 J Clin Aesthet Dermatol. 2018;11(11):36–39
J Clin Aesthet Dermatol. 2018;11(11):36–39
by James Q. Del Rosso, DO and Neal Bhatia, MD
Dr. Del Rosso is with JDR Dermatology Research/Thomas Dermatology in Las Vegas, Nevada and Touro University Nevada in Henderson, Nevada. Dr. Bhatia is with Therapeutics Clinical Research in San Diego, California.
FUNDING: No grants related to this project were provided. The authors received honoraria as consultants to IntraDerm Pharmaceuticals for data review and manuscript accuracy.
DISCLOSURES: Drs. Del Rosso and Bhatia have received honoraria as advisors and research consultants for IntraDerm Pharmaceuticals.
Abstract: In-vitro and in-vivo studies have supported antimicrobial, anti-inflammatory, and other biologic properties of hypochlorous acid (HOCl), which has led to its use in the treatment of skin wounds, pruritus, diabetic ulcers, and some inflammatory skin disorders. Research has also shown that the physiochemical properties of HOCl after application to skin are highly dependent on both pH and formulation stability. In this review, the authors discuss a core HOCI formulation (Microcyn® Technology, Sonoma Pharmaceuticals, Petaluma, California) that is stable for up to two years, noncytotoxic, and pH-neutralized to augment therapeutic activity, skin tolerability, and stability. The authors summarize relevant study outcomes and potential modes of action related to this core HOCI formulation, as well as describe its ready-to-use vehicles that are approved and available for topical application.
Introduction
Hypochlorous acid (HOCl), a naturally occurring molecule that is a component of the human innate immune response, is recognized as a major active component of bleach and demonstrates antimicrobial properties supported by both in-vitro and in-vivo studies.1–9 One important function of HOCl in host immunity is its release by neutrophils to destroy pathogenic organisms (i.e., respiratory burst). Over time, a variety of anti-inflammatory and other biologic properties of HOCl have led to applications for wound healing, pruritus, and diabetic ulcers, as well as applications for the management of some inflammatory skin disorders, such as seborrheic dermatitis and atopic dermatitis (AD).8–18 What has also come to light is that the physiochemical properties of HOCl and its impact after application to skin are highly dependent on both pH and formulation stability.8,11 The use of HOCl in the clinical setting is supported by a substantial body of research, which has led to the use of a core formulation—available in ready-to-use, approved topical vehicles—that is stable for up to two years, noncytotoxic, and, importantly, pH-neutralized to augment therapeutic activity, skin tolerability, and stability.8,11,18,19 This core formulation (often referred to in the literature as a superoxidized solution or, sometimes, slightly acidic electrolyzed water) has been termed and marketed as Microcyn® Technology (Sonoma Pharmaceuticals, Petaluma, California; also referred to in some publications as Dermacyn™ from Dyamed Biotech Pte Ltd., Singapore).8,11,20–22 In this article, we’ve summarized the available studies related to the core formulation, its available vehicles for topical application, its potential modes of action, and in-vitro and in-vivo study outcomes.
HOCl as an Integral Component of Topical Formulations Used in the Management of Various Skin Disorders
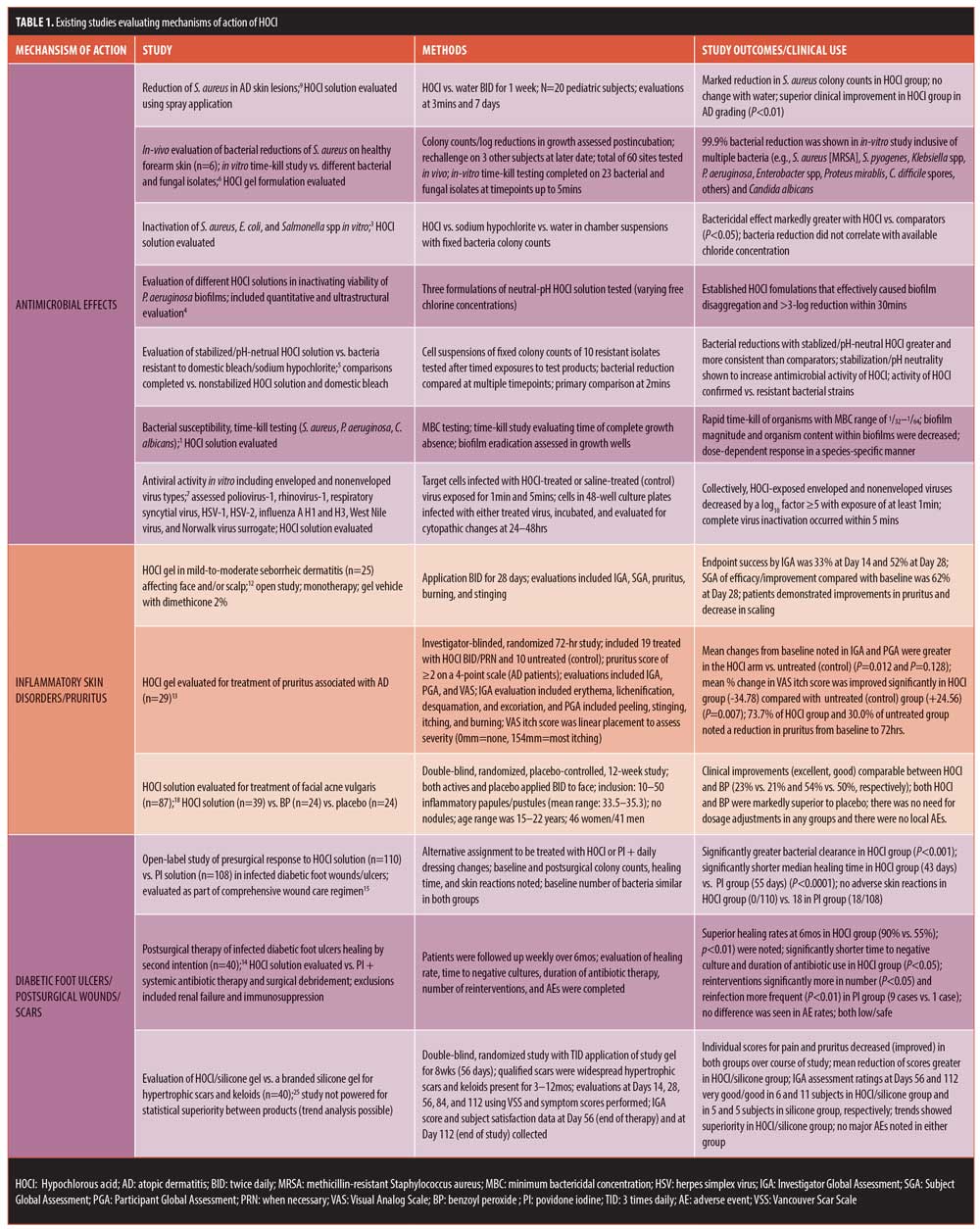
HOCl has been incorporated into topical formulations due to antimicrobial, anti-inflammatory, immunomodulatory, and wound healing properties.1,6,8,10,11,17,20–22 The central microbicidal role of HOCl as a component of an innate immune response to combat pathogens within human phagocytic cells (i.e., neutrophils, monocytes, macrophages) is a pivotal concept in appreciating the potential therapeutic value of HOCl.11 The antimicrobial activity of HOCl is not that of a conventional antibiotic but rather an agent that is directly toxic to microbial cells, including many gram-positive and gram-negative bacteria (e.g., Staphylococcus aureus, Pseudomonas aeruginosa) and their biofilms.1–7 However, it is important to understand the relative balance of HOCl versus other reaction byproducts that are produced in equilibrium after the HOCl solution is applied. Formulation stability, pH, and resultant chemical reactivity influence the relative concentrations of HOCl, as compared with other byproducts (e.g., hypochlorite [-OCl]), which directly impacts the magnitude of antimicrobial activity, clinical effects, and potential irritancy of topical HOCl formulations.11,20,21 In highly acidic pH (pH<3.5), HOCl markedly decreases with the production of multiple byproducts while, in a higher alkaline environment (pH>5.5), much of the HOCl is converted to -OCl.11 HOCl stability is optimized over a pH range of 3.5 to 5.5.11 Stabilized/pH-neutral HOCl is superior in terms of antimicrobial activity to nonstabilized HOCL and acidified bleach in vitro, including against hypochlorite-resistant strains.5,11,21 This cumulative fundamental understanding of the physiochemical properties of HOCl led to the development of the stabilized and pH-neutral core formulation of HOCl solution mentioned previously (Microcyn® Technology) and its incorporation into different vehicles that allow for ease of application in clinical practice.8,22 Existing studies evaluating the antimicrobial effects of HOCl are summarized in Table 1.
In addition to antimicrobial effects, other biologic properties of HOCl are likely to be clinically relevant. In a variety of laboratory studies, HOCl has been shown to decrease the activity of histamine, neutrophil-generated leukotrienes (i.e., LTB4), interleukin (IL)-6 and IL-2, and, in high concentrations, to downregulate some matrix metalloproteinases (MMPs) (e.g., MMP-7, collagenases), diminish mast-cell degranulation and cytokine release induced by immunoglobulin E, and induce favorable effects on keratinocyte and fibroblast migration.1,10,11,17,22,23 The reduction of superantigen-producing S. aureus on atopic skin by HOCl application might also be a mechanism for a decrease in cutaneous inflammation associated with both eczematous dermatitis and pruritus in AD.1,9,11 Both antimicrobial and anti-inflammatory/immunomodulatory properties appear to correlate with clinical benefits of HOCl when used for the treatment of various skin disorders, including AD/atopic skin, seborrheic dermatitis, lower extremity diabetic ulcers, pruritus, and acne vulgaris.1,6,9,11–18 Other clinical applications include promotion of wound healing and scar prevention.14–16,24
Completed Studies Supporting Antimicrobial Activity and Other Potential Clinical Applications of HOCl
There are several publications that include discussion and review of the antimicrobial properties of HOCl and topically applied stablilized/pH-neutral HOCl; methodologies have included preservative time-kill testing, microbial reduction studies on forearm skin with rechallenge, microbial analyses of diabetic foot ulcers, microbial analyses of atopic skin, and skin preparation studies.1–9,14–16 Other publications discuss specific clinical applications using a variety of parameters to determine study outcomes. Table 1 depicts details from major studies that address the antimicrobial effects and other therapeutic properties and applications with use of stablilized/pH-neutral HOCl.
Summary
HOCl exhibits broad-spectrum antimicrobial activity that is directly toxic to many bacteria and fungi and might also impart antiviral properties. Hypochlorous acid exhibits anti-inflammatory and immunomodulatory properties based on multiple laboratory analyses. These properties appear to correlate with the potential therapeutic benefits of topically applied HOCl for a variety of skin disorders. Topical formulations of stabilized, pH-neutral HOCl (e.g., solution, gel, spray) have been evaluated in several studies demonstrating both antimicrobial effects and therapeutic benefit in many cutaneous disorders, including seborrheic dermatitis, atopic dermatitis-associated pruritus, acne vulgaris, diabetic foot ulcers, and hypertrophic scars/keloids. Topical HOCl appears to be well tolerated and safe, without any major adverse events reported.
References
- Sakayra S, Gunay N, Karakulak M, et al. Hypochlorous acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing properties. Wounds. 2014;26(12):342–350.
- Day A, Abdulnaster A, Carney B, et al. Disruption of biofilms and neutralization of bacteria using hypochlorous acid solution: an in vivo and in vitro evaluation. Advances Skin Wound Care. 2017;30(12):543–551.
- Issa-Zacharia A, Kamitani Y, Tiisekwa A, et al. In vitro inactivation of Escherichia coli, Staphylococcus aureus, and Salmonella spp using slightly acid electrolyzed water. J Biosci Bioeng. 2010; 110(3):308–313.
- Sauer K, Thatcher E, Northey R, et al. Neutral super-oxidized solutions are effective in killing P. aeruginosa biofilms. Biofouling. 2009;(25(1): 45–54.
- Dardine J, Diaz L, Matinez C, et al. Activity of a pH neutral superoxidized solution against bacteria selected for sodium hypochlorite resistance. Poster L-1144 presented at the 47th ICAAC Meeting. Chicago, IL; 17–20 Sep 2007.
- Fowler J. Antibacterial activity of Microcyn gel formulation containing hypochlorous acid. Poster presented at Winter Clinical Dermatology Conference 2016; Koloa, Hawaii; 15–20 Jan 2016.
- Taketa-Graham M, Gutierrez AA, Thatcher E. The anti-viral efficacy of a new super-oxidized solution. Poster L-1144 presented at the 47th ICAAC Meeting. Chicago, IL; 17–20 Sep 2007.
- IntraDerm Pharmaceuticals. Data on file.
- Sasai-Takedatsu M, Kojima T, Yamamoto A, et al. Reduction of Staphylococcus aureus in atopic skin lesions with acid electrolytic water—a new therapeutic strategy for atopic dermatitis. Allergy. 1997;52(10):1012–1016.
- Medina-Tamayo J, Sanchez-Miranda E, Ballez-Tapia H, et al. Super-oxidized solution inhibits IgE-antigen induced degranulation and cytokine release in mast cells. Int Immunopharmacol. 2007;7(8):1013–1024.
- Pelgrift RY, Friedman AJ. Topical hypochlorous acid as a potential treatment for pruritus. Curr Derm Rep. 2013;2(3):181–190.
- Draelos Z. An evaluation of oculus sebuderm-Microcyn based gel on mild to moderate facial and scalp seborrheic dermatitis. Poster presented at the 10th Annual Coastal Dermatology Symposium; Sonoma, CA: 24–27 Sep 2014.
- Berman B, Nestor M. Investigator-blinded, randomized study evaluation of HOCl in the treatment of atopic dermatitis-associated pruritus. Poster presented at the Fall Clinical Dermatology Conference 2017. Las Vegas, Nevada; 12–15 Oct 2017.
- Piaggesi A, Goretti C, Mazzurco S, et al. A randomized controlled trial to examine the efficacy and safety of a new super-oxidized solution for management of wide postsurgical lesions of the diabetic foot. Int J Lower Extr Wounds. 2010;9(1):10–15.
- Dalla Paola L, Brocco E, Senesi A, et al. Super-oxidized solution (SOS) for infected diabetic foot ulcers. Wounds. 2006;18(9):262–270.
- Totoraitis K, Cohen JI, Friedman A. Topical approaches to improve surgical outcomes and wound healing: a review of efficacy and safety. J Drugs Dermatol. 2017;16(3):209–212.
- Friedman AJ, Cash K, Berman B. Hypochlorous acid is anti-inflammatory and immunomodulatory. Poster presented at the Winter Clinical Dermatology Conference 2013. Kauai, Hawaii; 18 Jan 2013.
- Tirado-Sanchez A, Ponce-Olivera RM. Efficacy and tolerance of superoxidized solution in the treatment of mild to moderate inflammatory acne: a double-blind placebo-controlled, parallel-group, randomized, clinical trial. J Dermatol Treat. 2009;20(5):289–292.
- Duc QI, Breetveld M, Middlekoop E, et al. A cytotoxic analysis of antiseptic medication on skin substitutes and autograft. Br J Dermatol. 2007;157(1):33–40.
- Wang L, Bassiri M, Najafi R, et al. Hypochlorous acid as a potential wound care agent: part 1. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6:e5.
- Hostynek JJ, Wilhelm KP, Cua A, et al. Irritation factors of sodium hypochlorite solutions in human skin. Contact Dermatitis. 1990;23(5):316–324.
- Oculus Innovative Sciences. Data on file.
- Mainnemare A, Megarbane B, Soueidan A, et al. Hypochlorous acid and taurine-N-monochloramine in periodontal disease. J Dent Res. 2004;83(11):823–831.
- Gold MH, Andriessen A, Dayan SH, et al. Hypochlorous acid gel technology—its impact on postprocedure treatment and scar prevention. J Cosmet Dermatol. 2017;16(2):162–167.
- Bucko AD, Draelos ZD, Dubois JC, et al. Double-blind, randomized study to determine the substantial equivalence of Microcyn scar management hydrogel vs Kelo-cote scar gel for the management of hypertrophic or keloid scars. Poster presented at the Winter Clinical Dermatology Conference 2016. Koloa, Hawaii; 15–20 Jan 2016.

