, and user’s awareness. Int J Dermatol. 2010;49(4):414–420.
- Jacobs M, Levine S, Abney K, Davids L. Fifty shades of African lightness: A bio-psychosocial review of the global phenomenon of skin lightening practices. J Public Health Afr. 2016;7(2):552.
- Dlova NC, Hamed S, Tsoka-Gwegweni JM, Grobler A. Skin lightening practices: an epidemiological study of South African women of African and Indian ancestries. Br J Dermatol. 2015;173:2–9.
- Wone I, Tal Dia A, Diallo OF, et al. Prevalence of the use of skin bleaching cosmetics in two areas in Dakar (Sénégal). Dakar Med. 2000;45(2):154–157.
- Li EPH et al. Skin lightening and beauty in four asian cultures. Adv Consum Res. 2008;35:444–449.
- Ly F, Kane A, Déme A, et al. First cases of squamous cell carcinoma associated with cosmetic use of bleaching compounds. Ann Dermatol Venereol. 2010;137(2):128–131.
- Mahé A. The practice of skin-bleaching for a cosmetic purpose in immigrant communities. J Travel Med. 2014;21(4): 282–287.
- Dlova N, Hamed SH, Tsoka-Gwegweni J, et al. Women’s perceptions of the benefits and risks of skin-lightening creams in two South African communities. J Cosmet Dermatol. 2014;13(3):236–241.
- Darj E, Infanti JJ, Ahlberg BM, Okumu J. “The fairer the better?” Use of potentially toxic skin bleaching products. Afr Health Sci. 2015;15(4):1074–1080.
- Dadzie OE, Petit A. Skin bleaching: highlighting the misuse of cutaneous depigmenting agents. J Eur Acad Dermatol Venereol. 2009;23(7):741–750.
- Mendoza RL. The skin whitening industry in the Philippines. J Public Health Policy. 2014;35(2):219–238.
- Gbetoh MH, Amyot M. Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada. Environ Res. 2016;150:403–410.
- Mahé A, Ly F, Aymard G, Dangou JM. Skin diseases associated with the cosmetic use of bleaching products in women from Dakar, Senegal. Br J Dermatol. 2003;148(3): 493–500.
- U.S. Department of Health and Human Services, Food and Drug Administration. Skin bleaching drug products for over-the-counter human use; proposed rule. Federal Register 2006; 71: 51146–51155 (codified at 21 CFR Part 310).
- Chan R, Park KC, Lee MH, et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br J Dermatol. 2008;159(3):
697–703.

