J Clin Aesthet Dermatol. 2023;16(8):47–50.
J Clin Aesthet Dermatol. 2023;16(8):47–50.
by Uzma Noureen, MD; Rohan R. Shah, BA; Nadia Waqas, MD, FCPS; Shawana Sharif, MD;
Amar Shah, BA; and Babar K. Rao, MD
Drs. Noureen, Waqas, and Sharif are with the Department of Dermatology at Rawalpindi Medical University in Rawalpindi, Pakistan. Mr. Rohan R. Shah and Mr. Amar Shah are with the Department of Dermatology at Rutgers New Jersey Medical School in New Brunswick, Jersey. Dr. Rao is with the Department of Dermatology at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey and the Department of Dermatology at Weill Cornell Medical School in New York, New York.
FUNDING: No funding was provided for this article
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Cutaneous warts are benign epithelial lesions caused by human papillomavirus and are common entities, affecting nearly 10 percent of the United States population. While most warts spontaneously resolve, the immunocompromised are susceptible to recalcitrant warts which often require medical treatment. Most current therapies use either physical or chemical destruction for wart removal, but these treatments are associated with adverse effects. Intralesional vitamin D3 has the potential to demonstrate a stronger treatment response due to its ability to stimulate the immune system at the injection site via cell-mediated immunity. We sought to test the efficacy of intralesional vitamin D3 for wart treatment in a sample size of 70 patients over a three-month period. Efficacy was determined as “excellent” if there was greater than a 90-percent reduction in both size and number of lesions, “good” if there was a 60 to 89-percent reduction, and “fair” if there was less than a 60-percent reduction. Treatment efficacy was excellent in 20 (28.6%) patients, good in 29 (41.4%) patients, fair in 18 (25.7%) patients, and poor in three (4.3%) patients. Patients in the younger age group had a higher treatment efficacy compared to other treatment groups. Thus, intralesional vitamin D3 has promising qualities as a treatment for cutaneous warts and should be considered at the clinician’s disposal. Vitamin D is an innovative approach for treating warts without the various side effects posed by other commonly used agents. The unique features of this treatment modality including its simplicity, safety, and efficiency make it a promising option for a very common cutaneous condition. Keywords: Verruca vulgaris, vitamin D3, warts, intralesional immunotherapy.
Cutaneous warts are benign epithelial lesions caused by human papillomavirus (HPV) and are transferred by skin-to-skin contact.1 Although warts are benign and approximately 66 percent spontaneously disappear within 24 months, patients often seek removal for cosmetic reasons. Warts can spontaneously recur and affect a diverse patient population, thus multiple treatment options at the clinician’s disposal can help to ensure treatment efficacy regardless of immunity status.2
Currently, there are many treatment options available for wart removal, including home remedies, over- the- counter products, and outpatient therapies. These therapies either focus on physical or chemical tissue destruction aimed to remove the wart rather than kill the virus. First-line options include salicylic acid, which must be applied daily for up to 12 weeks, and cryotherapy which also requires multiple treatments and is painful.3 Such destructive therapies often lead to recurrence since the primary source of infection is not being treated.4 Immunotherapy is promising in that it decreases the likelihood of wart recurrence by supplementing the immune response to the virus.4 There are currently many immunotherapy options available in treating warts including intralesional injections of mumps antigen, candida antigen, and the HBV vaccine.3,4
Topical vitamin D has been previously used in some cases for wart treatment and is believed to exert its effect through regulation of epidermal cell proliferation and modulation of cytokine production upon binding vitamin D receptors.5 Experimental evidence suggests that the immunomodulatory effects of vitamin D occur through a suppression of interleukin-6 (IL-6), IL-8, and tumor necrosis factor (TNF)-α. Interferon gamma levels have been shown to be elevated after vitamin D injection. Intralesional vitamin D3 injections have the potential to demonstrate a stronger treatment response due to their ability to stimulate the immune system at the injection site via cell-mediated immunity.6 We sought to test the efficacy of intralesional vitamin D3 for wart treatment.
Methods
This study was designed as a quasi-experimental trial. The sample size of 70 was determined using a confidence level of 95 percent and an absolute precision of 10 percent to determine the efficacy of intralesional vitamin D3 among patients with cutaneous warts. Efficacy was determined in terms of clearance of warts in a three-month period and was classified as “excellent” if all warts had resolved completely or there was greater than a 90-percent reduction in both size and number of lesions. Response was classified as “good” response if there was between 50 to 90-percent reduction in both size and number of lesions, “fair” if there was a 30 to 50-percent reduction in both size and number of warts, and “poor” if response was less than 30 percent.
Patients were included if they were between the ages of 12 and 70 with a clinical diagnosis of cutaneous warts including the plantar, palmar, and common types. Lactating and pregnant patients were excluded. Patients were also excluded if they were immunosuppressed, including a history of HIV. Other exclusion criteria included a history of hypersensitivity reactions to vitamin D3 or a history of incomplete treatment for cutaneous warts.
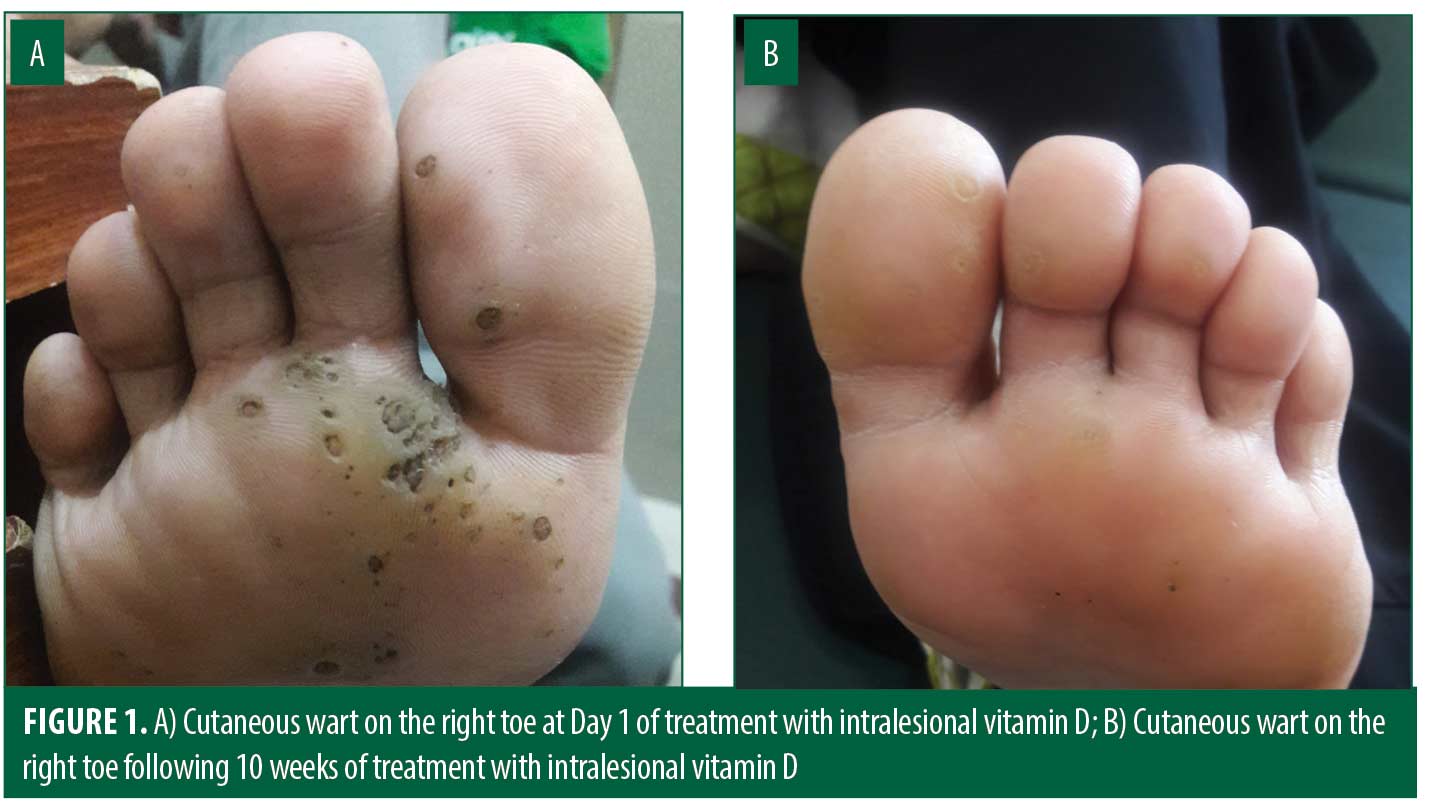
Seventy patients meeting selection criteria were selected by a consecutive sampling method. Patients with cutaneous warts were enrolled in this study after written informed consent. At the first visit, baseline evaluation and demographic data was collected and recorded. Patients with multiple warts were selected for immunotherapy. Vitamin D3 (0.2mL, 15mg/dl) was injected into the base of the warts after injecting with lidocaine (0.2mL, 15mg/dl). The injections were repeated two weeks apart for a maximum of four sessions or until complete clearance, whichever was earlier. All patients were followed-up for three months after the last injection. Clinical response was documented by recording decrease in number and size of lesions at each session and three months after the last injection.
All the data was analyzed using the SPSS 20 software. Quantitative data, like age and duration of warts, was presented as mean and standard deviation. Qualitative data, like sex and efficacy of the treatment, was presented by frequency and percentages. Data was stratified for age, gender, and duration of warts. A post-stratification, chi-square test was completed. P-values were considered significant if ≤0.05.
Results
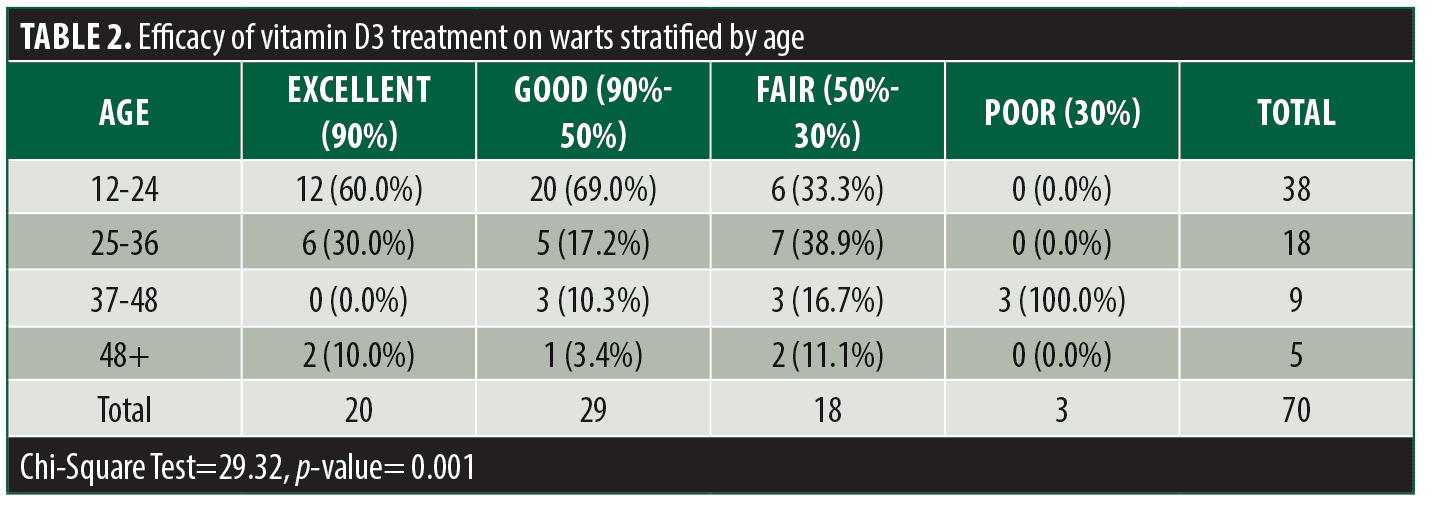
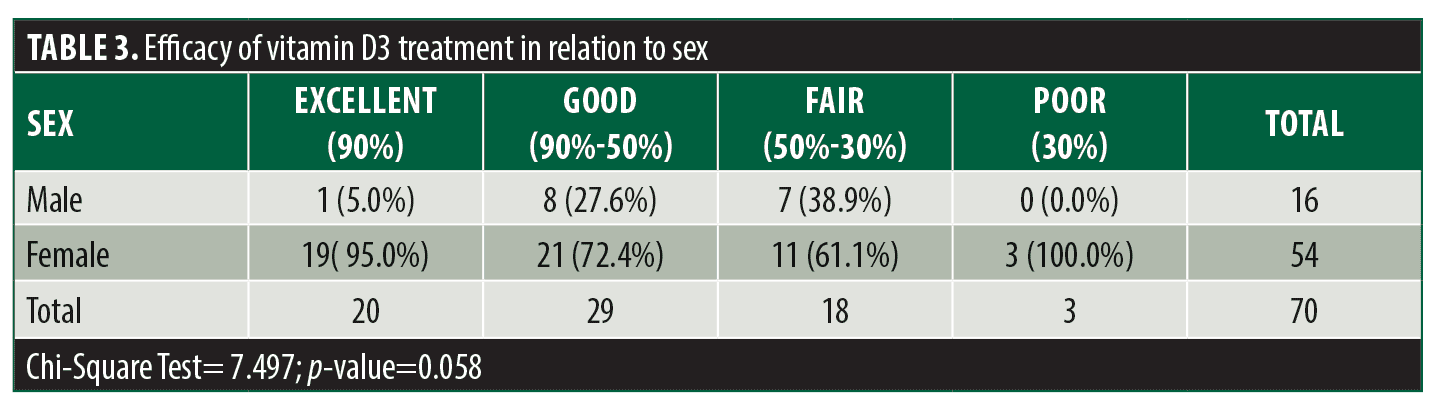
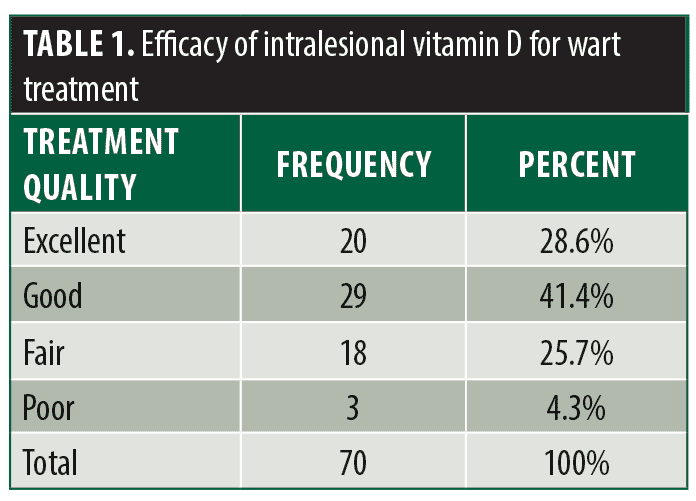
The mean age of the patient population was 26.82±11.54 years with a minimum and maximum age of 12 and 57 years respectively. 16 (22.9%) were male and 54 (77.1%) were female and the mean duration of cutaneous warts among patients was 2.71±1.50 months. Treatment efficacy was excellent in 20 (28.6%) patients, good in 29 (41.4%) patients, fair in 18 (25.7%) patients, and poor in three (4.3%) patients. (Table 1) Patients in the younger age group had a significantly higher treatment efficacy compared to other treatment groups (p=0.001, Table 2). The patient’s sex was correlated with treatment efficacy but was not statistically significant (p=0.058, Table 3). Disease duration had no significant impact on treatment efficacy.
Discussion
Destructive therapies such as cryotherapy, electrocautery, topical salicylic acid, and cantharidin are potential treatments for cutaneous warts, but are associated with side effects including pain, skin blistering, and scarring. Immunotherapy options include intralesional injections of candida antigen, HBV vaccine, tuberculin purified protein derivative, and the measles, mumps, rubella (MMR) vaccine.7 The HBV vaccine was shown to be an effective alternative method for intralesional wart treatment when compared to a control group receiving intralesional saline. Since it is not a live vaccine, the HBV vaccine can be considered a treatment option in immunocompromised patients.8 The MMR vaccine stimulates the immune system to fight HPV and some studies have shown a clearance rate of up to 85 percent. However, injection site pain and flu-like symptoms were common adverse effects.8 Candida antigen injections exert a therapeutic effect by stimulating a cell-mediated immune response. Patients responsive to therapy had higher levels of interferon-gamma compared to those who showed no response to treatment.3
Vitamin D has a well-documented role within the adaptive and innate immune system. Both, vitamin D receptors (VDR) and 1α-hydroxylase, are found on a variety of immune cells, including mononuclear cells.9 Vitamin D has been shown to play a role in mediating the immune response to tuberculosis infection. When M. tuberculosis binds to toll-like receptors on macrophages, VDR and 1α-hydroxylase are upregulated, which increases cathelicidin, an antimicrobial peptide. Cathelicidin upregulation enhances the destruction of M. tuberculosis.10 Vitamin D levels correlate with the degree of the immune system’s response against a viral infection; therefore, vitamin D levels can be related to the likelihood of developing a viral infection. Within the adaptive immune system, vitamin D has been shown to influence T-cells. When the VDR on T-cells is activated, vitamin D suppresses Th1 cell proliferation and promotes Th2 and T regulatory cell proliferation.10
As intralesional vitamin D3 is garners more attention, multiple studies have tested the clinical value of its role in the treatment of cutaneous warts. In a study of 64 patients with recurring warts, a dose of 0.2–0.5mL, 15mg/mL was injected in 3-week intervals. Ninety percent of patients reported complete resolution after 15 weeks. The average number of sessions for complete clearance was 3.66.11 Another study of 42 patients with multiple cutaneous warts showed complete response in 78.8 percent of patients who were treated with intralesional vitamin D3.12 A separate study in which 20 patients received injections of 7.5mg/mL of vitamin D along with 0.1ml of 20mg/mL of prilocaine found 80 percent of patients with a complete response after eight weeks. A two-month follow-up showed no signs of recurrence or adverse side effects.10 A recently published randomized control study from Egypt found that 40 percent of patients treated with intralesional vitamin D3 had complete clearance of the targeted warts, compared to 5 percent of patients in the control group who were injected with normal saline solution.13 Similarly, Abdel-Azim et al14 demonstrated that of 30 patients receiving intralesional saline for treatment of their wart, only one patient had partial improvement after four sessions. Six months after the last session, there was no evidence of improvement in any of the patients who received intralesional saline. Of the patients that received intralesional vitamin D injections, 56.25 percent had complete resolution of their warts. Six months after the last session, none of these patients had any evidence of recurrence. This study suggests that the mechanism behind the effectiveness of intralesional vitamin D injections in clearing viral warts is not solely due to injection-related irritation but may also result from an injection-induced immune response.14
The underlying mechanism of vitamin D injection in treating warts is still not completely understood; however, it has historically been used as a topical agent in the treatment of warts. By upregulating vitamin D receptors, it controls cell proliferation and differentiation of keratinocytes and immune cells of the skin.9 This leads to the activation of toll-like receptors on human macrophages and induction of antimicrobial peptide expression such as thymic stromal, lymphopoietin, and cathelecidin. It also reduces the synthesis of IL-1a and IL-6, resulting in reduced inflammation.3
In our study, efficacy of treatment was excellent among 20 (28.6%) patients, good for 29 (41.4%), fair for 18 (25.7%) and poor for three (4.3%) patients. Our results are consistent with the findings of Aktas et al10 and Raghukumar et al11 by showing promising efficacy for intralesional vitamin D3 in the treatment of cutaneous warts. According to our findings, intralesional vitamin D3 injections may be a viable mode of treatment for persistent warts. Though warts are often initially treated with methods such as cryotherapy or electrocautery, destructive methods may result in unnecessary scarring or recurrence.3 Given that viral warts can be resistant to the more routine treatment methods available, it is important to have a variety of therapeutic options available to increase the likelihood each patient can find an appropriate treatment method while also avoiding unnecessary adverse effects. Furthermore, patients of all age ranges may develop viral warts, including children. It was observed that patients aged 3 to 40 years old reported only mild injection-site pain which was well-tolerated.10 It is important to consider the child’s pain tolerance when determining which treatment method to use. Thus, having a variety of options can improve patient compliance as well. Intralesional vitamin D3 injection is a simple, well-tolerated treatment method that is easy to administer in outpatient clinics and should be considered by the clinician as a reliant option in the treatment of cutaneous warts.
Conclusion
Viral warts can be cleared by the immune system without any intervention. Common treatment options capitalize on this principle by stimulating the immune system to aid in clearing cutaneous warts. Vitamin D has a clear, distinct role within the immune system; as a result, its properties can be used in the treatment of viral warts. Results of our study show that 28.6 percent of total participants who received vitamin D injections had a greater than 90-percent clearance of warts, and 70-percent of total participants had at least a 50-percent clearance of their warts. Only 4.3 percent of patients had a poor response rate of less than 30-percent clearance. It was found that intralesional vitamin D3 had higher efficacy in younger age groups when compared to other treatment groups. Sex and duration of disease did not have a significant impact on the efficacy of the treatment. Given that most of the participants were women, it may be helpful to complete a similar study with more male subjects to determine if sex has a significant impact on treatment outcomes. Vitamin D, although perhaps unconventional, is an innovative approach for treating warts without the various side effects posed by other commonly used agents. The unique features of this treatment modality including its simplicity, safety, and efficiency make it a promising option for a very common cutaneous condition.
References
- Witchey DJ, Witchey NB, Roth-Kauffman MM, et al. Plantar Warts: Epidemiology, Pathophysiology, and Clinical Management. The Journal of the American Osteopathic Association. 2018;118:92–105.
- Aldahan AS, Mlacker S, Shah VV, et al. Efficacy of intralesional immunotherapy for the treatment of warts: A review of the literature. Dermatologic therapy. 2016;29:197–207.
- Muse ME, Stiff KM, Glines KR, et al. A review of intralesional wart therapy. Dermatology online journal. 2020;26.
- Shalaby ME, Hasan MS, Elshorbagy MS, et al. Diagnostic and therapeutic implications of vitamin D deficiency in patients with warts: A case-controlled study. Journal of cosmetic dermatology. 2022;21:1135–1142.
- Bikle DD. The Vitamin D Receptor as Tumor Suppressor in Skin. Advances in experimental medicine and biology. 2020;1268:285–306.
- Kareem IMA, Ibrahim IM, Mohammed SFF et al. Effectiveness of intralesional vitamin D(3) injection in the treatment of common warts: Single-blinded placebo-controlled study. Dermatologic therapy. 2019;32:e12882.
- Bruggink SC, Gussekloo J, Berger MY, et al. Cryotherapy with liquid nitrogen versus topical salicylic acid application for cutaneous warts in primary care: randomized controlled trial. CMAJ: Canadian Medical Association journal. 2010;182:1624–1630.
- Shaheen MA, Salem SA, Fouad DA, et al. Intralesional tuberculin (PPD) versus measles, mumps, rubella (MMR) vaccine in treatment of multiple warts: a comparative clinical and immunological study. Dermatologic therapy. 2015;28:194–200.
- Chia-Han Yeh M, Tsai TY, Huang YC. Intralesional vitamin D3 injection in the treatment of warts: A systematic review and meta-analysis. Journal of the American Academy of Dermatology. 2020;82:1013–1015.
- Aktaş H, Ergin C, Demir B, et al. Intralesional Vitamin D Injection May Be an Effective Treatment Option for Warts. Journal of cutaneous medicine and surgery. 2016;20:118–122.
- Raghukumar S, Ravikumar BC, Vinay KN, et al. Intralesional Vitamin D(3) Injection in the Treatment of Recalcitrant Warts: A Novel Proposition. Journal of cutaneous medicine and surgery. 2017;21:320–324.
- Fathy G, Sharara MA, Khafagy AH. Intralesional vitamin D3 versus Candida antigen immunotherapy in the treatment of multiple recalcitrant plantar warts: A comparative case-control study. Dermatologic therapy. 2019;32:e12997.
- Kavya M, Shashikumar BM, Harish MR, et al. Safety and Efficacy of Intralesional Vitamin D3 in Cutaneous Warts: An Open Uncontrolled Trial. Journal of cutaneous and aesthetic surgery. 2017;10:90–94.
- Abdel-Azim E, Abdel-Aziz R, Ragaie M, et al. Clinical and dermoscopic evaluation of intralesional vitamin D<sub>3</sub> in treatment of cutaneous warts: a placebo-controlled study. 2020;17:6–12.